Abstract
BACKGROUND
New treatments have improved outcomes for patients with relapsed chronic lymphocytic leukemia (CLL), but complete remissions remain uncommon. Venetoclax has a distinct mechanism of action; it targets BCL2, a protein central to the survival of CLL cells.
METHODS
We conducted a phase 1 dose-escalation study of daily oral venetoclax in patients with relapsed or refractory CLL or small lymphocytic lymphoma (SLL) to assess safety, pharmacokinetic profile, and efficacy. In the dose-escalation phase, 56 patients received active treatment in one of eight dose groups that ranged from 150 to 1200 mg per day. In an expansion cohort, 60 additional patients were treated with a weekly stepwise ramp-up in doses as high as 400 mg per day.
RESULTS
The majority of the study patients had received multiple previous treatments, and 89% had poor prognostic clinical or genetic features. Venetoclax was active at all dose levels. Clinical tumor lysis syndrome occurred in 3 of 56 patients in the dose-escalation cohort, with one death. After adjustments to the dose-escalation schedule, clinical tumor lysis syndrome did not occur in any of the 60 patients in the expansion cohort. Other toxic effects included mild diarrhea (in 52% of the patients), upper respiratory tract infection (in 48%), nausea (in 47%), and grade 3 or 4 neutropenia (in 41%). A maximum tolerated dose was not identified. Among the 116 patients who received venetoclax, 92 (79%) had a response. Response rates ranged from 71 to 79% among patients in subgroups with an adverse prognosis, including those with resistance to fludarabine, those with chromosome 17p deletions (deletion 17p CLL), and those with unmutated IGHV. Complete remissions occurred in 20% of the patients, including 5% who had no minimal residual disease on flow cytometry. The 15-month progression-free survival estimate for the 400-mg dose groups was 69%.
CONCLUSIONS
Selective targeting of BCL2 with venetoclax had a manageable safety profile and induced substantial responses in patients with relapsed CLL or SLL, including those with poor prognostic features. (Funded by AbbVie and Genentech; ClinicalTrials.gov number, NCT01328626.)
THE LANDSCAPE OF TREATMENT FOR RElapsed chronic lymphocytic leukemia (CLL) has recently changed with the introduction of agents that inhibit intracellular B-cell receptor signaling.1,2 Ibrutinib monotherapy3,4 and idelalisib in combination with rituximab5 induce responses in the majority of patients in whom chemoimmunotherapy has failed, and these patients have improved outcomes. Treatment is given indefinitely, and complete remission is uncommon, particularly in the first 2 years after the initiation of therapy. Persistent disease is a concern because of the potential development of resistance.6–8 Although many responses are durable and may deepen over time, relapses accumulate with ongoing follow-up,5,9 particularly in patients who have chromosome 17p deletions (deletion 17p CLL).9 The outcome for patients with disease progression while receiving ibrutinib treatment remains poor.7
Constitutively elevated expression of the antiapoptotic protein BCL2 renders CLL cells resistant to apoptosis, resulting in the accumulation of long-lived, clonal lymphocytes that characterize the disease.10–12 BH3-mimetic drugs are a new class of anticancer agents that mimic the activity of the physiologic antagonists of BCL2 and related proteins to trigger apoptosis.13–15 The first potent BH3-mimetic inhibitor of BCL2 that was evaluated in clinical trials, navitoclax,16 proved to be active against relapsed CLL, with partial responses observed in approximately 35% of the patients.17,18 Dose-limiting thrombocytopenia from concomitant on-target inhibition of BCL-xL, a related antiapoptotic protein critical for platelet survival,19 limited the ability to escalate the dose of navitoclax and precluded full clinical exploration of the potential of BCL2 antagonism.
Venetoclax (ABT-199/GDC-0199) is a highly selective inhibitor of BCL2 that is more potent than navitoclax but is less active against BCL-xL by a factor of more than 200.20 Venetoclax induced apoptosis in vitro against primary CLL cells and displayed efficacy in vivo in xenograft models of human lymphoid tumors that overexpressed BCL2, with minimal effects on platelets.20
On the basis of these preclinical data, we conducted a first-in-human phase 1 study in patients with relapsed or refractory CLL or small lymphocytic lymphoma (SLL). The primary objectives were to determine the safety profile, pharmacokinetic profile, and maximum tolerated dose, along with developing a potential dose and treatment schedule for a phase 2 trial. Secondary objectives were to assess response rates and other measures of efficacy. As the significant antitumor activity became apparent, an exploratory objective was added to evaluate minimal residual disease in patients who had a complete response.
METHODS
STUDY DESIGN
This study was designed as an open-label, multicenter, dose-escalation trial of venetoclax in patients with relapsed or refractory CLL or SLL or with non-Hodgkin’s lymphoma. All patients received active treatment. The results among patients with relapsed or refractory CLL or SLL are reported here. From June 2011 through December 2012, we enrolled patients in eight dose-escalation groups. From June 2013 through May 2014, we enrolled patients in an expansion cohort that received the dosing regimen that was based on data from the earlier part of the trial.
