Abstract
“An important objective of modern pharmaceutical research is the discovery of new medical uses for known molecules” (UKSC 2018), a component of secondary pharmaceuticals. This Viewpoint’s focus is the defense of the vulnerable strategy of secondary pharmaceutical patents (SPPs). Typical claims thereof are new medical uses, dosage, selection, and enatiomer patents. The attacks on secondary pharmaceuticals, including chiral switches, use negative-connotation terms, such as “evergreening”, “product hopping”, and “pejorative”. Most enantiomer patents, including the controversial Nexium patents, were challenged in courts worldwide yet validated. This Viewpoint considers the “teaching away” defense of nonobviousness of Nexium enantiomer patents due to “unexpected results”, applying stereochemistry principles. Physical organic chemistry arguments and the prediction of lower energy barriers of epimerization/racemization of benzylic anions of esomeprazole and dexlansoprazole (compared with their uncharged enantiomers) are a basis of the “teaching away”. This prediction is verified by DFT computations. “Obvious to try” of many SPPs should not prevail over “unexpected results”. A generalized concern about “evergreening” drugs should not be a justification for comprehensive attacks on SPPs. Following UKSC Lyrica decision (2018), plausibility, a condition of patent validity, may enter the arena of enantiomer patents, claiming second medical uses. Secondary pharmaceutical dosage, selection, improvement, and enantiomer patents are not necessarily obvious.
Keywords: Stereochemistry, chiral switch, evergreening, teaching away, second medical-use patent, Nexium
Introduction
1. In 2015, the United Nations Development Programme issued Guidelines for Pharmaceutical Patent Examination: Examining Pharmaceutical Patents from a Public Health Perspective (UN Guidelines). The heart of the UN Guidelines is a category-by-category examination of 12 types of secondary pharmaceutical patent claims: Markush claims; selection patents; polymorphs; enantiomers; salts; ethers and esters; compositions; doses; combinations; prodrugs; metabolites; and new medical uses.1,2Secondary pharmaceutical patent is a patent that protects a range of aspects, other than the direct active pharmaceutical ingredient (protected by the primary pharmaceutical patent). Pharmaceutical firms’ use of secondary patents to extend the period of exclusivity generates concerns among policymakers worldwide.3 Secondary patents are essential components of Pharmaceutical Lifecycle Management (LCM) (Box 1 and Box 2). The UN Guidelines postulate that many forms of pharmaceutical innovation are inherently routine and absent some sort of exceptional circumstance should be treated as obvious/noninventive and hence unpatentable.1,2
Box 1. DEFINITIONS OF TERMS.
Secondary Pharmaceutical Patent
A patent that protects a range of aspects other than the direct active pharmaceutical ingredient (protected by the corresponding primary pharmaceutical patent).
See Section 1.
Chiral Switch
See Section 3.
Enantiomer Patent
A patent that claims a single enantiomer of a chiral compound that has been claimed in the corresponding previous patent as a racemate or as a mixture of diastereomers.
Selection Patent
A patent claiming an invention that selects a group of individually novel members from a previously known class, on the basis of superior properties.
Improvement Patent
A patent that claims an invention in which its elements were disclosed in the prior art, yet includes an inventive step that was not suggested or disclosed in the prior art.
Dosage Patent
Patents directed to a product in the context of its dosage regimen (schedule of dose, frequency duration, etc.), which provides novelty and nonobviousness.
Method of Use Patent (Second Medical Use Patent)
A patent directed to a product in the context of its medical use (indication), which provides novelty and nonobviousness.
Person Skilled in the Art (PSITA)
A skilled practitioner (or a group of practitioners) in the relevant field of technology who is possessed of average knowledge and ability and is aware of what was common general knowledge in the art at the relevant date.
Obvious to Try
A patentability criterion for establishing prima facie obviousness wherein it is examined whether a person skilled in the art would have possessed any reasonable expectation of success of obtaining a beneficial technical effect of the claimed invention.
Unexpected Results
A term used to assess the nonobviousness of a claimed invention. It needs to be shown that the results were superior as compared with the prior art, to an nonobvious extent.
Teaching away
See Section 8.
Lifecycle Management (LCM)
Optimizing the marketing lifetime performance of pharmaceutical brands, within the context of the company’s overall business, product, and project portfolio.
Evergreening
See Section 4.
Box 2. DEFINITIONS OF TERMS (Continued).
Product Hopping
A term relating to a pharmaceutical manufacturer stopping the marketing of a drug formulation under patent expiry, yet concomitantly marketing a new formulation that is patent protected.
Pay for Delay (aka Reverse Payment)
A term that relates to drug manufacturer offering generic companies’ payments not to bring lower-cost alternatives to market.
Me-Too Drug
A drug within the same chemical class as another already on the market.
2. The United Kingdom Supreme Court (UKSC), in a landmark judgment in the case of the single-enantiomer drug Lyrica (pregabalin), stated (2018 UKSC 56): “An important objective of modern pharmaceutical research is the discovery of new medical uses for known molecules. This commonly involves expensive research programs, which will not be rewarded and will therefore not happen unless patent protection is available. Patent protection for second medical use patents (Box 1 and Box 2) is, however, difficult to accommodate within the traditional scheme of patent law”.
