Abstract
Hog-badgers (mustelid carnivorans classified in the genus Arctonyx) are distributed throughout East and Southeast Asia, including much of China, the eastern Indian Subcontinent, Indochina and the large continental Asian island of Sumatra. Arctonyx is usually regarded as monotypic, comprising the single species A. collaris F. Cuvier, 1825, but taxonomic boundaries in the genus have never been revised on the basis of sizeable series from throughout this geographical range. Based on a review of most available specimens in world museums, we recognize three distinctive species within the genus, based on craniometric analyses, qualitative craniodental features, external comparisons, and geographical and ecological considerations. Arctonyx albogularis (Blyth, 1853) is a shaggy-coated, medium-sized badger widely distributed in temperate Asia, from Tibet and the Himalayan region to eastern and southern China. Arctonyx collaris F. Cuvier, 1825, is an extremely large, shorter-haired badger, distributed throughout Southeast Asia, from eastern India to Myanmar, Thailand, Vietnam, Cambodia and Laos. The world's largest extant badger, A. collaris co-occurs with A. albogularis in eastern India and probably in southern China, and fossil comparisons indicate that its geographical range may have extended into central China in the middle Pleistocene. The disjunctly distributed species Arctonyx hoevenii (Hubrecht, 1891), originally described within the order ‘Edentata’ by a remarkable misunderstanding, is the smallest and darkest member of the genus and is endemic to the Barisan mountain chain of Sumatra. Apart from A. hoevenii, no other Arctonyx occurs on the Sunda Shelf below peninsular Thailand. The natural history of each species of Arctonyx, so far as is known, is briefly reviewed. No claim to original US Government works.
Keywords: biogeography, China, conservation, cryptic species, endemism, morphometrics, Sumatra, taxonomy, zoological gardens, zoonoses
INTRODUCTION
In a short note that appeared in the Notes of the Leyden Museum during the final decade of the 19th century, now largely forgotten, the famous Dutch comparative anatomist Ambrosius Arnold Willem Hubrecht of the University of Utrecht seized the interest of the zoological community in announcing the discovery of a new genus of edentate-like mammal from the mountains of Sumatra (Hubrecht, 1891). Hubrecht introduced the supposed new taxon as follows:
A few years ago a new and interesting mammal, which is exceedingly rare, even in its native haunts, was brought to the then Resident of Palembang, Mr. A. Pruys van der Hoeven. This gentleman who is not only an eager sportsman, but also well-versed in natural history, recognised it to be new to science and to be more closely allied to certain representatives of the Edentata, than to any other order of mammals. The type-specimen was held in captivity for several weeks, was fed on ants and afterwards on cooked rice and was sent alive to Europe in order to be examined, described and ultimately preserved in the Royal Museum at Leyden. It unfortunately died on board the vessel, on its way to Holland, and by an unaccountable blunder on the part of those in charge, its remains were not preserved, but thrown overboard.
During my own stay in Sumatra from February till May 1891 I took particular trouble to obtain further information concerning this animal and have found the fact of its existence - though at the same time of exceeding rarity. - confirmed in a way which does not allow me to doubt that ere long further specimens will be available for a thorough examination, also with respect to anatomical detail. My own attempts to secure a second specimen have as yet not been successful, but as they have turned the attention of many persons toward this animal I feel bound, in deference to the claims to priority of its original discoverer, who has put his preliminary description as well as sketches of the animal at my disposal, to introduce this peculiar mammal into science, notwithstanding the type-specimen has been lost. The generic name has been selected, not with a view of indicating any closer anatomical relation with the genus Manis, but only to indicate that a hairy anteater is meant.
TRICHOMANIS HOEVENII, N. G. ET N. SP.
Animal of the size of a very large cat. Fur grey, with a black longitudinal band along the middle of the back. Snout elongated and conical, with a small mouth at the extremity. A long cylindrical tongue, which is thrust out, serves the animal in the collecting of ants, which are its natural food. A more or less bushy tail. Ears not conspicuous. Legs higher than those of Manis, strong claws to the feet.