STUDY PATIENTS
Adults with CLL or SLL were eligible if they had relapsed or refractory disease requiring therapy according to standard criteria; an Eastern Cooperative Oncology Group performance score of 0 or 1 (on a 5-point scale, with higher scores indicating greater disability); adequate bone marrow function (defined as an absolute neutrophil count of 1000 per cubic millimeter or more and a platelet count of 50,000 per cubic millimeter or more, which was amended to 30,000 per cubic millimeter or more in the expansion cohort); a hemoglobin level of 8 g per deciliter or more; a creatinine clearance of 50 ml per minute or more; adequate hepatic function; and normal coagulation. The exclusion criteria included previous allogeneic or autologous stem-cell transplantation, major organ dysfunction, active infection, autoimmune cytopenias or other cancer, and current pregnancy or breast-feeding. (Details are provided in Table S1 in the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org.) All the study patients provided written informed consent.
STUDY TREATMENT
Patients were assigned sequentially to dose-escalation groups of three patients or more according to a 3+3 design,21 in which groups that received sequentially higher doses were opened after a minimum of three patients had completed 3 weeks of treatment at the preceding dose without having dose-limiting toxic effects. (See the Methods section in the Supplementary Appendix for details.) The first dose was 200 mg per day, and patients received a single initial dose followed by a washout period of at least 72 hours, which was followed by continuous daily administration. The occurrence of laboratory changes associated with tumor lysis in the first three patients led to the introduction of stepwise intrapatient increases in dose (ramp-up) to the designated group dose for the subsequent dose-escalation and expansion cohorts, respectively (Fig. 1A and 1B).
Figure 1. Venetoclax Schedules, Pharmacokinetic Response, and Activity against Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL).
Panel A shows the administration schedule for the 53 patients in the dose-escalation cohort who were enrolled after the first 3 patients (in a 3+3 design). On day –7 (7 days before week 1), an initial single 50-mg dose was administered to 50 patients and a 20-mg dose to 3 patients. Daily administration of a 50-mg dose started in week 1 and was stepped up to 100 mg in week 2 for patients in groups that were scheduled to receive 150 mg to 400 mg per day or to 150 mg for those in groups scheduled to receive 600 mg to 1200 mg per day before reaching the designated group dose in week 3; in the 600-mg group, an additional 400-mg step was incorporated. Panel B shows the administration schedule for the 60 patients in the expansion cohort. Daily administration started with 20 mg per day, followed by weekly ramp-up in three steps to 400 mg. Panel C shows plasma levels of venetoclax at steady state, grouped according to the dose at the time of collection. Panels D through F show the activity of venetoclax against CLL or SLL in blood (Panel D), lymph nodes (Panel E), and bone marrow (Panel F), which are shown as normalized changes from baseline. For each patient, the best response is presented, with color coding according to the designated dose group. Data for the absolute lymphocyte count in peripheral blood are included only for the 66 patients who had lymphocytosis immediately before the administration of venetoclax. Among 65 of these patients, the median time to a lymphocyte count of less than 4000 per cubic millimeter was 22 days (range, 1 to 451). Data for lymph-node disease are derived from the sums of the products of the perpendicular dimensions of target lesions as seen on computed tomography (CT) and as defined and reported by investigators for 110 patients who had at least one follow-up CT scan during the study. The median time to a 50% reduction in nodal size (as reported for 99 patients) was 42 days (range, 20 to 417), and the median time to a normalization in nodal diameter to less than 1.5 cm (as reported in 34 patients) was 8 months (range, 1 to 27). Data for changes in bone marrow infiltration in the 85 patients who underwent at least one bone marrow biopsy after the initiation of venetoclax are derived from hematopathological analysis of CLL infiltration. The median time until complete clearance of bone marrow infiltrate in 26 patients was 6 months (range, 2 to 22).
Patients continued to receive daily venetoclax until disease progression or unacceptable toxicity. Supportive care, antiinfection prophylaxis, and growth-factor support for substantial neutropenia were provided according to institutional standards of care. Prophylaxis against the tumor lysis syndrome and management of the condition if it occurred were specified in an early amendment to the study protocol, available at NEJM.org. For the expansion cohort, such prophylaxis required inpatient admission before and for 24 hours after the initial administration of the 20-mg dose and the first dose ramp-up to 50 mg. Patients underwent intravenous hydration, received allopurinol with or without rasburicase, and had strict monitoring of biochemical measures that was performed at 4, 6, 8, 10, 12, and 24 hours after each of the two doses. For subsequent dose increases, patients received oral hydration in an ambulatory care facility and had biochemical monitoring 8 and 24 hours after study-drug administration. In addition, patients with any lymph node measuring 10 cm in diameter or more or both bulky adenopathy measuring 5 cm or more and a lymphocyte count of 25,000 per cubic millimeter or more were considered to be at high risk for the tumor lysis syndrome and to require hospitalization for each ramp-up in dose (Tables S2 and S3 in the Supplementary Appendix).
ASSESSMENTS
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.22 Established criteria23 were used to evaluate and classify laboratory or clinical tumor lysis syndrome. Blood sampling for pharmacokinetic studies was performed before and 2, 3, 4, 6, and 8 hours after the first dose and after 3 or 6 weeks in the designated group. Measures of efficacy included overall response rate, progression-free survival, duration of response, time to progression, and overall survival.
Responses were evaluated by investigators on the basis of the International Workshop for CLL (IWCLL 2008) criteria24 or the International Working Group criteria for patients with SLL25 (Tables S4 and S5 in the Supplementary Appendix). According to the protocol, computed tomography was performed at the time of screening; at weeks 6, 12 or 16, and 24 and every 12 weeks thereafter; and at the final visit. Partial responses were confirmed with a second response assessment at least 2 months after first assessment. Marrow biopsies were performed at the time of screening, at week 24, and within 2 months after other criteria for complete response had been observed. Among patients who had a complete response, minimal residual disease was evaluated in marrow with the use of at least four-color flow cytometry,26 according to the protocol at each study site. Patients were followed for survival after discontinuation of the study drug.