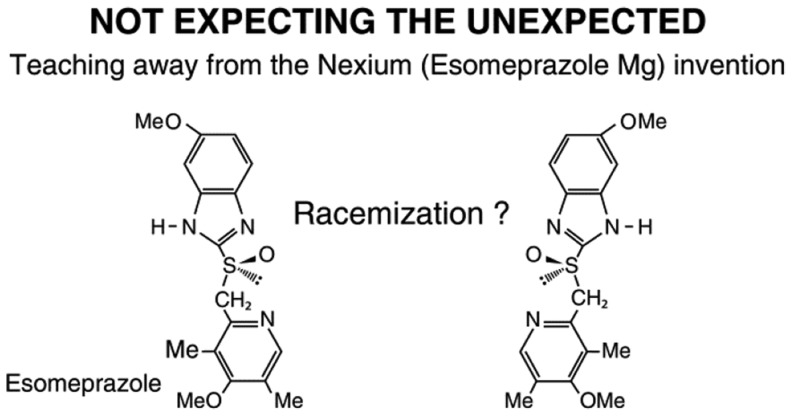
3. A chiral switch is the development of a single enantiomer from a chiral drug that has previously been developed (and often approved and marketed) as a racemate or as a mixture of diastereomers.4 The essential criterion of a chiral switch is a change in the status of chirality. The success of the chiral-switch strategy is manifested in the blockbuster drugs Lipitor (atorvastatin calcium), Plavix (clopidogrel hydrosulfate), and Nexium (esomeprazole magnesium) (Figure 1), which headed the list of global-annual sales of pharmaceutical products in 2008–2014. Since the emergence of chiral switches, this strategy, and in particular their enantiomer-patent components,4 which are necessary conditions for success, have been continuously under attack globally. Enantiomer patents (Box 1 and Box 2) are a type of secondary pharmaceutical patents.3 Most of the enantiomer patents of chiral-switch drugs have been challenged in courts in jurisdictions worldwide.4 (A list of these chiral-switch drugs is appended in Table 1). The attacks on chiral switches in scientific publications, books, magazines, and other news-media, have been using negative-connotation terms such as “evergreening” (vide infra), “product hopping”5 (Box 1 and Box 2), “me-too”6 (Box 1 and Box 2), “patent extenders”,6 “trivial patents”, and “dark side of pharma”.
Figure 1.
Enantiomers and Tautomers of Omeprazole.
Table 1. Chiral-Switch Drugs with Challenged Enantiomer Patents.
| Brand Name | Active Ingredient(s) |
|---|---|
| Lipitor | Atorvastatin Calcium |
| Plavix | Clopidogrel Bisulfate |
| Nexium | Esomeprazole Magnesium |
| Lexapro/Cipralex | Escitalopram Oxalate |
| Levaquin/Travanic | Levofloxacin |
| Xopenex | Levalbuterol Dihydrochloride |
| Focalin | Dexmethylphenidate Hydrochloride |
| Altace | Ramipril |
| Seractil | Dexibuprofen |
| Xyzal | Levocetirizine Hydrochloride |
| Lunesta | Eszopiclone |
| Nuvigil | Armodafinil |
| Exelon | Rivastigmine Tartarate |
| Azilect | Rasagiline Mesylate |
| Adderal XR 10 | Amphetamine Aspratate; Amphetamine Sulfate; Dextroamphetamine Succharate Dextroamphetamine ; Sulfate |
| Fusilev | Levoleucovorin |
| Precedex | Dexmedetomidine Hydrochloride |
| Ketanest | Esketimine Hydrochloride |
| Dexilant | Dexlansoprazole |
| Vimovo | Naproxen/Esomeprazole Magnesium |
| Amoxillin | Amoxycilin |
4. Evergreening
“Evergreening” is a controversial term. It is not a formal concept of patent law. It is best understood as a social idea, used to refer to the myriad ways in which pharmaceutical patent owners utilize patents. A 2014 Working Paper offered the following definition (without value judgment): “IP based evergreening is the business strategy to extend the duration of the effective protection derived or derivable from a portfolio of Intellectual Property Rights in order to increase the appropriability of an innovation or a set of business related innovations or technologies”.7 It highlighted the Losec (omeprazole) to Nexium chiral switch as a case particularly rich in many aspects of evergreening. The advantages of incremental innovation in drug development leading to me-too drugs have been highlighted.8 Drug makers often argue that additional patent applications filed prior to regulatory approval incentivize companies to invest in the development of a new drug and should not be characterized as evergreening. Evergreening has become a pejorative term to mean that innovator pharmaceutical companies abuse the patent and regulatory systems to delay the legitimate entry of generic competition. GlaxoSmithKline (GSK) labeled evergreening as an inherently pejorative term (2014). GSK rejected the accusations that later improvements unjustifiably delay generic competition, that improvements subject to later patents are not medically important and should not be encouraged and that improvement patents are not justified within patent law. The patent system allows for generic versions of the basic product to compete with the improved product. Therefore, secondary patents are not a barrier to generic competitors.
By “product hopping” (Box 1 and Box 2), a brand-name manufacturer delays generic competition and maintains market exclusivity.5 It may arguably occur in the patenting of a purified enantiomer after the patenting of its parent racemate,5 hence “enantiomer product hopping”. The UN Guidelines have recommended that “isolated enantiomers should not be deemed patentable when the racemic mixture was previously disclosed”.1 In certain jurisdictions, an enantiomer patent is considered a selection patent (Box 1 and Box 2).4 The Supreme Court of Canada held in the enantiomer patent litigation of the chiral-switch drug Plavix (clopidogrel bisulfate) that evergreening is a legitimate concern and, depending on the circumstances, strategies that attempt to extend the time limit of exclusivity of a patent may be contrary to the objectives of the Patent Act. However, the court noted that “a generalized concern about evergreening is not a justification for an attack on the doctrine of selection patents” (2008 SCC 61), including enantiomer patents. The court stated that selection patents encourage improvements over the subject matter of the original genus patent because selection does something better than or different from what was claimed in the genus patent.