I have no doubt that this description - however superficial - is more than sufficient to recognise the animal as soon as it will have been reobtained. The type-specimen was caught in the mountainous districts that separate the Residencies of Palembang and Bencoolen in Sumatra.
Zoological excitement over the discovery of ‘T richomanis’ was not to be long-lived. Four years later, Hubrecht (1895) wrote to inform the members of the Zoological Society of London that van der Hoeven's insectivorous beast was not actually an unknown edentate, but rather a hog-badger - that is, a montane Sumatran representative of the mustelid genus Arctonyx, previously recorded only from China and the Indian subcontinent:
A letter was read, addressed to the Secretary by Dr. A. A. W. Hubrecht, F.M.Z.S., calling attention to the account of a supposed new Mammal from Sumatra by him, published in the ‘Notes from the Leyden Museum’ (vol. xiii. p. 241), under the belief that it would turn out to be an unknown species of Edentate, and which he had proposed to call Trichomanis hoevenii. Further inquiries and information received from Mr. Pruys Van der Hoeven (after whom the supposed new animal had been named) had convinced Dr. Hubrecht that it was an Arctonyx (A. collaris), and that no further hopes could be entertained of the existence of an unknown Edentate in the forests of Sumatra.
A decade after Hubrecht's second letter was read, the Swiss zoologist Gustav Schneider became the first naturalist actually to obtain a museum specimen of a hog-badger from Sumatra - an adult male collected in the Karo Highlands of Sumatra, deposited in the collections of the Zoological Museum at Strasbourg, and discussed under the name Arctonyx hoevenii (Schneider, 1905). Unaware of Schneider's success,Oldfield Thomas (1910) later wrote that ‘if an Arctonyx occurs in the … mountain districts [of Sumatra], and its characteristics are in any way compatible with Hubrecht's animal, after elimination of the imaginary Edentate attributes, the name hoevenii may have to be used for it.’ In their report on an expedition to Mt Kerinci in west Sumatra, Robinson & Kloss (1918) redescribed the Sumatran hog-badger on the basis of newly collected series of skins and skulls deposited at the Raffles Museum in Singapore. In keeping with Thomas' recommendation, they designated a specimen in the Federated Malay States Museum (now the Raffles Museum of Biodiversity Research) as a neotype for hoevenii to anchor the name's association with the Arctonyx of Sumatra, because Hubrecht had, rather memorably, been unable to secure a type for the original description. Although they used the name Arctonyx collaris hoeveni in the text of their paper, Robinson and Kloss also figured a plate of the species bearing the name ‘Arctonyx hoeveni’, perhaps betraying their uncertainty regarding the Sumatran hog-badger's degree of taxonomic uniqueness. The only modern taxonomic review of the entire genus, provided byPocock (1941), recognized A. hoevenii as a distinctive species, a decision overlooked by all subsequent checklist-compilers (e.g. Ellerman & Morrison-Scott, 1951, 1966; Corbet & Hill, 1992; Wozencraft, 2005).
We began this project in an effort to evaluate the morphological and ecological distinctiveness of the Sumatran representative of Arctonyx. In addition to its unusual taxonomic introduction, this Arctonyx population intrigued us for several reasons. Apart from the Sumatran population, Arctonyx does not occur on the Sunda Shelf below Peninsular Thailand - that is, it is absent from the Malay Peninsula, Borneo and Java, rendering the Sumatran population a geographically isolated form. Second, it seemed clear from our reading of the literature and our preliminary examinations of museum specimens and their associated data that unlike other Arctonyx, the Sumatran population seems to be truly restricted to montane forests, suggesting an ecological distinction between this disjunct equatorial population and other Arctonyx. Finally, it was immediately clear from initial examinations of museum specimens that Sumatran Arctonyx are morphologically extremely distinctive relative to other Arctonyx, most notably when compared with the geographically nearest population of Arctonyx from southern Thailand. It became clear that full elucidation of the distinctive features of Sumatran Arctonyx necessitated a comprehensive taxonomic review of the genus - as Allen (1929) noted, ‘the precise relationships of the Asiatic hog-badgers still require to be more carefully worked out with adequate material.’ This long-needed review of museum material is the contribution that we present here.