STUDY OVERSIGHT
The study protocol was designed jointly by the sponsors (AbbVie and Genentech) and the investigators and approved by the institutional review board for each study site. The study was conducted according to the provisions of the Declaration of Helsinki and the International Conference on Harmonisation–Good Clinical Practice. Investigators and their research teams collected the clinical data. AbbVie confirmed and compiled the data and prepared summaries for analysis. All the authors had access to these data and analyses. The first author wrote the first draft of the manuscript, and subsequent drafts were prepared by the authors with assistance from a professional medical writer employed by AbbVie. All the authors made the decision to submit the manuscript for publication and vouch for adherence to the study protocol and for the accuracy and completeness of the data reported.
STATISTICAL ANALYSIS
The data cutoff for this report was August 25, 2015. All statistical and pharmacokinetic analyses are detailed in the Methods section in the Supplementary Appendix. Included in the safety and efficacy analyses were the patients who received at least one dose of venetoclax. Data were analyzed according to dose group and pooled for selected analyses, as specified. Descriptive statistics including medians, ranges, and standard deviations were calculated. Kaplan–Meier methods were used for time-to-event analyses. Data for the analysis of progression-free survival were censored at the time of the last tumor assessment for patients without an event or at the time of data cutoff if that assessment was performed after the cutoff.
RESULTS
STUDY PATIENTS
A total of 116 patients were enrolled in the study: 56 in the dose-escalation cohort and 60 in the expansion cohort. Patients had received a median of 3 previous therapies (range, 1 to 11), and 39% had resistance to the most recent therapy (Table 1). Disease features that were associated with an adverse prognosis with chemoimmunotherapy — resistance to fludarabine,27,28 chromosome 17p deletions,29,30 chromosome 11q deletions,29 unmutated IGHV,31 and bulky adenopathy32 — were present in 89% of the patients at study entry; 71% had two or more of these features.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Dose-Escalation Cohort (N = 56) | Expansion Cohort (N = 60) | All Patients (N = 116) |
|---|---|---|---|
| Age | |||
| Median (range) — yr | 67 (36–86) | 66 (42–84) | 66 (36–86) |
| ≥70 yr — no. (%) | 20 (36) | 14 (23) | 34 (29) |
| Sex — no. (%) | |||
| Male | 41 (73) | 48 (80) | 89 (77) |
| Female | 15 (27) | 12 (20) | 27 (23) |
| Diagnosis — no. (%) | |||
| Chronic lymphocytic leukemia | 49 (88) | 53 (88) | 102 (88) |
| Small lymphocytic lymphoma | 7 (12) | 7 (12) | 14 (12) |
| Rai stage III or IV — no. (%) | 28 (50) | 39 (65) | 67 (58) |
| Median no. of previous therapies (range)† | 4 (1–10) | 3 (1–11) | 3 (1–11) |
| Resistance to most recent therapy — no. (%)‡ | 23 (41) | 22 (37) | 45 (39) |
| Previous fludarabine-based therapy — no. (%) | |||
| Any previous fludarabine | 51 (91) | 49 (82) | 100 (86) |
| Resistance to fludarabine | 28 (50) | 42 (70) | 70 (60) |
| ECOG performance status — no. (%) | |||
| Grade 0 | 29 (52) | 27 (45) | 56 (48) |
| Grade 1 | 27 (48) | 31 (52) | 58 (50) |
| Missing data | 0 | 2 (3) | 2 (2) |
| Peripheral-blood lymphocytosis | |||
| Absolute lymphocyte count >5000 per mm3 — no. (%) | 31 (55) | 35 (58) | 66 (57) |
| Median count per mm3 (range) | 27,600 (5400–204,500) | 25,100 (5200–259,900) | 27,500 (5200–259,900) |
| Bulky nodes — no. (%) | |||
| >5 cm | 29 (52) | 38 (63) | 67 (58) |
| >10 cm | 10 (18) | 12 (20) | 22 (19) |
| Interphase cytogenetic abnormality — no./total no. with CLL (%)§ | |||
| Chromosome 17p deletion | 19/49 (39) | 12/53 (23) | 31/102 (30) |
| Chromosome 11q deletion | 13/49 (27) | 15/53 (28) | 28/102 (27) |
| No chromosome 17p or 11q deletion | 16/49 (33) | 27/53 (51) | 43/102 (42) |
| Data missing or indeterminate | 7/49 (14) | 3/53 (6) | 10/102 (10) |
| IGHV mutation status — no./total no. with CLL (%) | |||
| Unmutated | 26/49 (53) | 20/53 (38) | 46/102 (45) |
| Mutated | 6/49 (12) | 11/53 (21) | 17/102 (17) |
| Data missing | 17/49 (35) | 22/53 (42) | 39/102 (38) |
ECOG denotes Eastern Cooperative Oncology Group, and IGHV immunoglobulin heavy-chain variable region.
A total of 116 patients (100%) received anti-CD20 antibodies, 110 (95%) received alkylating agents, and 103 (89%) received purine analogues.