5. Chiral Switch of Losec/Prilosec to Nexium
One of the most successful chiral-switch drugs and yet the most defamed, infamous, heavily under-attack controversial drug3,6,9 is the “new purple pill”6 Nexium (esomeprazole magnesium), a single-enantiomer proton-pump inhibitor (PPI, H+, K+-ATPase inhibitor) (Figure 1). Nexium is indicated for gastric acid-related problems, such as gastroesophageal reflux disease (GERD). It was approved by EMA and FDA in 2000 and 2001, respectively, and reached average US$7.9bn global annual sales in 2008–2014 and US$72.5bn in 2001–2017. The pharmacological and clinical benefits of esomeprazole beyond those seen with the racemate omeprazole, emphasizing the enhanced bioavailability, specificity for the proton pump, and inhibition of acid secretion were noted. Nexium, called “Half-o’-Prilosec”,6 has been highlighted as a typical case of evergreening. A New Yorker article has alleged that “Nexium has become a symbol of everything that is wrong with the pharmaceutical industry” and “is little more than a repackaged version of an old medicine”.10 The chiral switch of Prilosec to Nexium has been characterized as “the most famous—yet infamous example of late-stage lifecycle management of recent years”.3 Nexium has been presented as the most globally famous case of “isomer patenting”, an application of the strategy of “successive patenting of improvements (evergreening)”.9 The “hop” from Prilosec to Nexium has been highlighted, whereas its claimed unexpected improved results have been challenged.5
“Paradoxically”, the courts in many jurisdictions worldwide have validated many enantiomer patents of chiral-switch drugs, including Nexium, rejecting the criticism and challenges. Recent decisions in Nexium-patent litigations which have verified the nonobvoiusness/inventive step of the Nexium invention are Federal Court of Canada 2014 FC 638 (see also Supreme Court of Canada (2017 SCC 36) striking down the Promise Doctrine and upholding Nexium patent as useful), Federal Court of Appeal of Canada 2015 FCA 258), Federal Court of Australia 2013 FCA 368, EPO Technical Board of Appeal, EPO-1760/11-3.3.1. Moreover, AstraZeneca and Ranbaxy prevailed in the Nexium “pay-for-delay” (“reverse payment”) (Box 1 and Box 2) U.S. antitrust litigation case (15-2005 (Ist Cir. 2016)). The validities of patents of the double chiral-switch drug Vimovo (naproxen/esomeprazole magnesium), an NSAID/PPI fixed-dose combination drug, have recently been litigated (2017-2473 (Fed. Cir. 2019)).
6. A recent essay entitled “Expecting the Unexpected”,11 put forward, inter alia, the frontal argument that chiral switches and their enantiomer patents are “obvious to try”4 (Box 1 and Box 2), a doctrine which must prevail over the doctrine of “unexpected results” and thus renders these patents obvious (vide infra).11 The result of the essay’s analysis and conclusions, if accepted, “will be a blow to pharmaceutical patent owners who have come to rely on patents for inventions that are obvious to try”.11
This Viewpoint focuses on the question whether secondary pharmaceutical patents are inherently obvious/noninventive. We apply here the “teaching away” defense of nonobviousness of chiral switches due to the “unexpected results” doctrine using the case of Nexium (esomeprazole magnesium). The second medical use patent of Lyrica (pregabalin) and the patentability of dosage patents (Box 1 and Box 2) are briefly discussed in the light of recent UKSC decisions. As such, this Viewpoint can also be applied to other secondary pharmaceutical patents, validating their patentability.
Results and Discussion
7. The popular argument against the patentability of chiral switches is that their case is “obvious-to-try”, even when the actual effects of the switch may turn out to be unexpected.11 The mentioned essay11 argued that enantiomers and salts that are known variant types on existing drugs are an important example of the conflict between the two doctrines in patent law, “obvious-to-try” and “unexpected results”; when the two conflict, “obvious-to-try” must prevail, because it is consistent with the logic of the obviousness doctrine, a cornerstone of patent law. It has been argued that “Any enantiomer of a known drug will either have a different effect than the racemate or not, but that is a fact that is inherent to the enantiomer, not a product of inventiveness” (quoted in ref (11), footnote 104). The essay’s analysis and conclusions are based on an interpretation of the U.S. Supreme Court KSR decision ((127 S.Ct. 1727 (2007)) wherein the US court tried to establish the test for obviousness noting that an invention was not patentable if it was obvious to try. The KSR decision coined this test on an invention from the mechanical field, however overreaching to implicate directly in evaluating the validity of claims covering single enantiomers.12 Sweet has argued that KSR has not resulted in a major change in the substantive standard of nonobviousness related to enantiomers.12 A prima facie case of obviousness for enantiomer patents is still rebuttable post-KSR by demonstrating objective indicia of nonobviousness; secondary considerations are still significant evidence for this purpose.12
8. Teaching away Defense
A reference may be said to teach away when a person skilled in the art, upon reading the reference, would be discouraged from following the path set out in the reference or would be led in a direction divergent from the path that was taken by the applicant. In general, a reference will teach away if it suggests that the line of development flowing from the reference’s disclosure is unlikely to be productive of the result sought by the applicant (27 F.3d. 551 (Fed, Cir. 1994)). “A finding that a prior art reference teaches away from combining references can alone defeat an obviousness claim” (388 F. Supp. 3d 717 (N.D. VA 2005)). Proper evidence of teaching away would discourage scientists from trying the invention and therefore could rebut a case of obvious to try. Enantiomer patents have been “obvious to try” (unless taught away) since the 1980s.4 “Teaching away” should be distinguished from the recently applied “absence of any teaching”.13
9. Considerations of Tautomerism and Stereochemistry in Omeprazole and Esomeprazole
a. Tautomerism
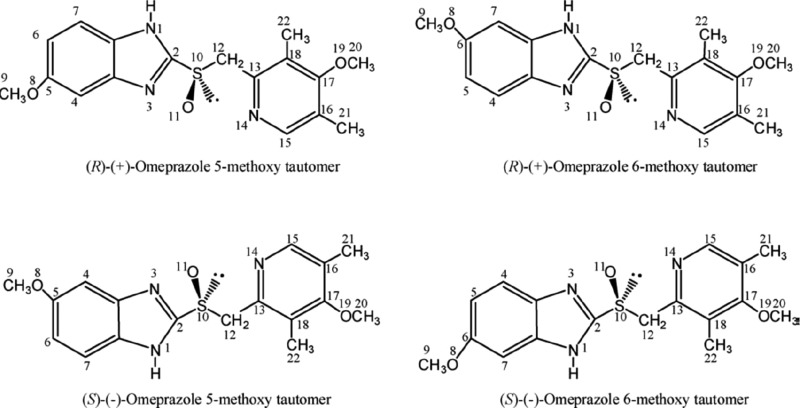
Esomeprazole and esomeprazole C12-anion exists each as a mixture of two tautomers: 5-methoxy- and 6-methoxy-[4-methoxy-3,5-dimethyl-(2-pyridinyl)methyl]sulfinyl-1H-benzimidazole (Figure 1); they undergo fast isomerization in solution. Tautomerism does not exist in esomeprazole N1-anion.
b. Stereochemistry
i. Chiral (Stereogenic) Center at S10
The chirality of omeprazole stems primarily from the presence of a chiral center at the sulfur atom S10 of the methylsulfinyl bridge between the 1H-benzimidazole and the pyridine moieties. In view of the additional stereogenic elements in esomeprazole (vide infra), the pyramidal inversion of S10 in esomeprazole, (S)-S10 → (R)-S10, is an epimerization, not an enantiomerization. Likewise, (S)-S10 ⇌ (R)-S10 per se is not considered a racemization.
ii. Chirality Axis at the Pyridine Ring
The rotation around the chirallty axis in the pyridine ring of esomeprazole and its paired enantiomer gives rise to diastereomerizations of (S)-omeprazole and (R)-omeprazole, (S)-S10,M) ⇌ ((S)-S10,P) and (R)-S10,M) ⇌ (R)-S10,P), respectively. The methoxy substituent at C17 of the pyridine ring is not coplanar with the pyridine ring, due to the presence of the two ortho-methyl substituents (at C16 and C18). This spatial orientation introduces a chirality axis and, in principle, may lead to atropisomers (conformers which owing to steric or electronic constraints, interconvert slowly enough that they can be isolated). In the present study, the effective energy barrier (ΔG‡) for the (S)-S10,M) ⇌ (S)-S10,P) diastereomerization of 6-methoxy tautomer of esomeprazole proved to be very low, 5.8 kcal/mol at B3LYP/6-311G(d,p). The (S)-S10,M) and (S)-S10,P diastereomers should not be considered atropisomers.
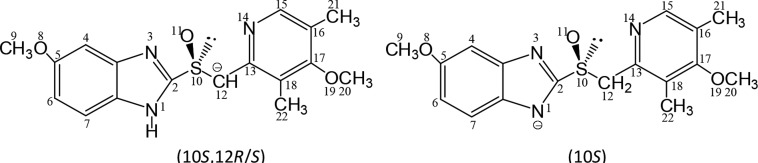
iii. Chiral Center at C12 of the C12-Anion
This second chiral center is formed at the prochiral C12 of esomeprazole (in addition to the sulfur atom S10). Neutral esomeprazole gives in the presence of a base the diastereomeric benzylic (S)-S10,(R)- C12 and (S)-S10,(S)-C12- anions.
iv. Chiral Pathway
The transition state for pyramidal inversion of the C12-anion is chiral. This is due to the presence of the additional chiral center C12 and a slight deviation from the plane containing the four atoms S10, C12, and H12 and C13, so that the C12-anion lacks mirror symmetry. Racemization processes may take place also via intermediates and/or transition states which do not contain mirror symmetry. This phenomenon is known as “chiral pathway”. A chiral pathway of enantiomerization is defined as a pathway that connects two enantiomers but does not pass through an achiral conformation, including transition states and intermediates. The pathways of enantiomerization/epimerization of the C12-anion of esomeprazole, (S)-S10,(S)-C12⇌ (R)-S10,(R)-C12/(R)-S10,(S)-C12, are chiral pathways.
v. Racemization vs Epimerization and Diastereomerization
There are up to three stereogenic elements (aka elements of stereoisomerism) in esomeprazole and its anions: chiral center at S10, chirality axis at the pyridine ring, and chiral center at C12 (only at C12-anion). The considerations of stereochemistry in the present outline are mainly concerned with the chiral inversion of the sulfur chiral center (S10) of esomeprazole ((S)-S10⇌(R)-S10)). The energy barriers for M⇌P rotation of the chirality axis at the pyridine ring (paragraph iii above) and for the inversions of the chiral center C12 at the C12-anion ((S)-C12⇌(R)-C12) (section iv above) are very low. Therefore, chiral inversion of S10 is expected to be accompanied by (S)-C12⇌(R)-C12 inversion and M⇌P rotation, namely, enantiomerization, leading to racemization. In neutral esomeprazole, there are only two stereogenic elements (the chiral center at S10 and the chirality axis at the pyridine ring), so that only epimerization of S10 ((S)-S10,(M)⇌(R)-S10,(M)/ (S)-S10,(P)⇌(R)-S10,(P))) and enantiomerization leading to racemization ((S)-S10,(M)⇌(R)-S10,(P))/((S)-S10,(P)⇌(R)-S10,(M)) are taken into account.
10. Teaching Away from the Nexium Invention
There are three aspects of teaching away from the invention of the chiral switch of the racemate omeprazole to (S)-omeprazole (esomeprazole), taking into account considerations of stereochemistry and tautomerism (vide supra).
i. A Teaching Away from the Invention of Omeprazole Single Enantiomers.
It had been confirmed in 1990, before the priority date of the first esomeprazole patents, that both enantiomers of omeprazole (Figure 1) had the same inhibitory effect on the proton pump in an in vitro gastric gland model.14
ii. Epimerization/Racemization of Each of the Single Enantiomers of Omeprazole under Acidic Conditions.