Resistance was defined as either a lack of at least a partial response or disease progression while receiving therapy or within 6 months after the completion of therapy. Nineteen patients with resistance to fludarabine were also resistant to the combination of fludarabine, cyclophosphamide, and rituximab.
A total of 11 patients — 7 in the dose-escalation cohort and 4 in the expansion cohort — had both chromosome 17p and chromosome 11q deletions.
DOSE-ESCALATION AND EXPANSION COHORTS
Patients were enrolled in eight groups in the dose-escalation cohort. Laboratory tumor lysis was observed after a single initial dose of 200 mg or 100 mg in all three patients in the first group.20 Subsequent patients started with a test dose of 50 mg or 20 mg and in the absence of tumor lysis syndrome underwent a ramp-up in dose to designated doses of 150 mg, 200 mg, 300 mg, 400 mg, 600 mg, 800 mg, and 1200 mg per day (Fig. 1A). Sixty patients then received 400 mg per day in the expansion cohort, after starting at 20 mg per day in an extended stepwise ramp-up (Fig. 1B). The median duration of follow-up for all 116 patients at the data cutoff was 17 months (range, 1 to 44). The median follow-up was 21 months in the dose-escalation cohort and 17 months in the expansion cohort (Table S6 in the Supplementary Appendix). Of the 116 patients, 51 (44%) continued to receive venetoclax as of the data cutoff for this report (Fig. S1 in the Supplementary Appendix). The most common reasons for discontinuation were progressive disease (35%), toxicity (11%), and eligibility for allogeneic stem-cell transplantation (6%) (Table S6 in the Supplementary Appendix).
PHARMACOKINETIC PROFILE
Peak venetoclax levels were attained 6 to 8 hours after the first dose (Fig. 1C, and Table S7 and Fig. S2 in the Supplementary Appendix). The terminal half-life of venetoclax after a single 50-mg dose was approximately 19 hours. At steady state, venetoclax exposure (maximal level and area-under-the-curve value) was approximately proportional to the dose for amounts ranging from 150 mg to 800 mg per day (Table S7 in the Supplementary Appendix). No trend was observed in dose-normalized steady-state trough levels over time.
SAFETY
The most important toxic effect in the dose-escalation cohort was the tumor lysis syndrome, which occurred in 10 of 56 patients (18%). The occurrence was either clinical (in 3 patients) or laboratory-only with no clinically important sequelae (with 8 episodes in 7 patients) and occurred either after the administration of the first dose (200 mg in 2 patients, 100 mg in 1 patient, and 50 mg in 4 patients) or immediately after ramp-up of the dose to 150 mg (in 2 patients), to 800 mg (in 1 patient), or to 1200 mg (in 1 patient) (Table 2, and Tables S8 and S9 in the Supplementary Appendix). Of the 3 patients with clinical tumor lysis syndrome, 2 had severe sequelae: acute renal failure requiring dialysis and hospitalization for 24 days after an initial 50-mg dose in one patient and sudden death on the second day after stepping up to 1200 mg per day in another patient. The third patient had a transient elevation in serum creatinine, which resolved within 2 days. After resolution of the tumor lysis syndrome, 9 of 10 patients resumed taking venetoclax. Of these patients, 8 had no recurrence of the syndrome at subsequent doses.
Table 2.
Adverse Events and Serious Adverse Events in the 116 Study Patients.
| Event | Any Grade | Grade 3 or 4 |
|---|---|---|
| no. of patients (%) | ||
| Adverse event* | ||
| Any | 115 (99) | 96 (83) |
| Diarrhea | 60 (52) | 2 (2) |
| Upper respiratory tract infection | 56 (48) | 1 (1) |
| Nausea | 55 (47) | 2 (2) |
| Neutropenia | 52 (45) | 48 (41) |
| Fatigue | 46 (40) | 4 (3) |
| Cough | 35 (30) | 0 |
| Pyrexia | 30 (26) | 1 (1) |
| Anemia | 29 (25) | 14 (12) |
| Headache | 28 (24) | 1 (1) |
| Constipation | 24 (21) | 1 (1) |
| Thrombocytopenia | 24 (21) | 14 (12) |
| Arthralgia | 21 (18) | 1 (1) |
| Vomiting | 21 (18) | 2 (2) |
| Peripheral edema | 18 (16) | 0 |
| Hyperglycemia | 17 (15) | 10 (9) |
| Serious adverse event† | ||
| Any | 52 (45) | |
| Febrile neutropenia | 7 (6) | |
| Pneumonia | 5 (4) | |
| Upper respiratory tract infection | 4 (3) | |
| Immune thrombocytopenia | 3 (3) | |
| Tumor lysis syndrome | 3 (3) | |
| Diarrhea | 2 (2) | |
| Fluid overload | 2 (2) | |
| Hyperglycemia | 2 (2) | |
| Prostate cancer | 2 (2) | |
| Pyrexia | 2 (2) | |
Listed are adverse events that were reported in at least 15% of the patients. Preexisting grade 1 or 2 laboratory abnormalities are not reported, unless the grade increased during the study.
Listed are serious adverse events that were reported in at least two patients. Excluded are serious adverse events that were related to disease progression in two patients.