The first US patents of esomeprazole, US5,693,828, US5,714,504, and US5,877,192, priority date 28.05.1993, claimed the optically pure salts of the (−)-enantiomer of omeprazole, esomeprazole, a process for the preparation of the single (+)- and (−)-enantiomers comprising separating diastereomeric esters followed by hydrolysis under basic conditions, and their method of use. A person skilled in the art (PSITA) (Box 1 and Box 2) was aware on the priority date of these patents that omeprazole is in fact a prodrug acting in vivo as a proton-pump inhibitor (PPI) by means of the “omeprazole cycle”.15 This mechanism involves the active achiral tetracyclic sulphenamide sulfenic intermediate (and/or the achiral sulfenic acid), formed rapidly and reversibly under acidic conditions in the stomach, which then attacks the gastric proton-pump enzyme, H+,K+-ATPase, forming irreversibly a disulfide complex with the thiol group of Cys-813 (by a nucleophilc reaction of the thiol with the reactive sulfur atom of the sulfenamide) to inhibit the enzyme.15 Indeed, each of the omeprazole enantiomers is not stereochemically stable and has been shown to undergo racemization in vivo, under acidic conditions.15 Therefore, there has been a teaching away from any process for a resolution of the racemic drug omeprazole under acidic conditions.
iii. Epimerization/Racemization of Each of the Single Enantiomers of Omeprazole under Basic Conditions.
US Patent 5,693,828 claimed (inter alia) a process for the preparation of pure (+)- and (−)-enatiomers of omeprazole comprising separating diastereomeric N-acyloxymethyl esters wherein acyl (e.g., mandeloyl) designates a chiral acyl group, having either R or S configuration and dissolving each of the separated diastereomers in an alkaline solution of above about pH 7 so as to hydrolyze the acylmethyl group off from the separated diastereomers to give the optically pure intact enantiomers. The solvolysis is also performed in alkaline solution wherein the pH is more than about 7, containing a base in a protic or an aprotic solvent. A PSITA was aware on the priority date of the patents that in a neutral environment, the single enantiomers of omeprazole were not expected to undergo racemization at ambient temperature. Mislow et al. showed in the late 1960s that the experimentally determined energy barriers for racemizations of simple sulfoxides are 35–43 kcal/mol, meaning their single enantiomers are stable at ambient temperatures. Erlandsson et al. reported in 1990 a chiral-HPLC resolution of omeprazole and derivatives thereof, using a basic moving phase, and an experimental racemization barrier of 26 kcal/mol at 75 °C.14 However, this reported experimental racemization barrier should be taken with a grain of salt. The authors argued that the separation character was dependent on the benzimidazole-N1 proton-donating power, as expressed in the pKa values of the tested compounds. For omeprazole, pKa = 8.72. Even though the protons of the methylene C12 of omeprazole were less acidic than the proton of the benzimidazole N1, they were sufficiently acidic to be exchanged with a deuteron in a reaction with D2O and NaOD. The accepted mechanism of thermal racemization of sulfoxides is the pyramidal inversion mechanism. The transition state for pyramidal inversion of the chiral center S10 of the C12-anion of esomeprazole was expected to be stabilized relative to the ground-state conformation. In other words, the energy barrier for racemization/epimerization of the C12-anion was expected to be considerably lower than the corresponding barrier of uncharged esomeprazole. A PSITA who have applied considerations of physical organic chemistry established at the priority date of the patents, would have known that the single enantiomers of omeprazole would be converted under basic conditions to their respective benzimidazole N1- and benzylic C12-anions and N1, C12-dianion (Figure 2). Moreover, the energy barriers for epimerization/enantiomerization of the benzylic C12-anion and the N1,C12-dianion would be substantially and significantly lower, as compared with the uncharged enantiomers. The reason for this expected lower energy barriers was based on qualitative electron delocalization considerations and on the relative stabilization of the transition state for pyramidal inversion, the accepted mechanism of thermal racemization of sulfoxides, as compared with the ground state. These considerations are elaborated in the next section. Hence, on the priority date of the first esomeprazole patents, the resolution of omeprazole under basic conditions was deemed to be unsuccessful. There was thus a teaching away from a resolution of omeprazole to its single enantiomers under basic conditions. From this point of view, the process for the preparation of pure (+)- and (−)-enantiomers of omeprazole under basic conditions was nonobvious. When a teaching away from an invention leads to unexpected results, it nullifies the necessary condition for “obvious-to-try”. The doctrine of “unexpected results” is then consistent with the logic of nonobviousness, and must prevail over the doctrine “obvious-to-try” in the conflict between these two doctrines in patent law. It has recently been argued that (in enantiomer inventions) “the illustration of unexpected results and/or a showing of teaching away are the only available option to successfully rebut the grounds of prima facie obviousness and hence ultimate nonobviousness”.13
Figure 2.
C12-anion (5-methoxy tautomer) and N1-anion of (S)-omeprazole.
11. In the transition state of the pyramidal inversion mechanism of thermal racemization of chiral sulfoxides, the four central atoms of the sulfinyl group, sulfur, oxygen, and the two carbon atoms bonded to sulfur, form one plane, and the nonbonding electron pair of the sulfur atom is in a p orbital perpendicular to this plane. In the corresponding transition state in esomeprazole (neutral), the sulfur atom S10 is essentially not a chiral center, because its configuration is not pyramidal. In the transition state for the pyramidal inversion of the C12-anion of esomeprazole, there is an enhanced electron delocalization of the S10=O11 π-bond electrons, the benzimidazole C2=N3 π-bond electrons, and the nonbonding electrons of the negatively charged C12. In addition, the π-electrons of the pyridine ring (e.g., C13=N14), which is coplanar with the sulfinyl group, can also participate in the electron delocalization of the C12-nonbonding electrons. This latter effect of electron delocalization was not expected in the ground-state conformation of the C12-anion, because its pyridine ring is not coplanar with the sulfinyl group anion.
12. The teaching way argument has previously been applied in the Plavix (clopidogrel bisulfate) enantiomer-patent litigation where the U.S. Federal Circuit affirmed the District Court decision that clopidogrel bisulfate was nonobvious (whether or not it may have been “obvious to try” separating the enantiomers), holding that “the prior art taught away from the use of sulfuric acid with an enantiomer, for strong acids could encourage racemization” (2007-1438 (Fed. Cir. 2008)).