In the expansion cohort, an extended stepwise ramp-up starting at 20 mg was used (Fig. 1B). Patients were admitted to the hospital for the administration of the first doses at 20 mg or 50 mg and received prophylaxis against the tumor lysis syndrome and management of any symptoms according to their level of risk. Of the 60 patients in the expansion cohort, 21 (35%) were considered to be at high risk for the tumor lysis syndrome and thus were hospitalized for subsequent dose ramp-ups. One of the patients had laboratory evidence of the tumor lysis syndrome, and none of the patients had clinical sequelae. The extended ramp-up schedule was associated with a more gradual reduction in the peripheral-blood lymphocyte count, which suggested a more controlled cytotoxic effect than that in the escalation cohort (Fig. S3 in the Supplementary Appendix).
Additional toxic effects that were reported during ongoing venetoclax therapy in all 116 patients are summarized in Table 2. The most common adverse events were of grade 1 or 2; the most common of these events were self-limited diarrhea and nausea, along with upper respiratory tract infection. Neutropenia was the most common grade 3 or 4 adverse event. Grade 4 neutropenia was observed in 33 patients (28%), predominantly in those who entered the study with a reduced neutrophil count. Of these patients, 28 received growth factor (either filgrastim or pegfilgrastim) and had a response; nine patients required a reduction in the venetoclax dose. The most common serious adverse event was febrile neutropenia, which was reported in 7 patients (6%). Other serious adverse events of note included pneumonia (in 5 patients [4%]), upper respiratory tract infection (in 4 patients [3%]), and immune thrombocytopenia (in 3 patients [3%]) (Table 2). Twenty patients had any type of infection of grade 3 or higher, at an exposure-adjusted rate of 1.4 per 100 patientmonths. Dose-limiting toxic effects were observed in 8 patients across the dose-escalation groups; the most common was the tumor lysis syndrome (Tables S8 and S9 in the Supplementary Appendix). A maximum tolerated dose was not identified. Two deaths that were unrelated to progressive disease occurred within 30 days after the last administration of venetoclax: one from the tumor lysis syndrome, as described previously, and one from intestinal obstruction from a strangulated abdominal hernia.
EFFICACY
Venetoclax was active at all doses that were studied and induced deep reductions in the CLL burden in the blood, lymph nodes, and marrow (Fig. 1D, 1E, and 1F). Responses according to IWCLL criteria were observed in all dose-escalation groups (Table S9 in the Supplementary Appendix). Among the 56 patients in the dose-escalation cohort, the pooled overall response rate was 77%, with 30% having either a complete response or a complete response with incomplete count recovery (hereafter collectively referred to as a complete response) (Table 3, and Table S10 in the Supplementary Appendix). The median time until the first objective response was 6 weeks (range, 5 to 24). The median time until the determination of a complete response was longer (median, 6 months; range, 3 to 19); three complete responses were first reported more than 1 year after the initiation of treatment (Fig. S4 in the Supplementary Appendix).
Table 3.
Complete and Overall Response Rates, According to Cohort and Subgroup.
| Variable | No. of Patients | Complete Response Rate* | Overall Response Rate |
|---|---|---|---|
| percent of patients (95% CI) | |||
| All patients | 116 | 20 (13–28) | 79 (71–86) |
| Dose-escalation cohort | 56 | 30 (19–44) | 77 (64–87) |
| Expansion cohort | 60 | 10 (4–21) | 82 (70–91) |
| Age | |||
| ≥70 yr | 34 | 21 (9–38) | 71 (53–85) |
| <70 yr | 82 | 20 (12–30) | 83 (73–90) |
| No. of previous therapies | |||
| ≥4 | 56 | 16 (8–28) | 73 (60–84) |
| <4 | 60 | 23 (13–36) | 85 (73–93) |
| Fludarabine resistance | |||
| Yes | 70 | 16 (8–26) | 79 (67–88) |
| No | 44 | 27 (15–43) | 82 (67–92) |
| Bulky nodes of >5 cm | |||
| Yes | 67 | 8 (3–17) | 78 (66–87) |
| No | 48 | 38 (24–53) | 83 (70–93) |
| Chromosome 17p deletion | |||
| Yes | 31 | 16 (6–34) | 71 (52–86) |
| No | 60 | 18 (10–30) | 80 (68–89) |
| Chromosome 11q deletion | |||
| Yes | 28 | 11 (2–28) | 82 (63–94) |
| No | 62 | 21 (12–33) | 76 (63–86) |
| IGHV status | |||
| Unmutated | 46 | 17 (8–31) | 76 (61–87) |
| Mutated | 17 | 29 (10–56) | 94 (71–100) |
A complete response includes complete remission with incomplete count recovery.
In the 400-mg expansion cohort, data were mature for the overall response rate (82%) but less mature for the complete response rate (10% at the time of data cutoff). The pooled overall response rate across all doses for all 116 patients was 79%, with a complete response reported in 20% of the patients.