13. The physical organic chemistry considerations and prediction of lower energy barriers of epimerization/racemization of the C12-anion of esomeprazole enantiomers, outlined in Sections 10 and 11, are the basis of the teaching away argument which leads to the inventive step/nonobviousness of the Nexium invention/patent. This prediction of a PSITA (at the priority date of the Nexium original enantiomer patents) is now verified by the results of our DFT computational study of the energy barriers of the epimerization/enantiomerization of the C12-anion of the esomeprazole enantiomers, as compared with the corresponding energy barriers of uncharged esomeprazole tautomers, using Gaussian 09.16 According to the pyramidal inversion mechanism, in each of these anion-epimers, an epimerization gives the paired epimer: (S)-S10, (R)-C12 → (R)-S10, (R)-C12 and (S)-S10, (S)-C12 → (R)-S10, (S)-C12. This epimerization is accompanied by fast P → M and M → P and fast (R)-C12 → (S)-C12/ (S)-C12 → (R)-C12 due to rotations around the chiral axis, resulting in enantiomerization, e.g., (S)-S10, (R)-C12, P → (R)-S10, (S)-C12, M, leading ultimately to racemization: (S)-S10, (R)-C12, P⇌ (R)-S10, (S)-C12, M. The analysis of the results of our computational study takes into account considerations of stereochemistry and tautomerism, as applied to omeprazole and its enantiomers (vide supra, Section 9).
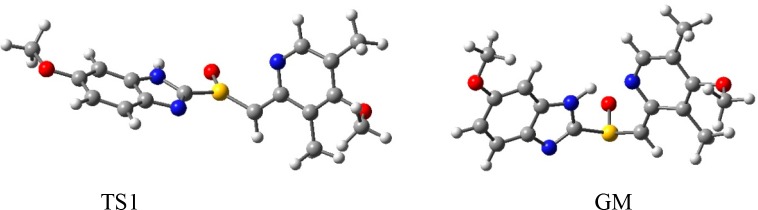
DFT calculations at B3LYP/6-311++G(d,p)/SMD(water) of (S)-omeprazole and its C12-anion gave the following results. In uncharged esomeprazole, the Gibbs free-energy barriers (ΔG298‡) of the pyramidal inversion of the chiral center S10 ((S)-S10,(R)-C12 → (R)-S10,(R)-C12), an epimerization) is 39.4 kcal/mol (5-methoxy tautomer) and 37.8 kcal/mol (6-methoxy tautomer). The respective ΔG298‡ barriers of epimerization in the C12-anion of esomeprazole are 29.4 kcal/mol (5-methoxy tautomer) and 29.8 kcal/mol (6-methoxy tautomer). Thus, ΔΔG298‡ = ΔG298‡ (esomeprazole) – ΔG298‡ (esomeprazole C12-anion) = 10.0 kcal/mol (5-methoxy tautomer) and 8.0 kcal/mol (6-methoxy tautomer). Very similar ΔΔG298‡ values for the corresponding enantiomerization processes are inferred, in view of the very low energy barriers for the M⇌P rotation of the chirality axis at the pyridine ring and for the inversions of the chiral center C12 at the C12-anion ((S)-C12⇌ (R)-C12) (vide supra) The striking computational results of substantial lowering of the energy barrier of epimerization/enantiomerization in the C12-anion of esomeprazole as compared with neutral esomeprazole verifies the prediction based on qualitative considerations (vide supra, Sections 10 and 11). Also, the predicted enhanced electron-delocalization effect in the transition state of the C12-anion (vide supra) is borne out by the lengthening of the S10=O12 bond and the shortening of the S10—C12 and S10–C2 bonds. Consequently, the teaching away considerations proved to be justified, leading to the conclusion that the invention of Nexium was nonobvious. The global minimum (GM) and the first transition state (TS1) for the pyramidal inversion of the 6-methoxy-tautomer of the C12-anion of (S)-omeprazole at B3LYP/6-311++G(d,p) are depicted in Figure 3. (See also ref (15).)
Figure 3.
Global minimum (GM) and the first transition state (TS1) for the pyramidal inversion of the 6-methoxy-tautomer of the C12-anion of (S)-omeprazole at B3LYP/6-311++G(d,p).
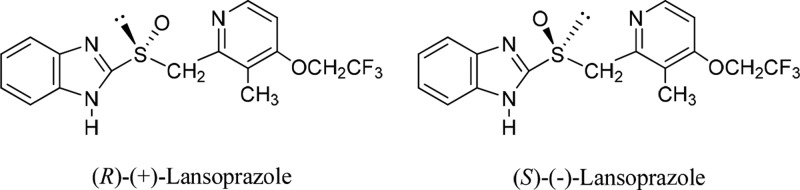
14. The chiral-switch drug Dexilant (dexlansoprazole), the (R)-(+)-enantiomer of the PPI racemic drug Prevacid (lansoprazole) (Figure 4), an omeprazole analogue, has been developed and marketed. Dexlansoprazole and esomeprazole differ in the absolute configuration of their chiral sulfur centers, R versus S. This unexpected result is a manifestation of nonobviousness: “not expecting the unexpected”. Dexlansoprazole is devoid of tautomers and a chiral axis; the pyramidal inversion of its sulfur chiral center is a true enantiomerization: (R)-S → (S)-S. The difference ΔΔG298‡ = ΔG298‡ (dexlansoprazole) - ΔG298‡ (dexlansoprazole benzylic C-anion) at B3LYP/6-311++(d,p) is 9.3 kcal/mol, very similar to the respective values of esomeprazole tautomers. FDA has determined that Nexium and Dexilant did not contain a New Chemical Entity (NCE); they were thus not eligible for 5-year regulatory exclusivity and have not been considered New Molecular Entities (NMEs). By contrast, EMA grants chiral-switch drugs, e.g. Nexium, under certain conditions, the status of New Active Substance (NAS), thus providing 10-year regulatory exclusivity. Patents covering the enantiomeric products subsequent to approval of their corresponding racemates, including Nexium, were consistently granted statutory patent term extensions by USPTO, “for the enantiomer is a different drug product from the corresponding racemate” (603 F.d 1377 (Fed. Cir. 2010)).