The overall response rate did not vary on the basis of age, the number of previous therapies, or the risk factors typically associated with a poor outcome with chemoimmunotherapy-based treatments (Table 3). Notably, among patients with deletion 17p CLL, the response rate was 71%, with 16% having a complete response. Similarly, patients with resistance to fludarabine or unmutated IGHV had overall response rates of 79% and 76%, respectively, with 16% and 17%, respectively, having a complete response (Table 3). Among patients with bulky disease, the response rate was 78%, with a complete response rate of 8%; in the absence of bulky lymphadenopathy, the response rate was 83%, with 38% of patients having a complete response. Of the 23 patients who had a complete response, 17 underwent multicolor flow cytometry to evaluate minimal residual disease in bone marrow; of those who were tested, 6 (35%) had negative results according to standard criteria (i.e., 5% of all the study patients).26
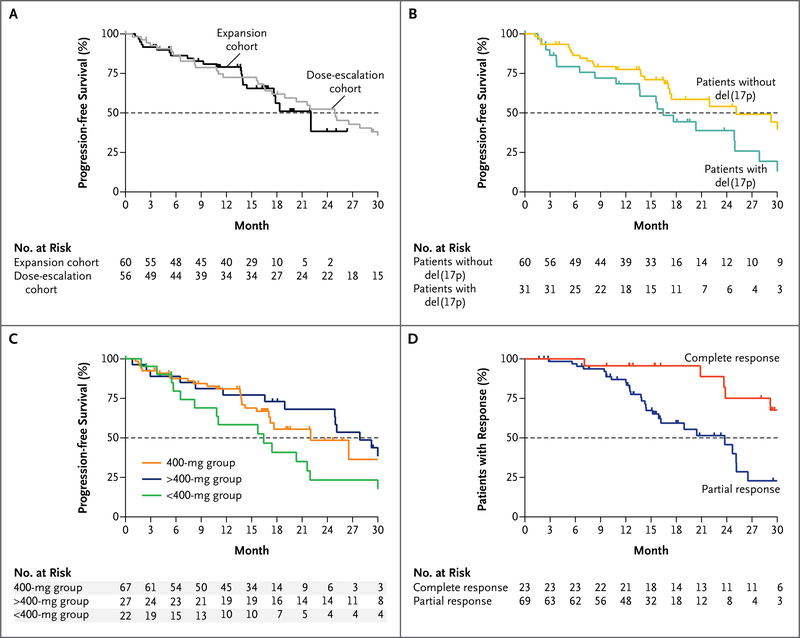
A median duration of progression-free survival of 25 months (95% confidence interval [CI], 17 to 30) was observed for patients in the dose-escalation cohort. In the expansion cohort, the median duration of progression-free survival cannot be reliably estimated because of the short follow-up (median follow-up duration, 17 months; range, <1 to 26). The rate of progression-free survival at 15 months was estimated to be 66% (95% CI, 51 to 77) (Fig. 2A, and Table S10 in the Supplementary Appendix). Disease progression occurred in 41 patients (35%), including Richter’s transformation (i.e., conversion into an aggressive lymphoma, typically diffuse large-B-cell lymphoma) in 18 (16%) (Table S6 in the Supplementary Appendix). Richter’s transformation was diagnosed within the first year of the study in 11 of these patients. Progression including Richter’s transformation (in 10 of the 18 patients) was more common among patients with deletion 17p CLL. The median progression-free survival was 16 months (95% CI, 11 to 25) for patients with deletion 17p CLL across all doses, whereas 71% of patients (95% CI, 57 to 81) without deletion 17p CLL were progression free at 15 months (Fig. 2B).
Figure 2. Durability of Benefit with Ongoing Venetoclax Therapy.
The proportions of patients with progression-free survival are shown for the dose-escalation and expansion cohorts (Panel A), for patients with CLL with or without chromosome 17p deletions, abbreviated del(17p) (Panel B), and for patients according to dose group (Panel C). Panel D shows the duration of response, according to whether patients had a complete response (including a complete remission with incomplete marrow recovery) or a partial response as their best response during the trial. Tick marks represent censored data. The number of patients at risk for an event at each time point is shown for the first 30 months of follow-up. When the numbers of patients at risk are low because of data censoring, estimates of median values may be unstable. Point estimates at 15 months are provided in Table S10 in the Supplementary Appendix.
To explore whether dose influenced the durability of disease control, patients were grouped according to the assigned dose (<400 mg, 400 mg, and >400 mg), and progression-free survival was analyzed to the point at which data for the 400-mg group were mature. The 15-month progression-free estimates were 58% (95% CI, 34 to 77) for the patients who received less than 400 mg per day, 69% (95% CI, 55 to 79) for those who received 400 mg per day, and 77% (95% CI, 56 to 89) for those who received more than 400 mg per day (Fig. 2C). Similar patterns were observed for the duration of response and the time to progression (Table S10 in the Supplementary Appendix). Among all the patients who had a response, the estimated durability of response was 75% (95% CI, 64 to 84) at 15 months. The duration of response was longer among the patients who had a complete response than among those whose best response was a partial response (Fig. 2D). The 2-year overall survival estimate for all the patients was 84%.
Discussion
Targeted therapies that inhibit signaling from the B-cell receptor have improved the survival of patients with relapsed CLL. This first trial of venetoclax showed the potential of BCL2 antagonism as an additional therapeutic avenue for patients with relapsed CLL. Across a range of doses, venetoclax induced major reductions in tumor burden in all tissue compartments, and side effects were generally limited to low-grade nausea and diarrhea. The most important toxic effect that we observed, the tumor lysis syndrome, was a consequence of the potency of venetoclax in inducing apoptosis in CLL cells.20 When treatment was initiated in patients with a high tumor burden at doses of 50 mg per day or more, clinical tumor lysis syndrome was observed in three patients, two of whom had severe sequelae. Laboratory evidence of the tumor lysis syndrome was seen in an additional seven patients. The adoption of a stepwise ramp-up phase, beginning at a daily 20-mg dose with weekly increases to 50 mg, 100 mg, and 200 mg per day to the target dose of 400 mg per day, coupled with strict adherence to prophylaxis and monitoring on the first day of dose increases, reduced the incidence of laboratory evidence of the tumor lysis syndrome with no clinical tumor lysis syndrome. Current phase 2 and 3 trials of venetoclax in patients with CLL have been designed to confirm that this risk can be mitigated with the use of protocols amenable to routine application in the community.