Figure 4.
Enantiomers of lansoprazole.
15. The validities of enantiomer patents of drugs developed by application of the strategy of chiral switches should be evaluated on a case-by-case basis. Irrespective of the teaching away analysis (vide supra), the argument11 that in enantiomer patents, when the two doctrines “obvious-to-try” and “unexpected results” conflict, “obvious-to-try” must prevail, because it is consistent with the logic of the obviousness doctrine, (vide supra), should be dismissed. When the description in an enantiomer patent includes superior pharmacological and/or pharmaceutical properties of the claimed single enantiomer, versus the racemate (efficacy, and/or reduced toxicity and/or solubility and other pharmaceutical properties), well above the expected ratio of 2:1,4 “unexpected results” wins over, so that inventiveness/nonobviousness is established. The UKSC stated in the Cialis (tadalafil) dosage patent (Box 1 and Box 2) case ([2019] UKSC 15) (vide infra, Section 18) that “The relevance of the ‘obvious to try’ consideration and its weight when balanced against other relevant considerations depend on the particular facts of the case”. “The fact that the results which the inventor actually carried out are unexpected or surprising is a relevant consideration as it may point to an inventive step···”.
16. As long as “the predicated demise of racemic new molecular entities remains an exaggeration”, chiral switches are here to stay. Drugs and drug candidates are still being marketed/developed as racemates, including the class of immunomodulatory drugs derived from thalidomide. The chiral center of these thalidomide analogs is chemically unstable, resulting in interconversion of the enantiomers both in vitro and in vivo. There has therefore been a teaching away from developing single enantiomers of racemic-thalidomide analogs. The teaching away argument may also hold true in the cases of deuterium-stabilized single enantiomers of thalidomide analogs, rendering these derivatives nonobvious. Through stabilization of the chiral center with deuterium, DeWitt et al. have recently shown that the in vitro antiinflammatory and in vivo antitumorigenic activities of a thalidomide analog currently in clinical development (CC-122) are caused exclusively by one enantiomer. Their findings enable the development of improved thalidomide analogs as therapeutics following stated regulatory guidance for the development of single enantiomers.
17. The UKSC landmark judgment in the single-enantiomer drug Lyrica litigation ([2018] UKSC 56, vide supra, Section 2) defines a role for plausibility in the experiment for sufficiency of disclosure of a second medical-use patent (Box 1 and Box 2) (aka Swiss-form patent). Plausibility is not a distinct condition of validity, but one element in the test of sufficiency. Warner-Lambert is the proprietor of EP(UK) 0934061 for Lyrica (pregabalin), covering a second medical use of pregabalin for the treatment of inflammatory pain and neuropathic pain (and other indications). The validity of the patent of pregabalin (EP(UK) 0641330), which disclosed the indications seizure disorders, notably epilepsy, and generalized anxiety disorder (GAD), expired in 2013. The validity of the second medical-use patent has been challenged on the grounds of insufficient disclosure. The UKSC held that plausibility of disclosure is a requirement for sufficiency under UK Law. “The proposition that a product is efficacious for the treatment of a given condition must be plausible.” “It must always be necessary for the patentee to demonstrate that he has included in the specification something that makes the claim to therapeutic efficacy plausible. Otherwise a mere assertion of efficacy would be enough.” “The specification must disclose some reason for supposing that the implied assertion of efficacy in the claim is true.” It has not escaped our minds that the concept of plausibility as a condition of patent validity may enter the arena of enantiomer patents of chiral-switch drugs claiming a second medical use.
Lyrica, the (S)-(+)-enantiomer, may be considered a chiral-switch drug. US Patent 6,197,819 B1 claiming pregabalin is based on US Application No. 07/681,692, filed on November 27, 1990 (the priority date). This original patent application claimed, inter alia, (R)-, (S)-, and (RS)-4-amino-3-(2-methylpropyl)butanoic acid, without a selection and a preference of any one of them. The patent prosecution first included Application No. 07/886.080 (the continuation-in-part of Application 07/681,692) filed on May 20, 1992 (later abandoned), which claimed (claim 4) the single enantiomer “S-(+)-4-amino-3-(2-methypropyl)butanoic acid” (pregabalin, aka (S)-(+)-3-isobutyl GABA). Thus, US Application No. 07/886,080 may have constituted a selection patent application. The selection of the (S)-(+)-enantiomer eventually led to the single claim of a method of treating a patient having seizure disorders which comprises administering to said patient an effective amount of substantially pure compound of the formula (S)-(+)- 4-amino-3-(2-methylpropyl)butanoic acid (US Patent 5,563.175). Thus, from the point of view of stereochemistry, the single (S)-(+)-enantiomer was selected from a group of three: the (R)-enantiomer, the (S)-enantiomer, and the (RS)-racemate.
18. The UKSC has recently addressed in an appeal concerning the application of the test of obviousness to a dosage patent (Box 1 and Box 2) and added few general remarks on selection patents (Box 1 and Box 2) and improvement patents (Box 1 and Box 2) ([2019] UKSC 15). These are types of secondary pharmaceutical patents. The dosage patent in suit relates to the use of Cialis (tadalafil) in a dosage form for the treatment of sexual dysfunction. The UKSC held, quoting the judgment on appeal ([2017] EWCA 15) that “it was not the law that investigations into appropriate dosage regimes cannot yield patentable inventions”. The UKSC stated the possibility that a dosage patent with such claims may be valid has been recognized both by the EPO and in the UK courts. “There is no policy reason why a novel and inventive dosage regime should not be rewarded by a patent”. The UKSC held that the patent-in-suit (EP(UK) 1,173,181) was invalid for lacking an inventive step. The UKSC also noted that it does not militate against selection patents or improvement patents. “Selection patents are patentable as involving an inventive step if the selection is not arbitrary and is justified by a hitherto unknown technical effect...or, in other words, when they make a real, novel and nonobvious technical advance...”. “Improvement” in the context of the law of patents is “in the most technical sense...an invention which comes within the claims of an earlier patent but contains a further inventive step... The use of well-known research tests of itself does not render such selections and improvements obvious”. Thus, according to the UKSC, secondary pharmaceutical dosage, selection, and improvement patents are not necessarily obvious; in principle, they may be patentable.