The other notable toxic effect that we observed was neutropenia, with grade 3 or 4 neutropenia developing in 41% of the patients during the trial. Neutropenia was previously observed in heavily pretreated patients with CLL or non-Hodgkin’s lymphoma in trials of navitoclax17,33 and may be a class effect of BCL2-inhibiting BH3-mimetic drugs.34 This condition responded to intermittent treatment with neutrophil growth factor and enabled uninterrupted venetoclax delivery in most patients who had grade 4 neutropenia. Infectious complications of neutropenia were uncommon, in contrast to the historical experience with infections after chemoimmunotherapy.35–37 Whether neutropenia will be less common in patients who have not been extensively exposed to alkylating agents or fludarabine needs to be determined.
A maximum tolerated dose was not identified in this study. On the basis of short-term exposure, doses that were as high as 800 mg per day were not associated with serious toxicity. However, most long-term experience was with doses of 600 mg per day or less. Overall response rates appeared to be similar among patients who initially received doses ranging from 400 to 1200 mg per day in the dose-escalation cohort. The selection of 400 mg per day as the dose for ongoing evaluation was informed by the balance of overall response and safety data; the selection of this dose was subsequently supported by the safety and efficacy analyses of data from the expansion cohort after a minimum of 15 months of follow-up.
The overall response rate of 79% that we observed provides support for further development of venetoclax as a treatment option for patients with heavily pretreated relapsed or refractory CLL or SLL. It is difficult to generalize the results of this unblinded, dose-escalation study involving patients who were selected as being fit for a phase 1 trial to all patients with relapsed disease. However, we observed that venetoclax induced deep responses, including complete responses without minimal residual disease, in patients up to the age of 86 years and including those with disease characteristics that are associated with poor outcomes with chemoimmunotherapy. In particular, we saw overall response rates of 79% among patients with fludarabine-resistant disease and 71% among those with deletion 17p CLL, in whom loss of function of the tumor suppressor TP53 represents a major obstacle to successful therapy.28,30 Complete response rates of 16% were observed in these two subgroups. Median progression-free survival among patients with deletion 17p CLL was 16 months with a range of doses.
Transformation to aggressive lymphoma accounted for 18 of 41 progressions and appears to represent a mechanism of tumor escape from suppression by the inhibition of BCL2, particularly for patients with deletion 17p CLL. Richter’s transformation was also observed in 8 of 25 patients with disease progression who were receiving ibrutinib monotherapy in an extended follow-up study.9
In conclusion, venetoclax was shown to have substantial antitumor activity in patients with relapsed CLL, including those with poor prognostic features. Responses appeared to be more durable among those who had a complete response than among those with a partial response. Gradual dose escalation appeared to minimize the risk of the tumor lysis syndrome, the major toxicity associated with venetoclax.
Supplementary Material
Acknowledgments
Supported by AbbVie and Genentech.
Dr. Roberts reports receiving grant support and study drugs from AbbVie, serving as an investigator in trials sponsored by Genentech, AbbVie, Janssen, and Beigene, and receiving institutional research funding from Genentech for the development of venetoclax; Dr. Davids, receiving fees for serving on advisory boards from Infinity, Pharmacyclics, TG Therapeutics, Genentech, and Gilead Sciences, consulting fees from Infinity, Pharmacyclics, Janssen, and AbbVie, travel support from TG Therapeutics, Janssen, and AbbVie, and grant support from Infinity, Pharmacyclics, TG Therapeutics, and Genentech; Drs. Pagel and Kahl, receiving consulting fees from Gilead Sciences and Pharmacyclics; Dr. Puvvada, receiving fees for serving on advisory boards and consulting fees from Genentech and AbbVie, travel support from Genentech, and institutional research funding from Genentech, AbbVie, Spectrum, Janssen, and Takeda; Dr. Gerecitano, receiving fees for serving on advisory boards from AbbVie and Genentech and consulting fees from AbbVie; Dr. Kipps, receiving consulting fees and grant support from AbbVie; Dr. Anderson, serving as an investigator in trials sponsored by AbbVie and receiving institutional research funding from Genentech for the development of venetoclax; Dr. Brown, receiving consulting fees from Genentech, ProNAi, Sun BioPharma, Janssen, Infinity, Gilead Sciences, Celgene, and Pharmacyclics; Ms. Gressick and Drs. Wong, Dunbar, Zhu, Desai, Cerri, Heitner Enschede, and Humerickhouse, being employees of and having an equity interest in AbbVie; Dr. Wierda, participating on an advisory board for AbbVie; and Dr. Seymour, receiving fees for serving on advisory boards from AbbVie, Celgene, Genentech, Gilead Sciences, Infinity, Mundipharma, Pharmacyclics, Roche, and Takeda, consulting fees from Phebra, lecture fees from AbbVie, Gilead Sciences, and Roche, travel support from Celgene, Gilead Sciences, Janssen, and Roche, and grant support from AbbVie and Janssen. No other potential conflict of interest relevant to this article was reported.