In any event, the attempt to invalidate all enantiomer patents on the basis of obviousness (vide supra)11 (an attempt which is criticized in the present Viewpoint), is not necessarily relevant to second medical-use enantiomer patents and to patents of chiral-switch combination drugs, including double chiral-switch combination drugs such as Vimovo (vide supra, Section 5).
Conclusion
19. In conclusion, the teaching away defense of enantiomer patents should be considered and explored in order to maintain the strategy of chiral switches. “An applicant should attempt to find any teaching away that discourages a resolution or use of a particular enantiomer”.17 “The fallacy of the premise that patents on enantiomers somehow provide “evergreened” protection for products whose patents have expired”2 has thus been verified. The arguments and conclusions against chiral switches and enantiomer patents should be rejected so that drug discovery will not be handicapped. Nonobviousness of enantiomers was and continues to be the key issue in deciding the patentability of enantiomers.13 Hopefully, our application of the teaching away defense in chiral-switch inventions will contribute to the continuation of the strategy of secondary pharmaceuticals in drug discovery and development. As a corollary, a generalized concern about evergreening should not be a justification for comprehensive attacks on the doctrine of secondary pharmaceutical patents, including enantiomer patents.
Author Present Address
† Mailing address of Hili Marom: Dr. Hili Marom, Principal, Nofey Golan High School, P.O. Box 18, Qatsrin 1290005, Israel.
Views expressed in this editorial are those of the authors and not necessarily the views of the ACS.
The authors declare no competing financial interest.
References
- Correa C. M.Guidelines for Pharmaceutical Patent Examination: Examining Pharmaceutical Patents from a Public Health Perspective; United Nations Development Programme: New York, 2015; pp 1–44. [Google Scholar]
- Holman C. M. In Defense of Secondary Pharmaceutical Patents: A Response to the UN’ Guidelines for Pharmaceutical Patent Examination. Indiana Law Rev. 2017, 50, 759–811. 10.18060/4806.1153. [DOI] [Google Scholar]
- Ellery T.; Hansen N.. Pharmaceutical lifecycle management: making the most of each and every brand; Wiley: Hoboken, NJ, 2012; pp 161, 344–351. [Google Scholar]
- Agranat I.; Wainschtein S. R. The Strategy of Enantiomer Patents of Drugs. Drug Discovery Today 2010, 15, 163–170. 10.1016/j.drudis.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Metzke M. Targeting Enantiomer Product Hopping with a New “Obviousness” Standard. UCLA J. Law & Technology. 2010, 14, 1–31. [Google Scholar]
- Angell M.The Truth About the Drug Companies: How They Deceive Us and What to Do About It; Random House: New York, 2004; pp 74–93. [Google Scholar]
- Granstrand O.; Tietze F.. IP strategies and policies for and against evergreening. European Policy for Intellectual Property, Brussels Conference. University of Cambridge: Cambridge, CIM Working Paper, 2014; Vol. 4, pp 1–36. https://www.ifm.eng.cam.ac.uk/uploads/Research/CTM/working_paper/2015-01-Granstrand-Tietze.pdf (Last accessed 10th March 2019). [Google Scholar]
- Wertheimer A. I.; Santella T. M.. Pharmaceutical Evolution: The Advantages of Incremental Innovation in Drug Development. Issue Analysis; Competitive Enterprise Institute: Washington, DC. Issue Analysis, 2009, No. (2), , 1–19. [Google Scholar]
- Moir H. V. J.Empirical evidence on patents and data protection: Response to the Productivity Commission’s Issues Paper on Intellectual Property Arrangements, Attachment C: Pharmaceuticals: an insight into patent standards and costs. 2015; pp 1−19. https://www.pc.gov.au/__data/assets/pdf_file/0006/195792/sub130-intellectual-property-attachmentc.pdf Last accessed 31st December, 2019. [Google Scholar]
- Gladwell M.High Prices. How to think about prescription drugs. New Yorker, 25th October, 2004. https://www.newyorker.com/magazine/2004/10/25/high-prices. Last accessed 5th October. 2019. [Google Scholar]
- Lemley M. A. Expecting the Unexpected. Notre Dame Law Rev. 2017, 92, 1369–1394. [Google Scholar]
- Sweet M. J. The Patentability of Chiral Drugs Post-KSR: The More Things Change, The More They Stay The Same. Berkeley Technology Law J. 2009, 24, 129–147. [Google Scholar]
- Dhulap S.; Kulkarni M. G. Prima facie obviousness of pharmaceutical patents inplications for enantiomers. World Pat. Inf. 2018, 54, 39–45. 10.1016/j.wpi.2018.07.008. [DOI] [Google Scholar]
- Erlandsson P.; Isaksson R.; Lorentzon P.; Lindberg P. Resolution of the enantiomers of omeprazole and some of its analogues by liquid chromatography on a triphenylcarbamoylcellulose-based stationary phase. J. Chromatogr., Biomed. Appl. 1990, 532, 305–319. 10.1016/S0378-4347(00)83781-0. [DOI] [PubMed] [Google Scholar]
- Jana K.; Bandyopadhyay T.; Ganguly B. Revealing the Mechanistic Pathway of Acid Activation of Proton Pump Inhibitors To Inhibit the Gastric Proton Pump: A DFT study. J. Phys. Chem. B 2016, 120, 13031–13038. 10.1021/acs.jpcb.6b09334. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr., Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, 2009. [Google Scholar]
- Spenner J. M. Obvious-to-Try Obviousness of Chemical Enantiomers in view of Pre- and Post-KSR Analysis. J. Patent and Trademark Office Society. 2008, 90, 475–540. [Google Scholar]