We thank the patients who participated in this trial and their families; the study coordinators and the support staff at the clinical sites; venetoclax team members at AbbVie and Genentech; Joseph Beason, Srikanth Birru, Vinay Tavva, Mani Mudumba, and Jingwen Jia of AbbVie for their statistical programming support; and Leanne Lash, Ph.D., of AbbVie for her assistance in the preparation of the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Andrew W. Roberts, Department of Clinical Haematology and the Bone Marrow Transplantation Unit, Royal Melbourne Hospital, Melbourne, VIC, Australia Division of Cancer and Haematology, Walter and Eliza Hall Institute of Medical Research, Melbourne, VIC, Australia; Victorian Comprehensive Cancer Centre, Melbourne, VIC, Australia; Parkville, VIC, and the University of Melbourne, Melbourne, VIC, Australia.
Matthew S. Davids, Dana–Farber Cancer Institute, Boston
John M. Pagel, Swedish Medical Center, Seattle
Brad S. Kahl, Washington University, St. Louis
Soham D. Puvvada, University of Arizona, Tucson
John F. Gerecitano, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York
Thomas J. Kipps, University of California, San Diego, San Diego
Mary Ann Anderson, Department of Clinical Haematology and the Bone Marrow Transplantation Unit, Royal Melbourne Hospital, Melbourne, VIC, Australia Division of Cancer and Haematology, Walter and Eliza Hall Institute of Medical Research, Melbourne, VIC, Australia.
Jennifer R. Brown, Dana–Farber Cancer Institute, Boston
Lori Gressick, AbbVie, North Chicago, IL
Shekman Wong, AbbVie, North Chicago, IL
Martin Dunbar, AbbVie, North Chicago, IL
Ming Zhu, AbbVie, North Chicago, IL
Monali B. Desai, AbbVie, North Chicago, IL
Elisa Cerri, AbbVie, North Chicago, IL
Sari Heitner Enschede, AbbVie, North Chicago, IL
Rod A. Humerickhouse, AbbVie, North Chicago, IL
William G. Wierda, University of Texas M.D. Anderson Cancer Center, Houston
John F. Seymour, Victorian Comprehensive Cancer Centre, Melbourne, VIC, Australia Parkville, VIC, and the University of Melbourne, Melbourne, VIC, Australia; Peter MacCallum Cancer Centre, Melbourne, VIC, Australia.
REFERENCES
- 1.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013; 31: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood 2014;123:3390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371: 213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood 2015; 125: 2062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol 2015; 1: 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014; 370: 2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015; 125: 2497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 2005; 102: 13944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia 1996; 10:456–9. [PubMed] [Google Scholar]
- 12.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest 2007; 117: 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677–81. [DOI] [PubMed] [Google Scholar]
- 14.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006;10: 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green DR, Walczak H. Apoptosis therapy: driving cancers down the road to ruin. Nat Med 2013; 19: 131–3. [DOI] [PubMed] [Google Scholar]
- 16.Park CM, Bruncko M, Adickes J, et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J Med Chem 2008; 51: 6902–15. [DOI] [PubMed] [Google Scholar]
- 17.Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 2012; 30: 488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seymour F, Roberts A, Carney DA, et al. Phase II study of navitoclax (ABT-263) safety and efficacy in patients with relapsed or refractory chronic lymphocytic leukemia (CLL): interim results. Haematologica 2011; 96(s2): 227 abstract. [Google Scholar]
- 19.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell 2007;128:1173–86. [DOI] [PubMed] [Google Scholar]
- 20.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013; 19: 202–8. [DOI] [PubMed] [Google Scholar]
- 21.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 2009; 101: 708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. May 2009. (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf).
- 23.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011; 364:1844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol 1999;17:1244. [DOI] [PubMed] [Google Scholar]
- 26.Rawstron AC, Böttcher S, Letestu R, et al. Improving efficiency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia 2013;27:142–9. [DOI] [PubMed] [Google Scholar]
- 27.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002;99:3554–61. [DOI] [PubMed] [Google Scholar]
- 28.Zenz T, Gribben JG, Hallek M, Döhner H, Keating MJ, Stilgenbauer S. Risk categories and refractory CLL in the era of chemoimmunotherapy. Blood 2012; 119: 4101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol 2006;24:437–43. [DOI] [PubMed] [Google Scholar]
- 30.Tam CS, O’Brien S, Plunkett W, et al. Long-term results of first salvage treatment in CLL patients treated initially with FCR (fludarabine, cyclophosphamide, rituximab). Blood 2014;124:3059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin KI, Tam CS, Keating MJ, et al. Relevance of the immunoglobulin VH somatic mutation status in patients with chronic lymphocytic leukemia treated with fludarabine, cyclophosphamide, and rituximab (FCR) or related chemoimmunotherapy regimens. Blood 2009;113:3168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fornecker LM, Aurran-Schleinitz T, Michallet AS, et al. Salvage outcomes in patients with first relapse after fludarabine, cyclophosphamide, and rituximab for chronic lymphocytic leukemia: the French Intergroup experience. Am J Hematol 2015;90:511–4. [DOI] [PubMed] [Google Scholar]
- 33.Wilson WH, O’Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 2010; 11: 1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leverson JD, Phillips DC, Mitten MJ, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 2015;279279ra40. [DOI] [PubMed] [Google Scholar]
- 35.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2011;29:3559–66. [DOI] [PubMed] [Google Scholar]
- 36.Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol 2010;28:1756–65. [DOI] [PubMed] [Google Scholar]
- 37.Wierda W, O’Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol 2005;23:4070–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.