MATERIAL AND METHODS
We studied Arctonyx skins and skulls in the collections of the American Museum of Natural History, New York (AMNH); the Academy of Natural Sciences, Philadelphia (ANSP); the Natural History Museum, London (BMNH); the Field Museum of Natural History, Chicago (FMNH); the Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts (MCZ); the Muséum national d'Histoire naturelle, Paris (MNHN); the Naturalis Museum, Leiden (RMNH); the United States National Museum of Natural History, Smithsonian Institution, Washington, DC (USNM); and the Raffles Museum of Biodiversity Research, National University of Singapore, Singapore (ZRC). Other museums mentioned by abbreviation are the Royal Ontario Museum, Toronto (ROM) and the Museum of Vertebrate Zoology, University of California, Berkeley, California (MVZ).
We classified Arctonyx skulls into four rough age categories. We categorized as ‘immature’ those individuals in which the basioccipital–basisphenoid (basilar) suture in particular (and many other cranial sutures in general) was clearly evident and not yet fusing, and in which the dentition was not yet fully erupted or in which the molars showed no signs of wear. Our category of ‘young adult’ was used for skulls in which the basilar was fusing but not yet completely ossified, and in which the dentition was fully erupted with the molars showing little wear (Fig. 1). ‘Adult’ animals were those in which the basilar was fully fused (signifying cessation of elongating growth) and the molar cusps showed considerable wear. Finally, animals classified as ‘old adults’ were those in which the molars were so heavily worn as to lose entirely their occlusal features, and in which all cranial sutures were usually completely obliterated. Only skulls of young adult, adult and old adult animals were used in craniometric comparisons. In morphometric comparisons of molar dimensions, measurements for younger animals were included in cases where their molars were fully erupted.
Figure 1.
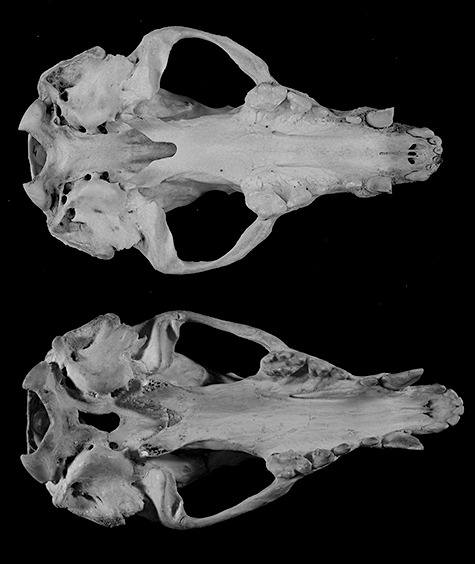
Some characteristic craniometric differences between young adult and older Arctonyx, as demonstrated by two female skulls of A. hoevenii from northern Sumatra. Above, USNM 269073, adult female from ‘Atjeh’; below, ANSP 20232, young adult female from Gunung Leuser. Young adults generally match adults/old adults in overall length, but have the zygomata weakly expanded in comparison, the interorbital and postorbital regions less constricted, cranial cresting less pronounced, and the surfaces of the teeth less worn.
For each young adult, adult and old adult skull we measured a series of 14 craniodental variables: condylobasal length (CBL); external alveolar distance across the upper canines (CC); maximum zygomatic width (ZYG); maximum breadth of braincase (BBC); height of braincase from the basioccipital floor to the apex of the braincase, including the sagittal crest when present (HBC); minimum interorbital width (IOB); minimum postorbital width (POB); minimum breadth across the rostrum behind the canines (ROST); palatal length (PAL); palatal width, measured across the external alveolar margins of the upper first molars (MM); maximum length of M1 (M1L); maximum width of M1 (M1W); maximum length of m1 (m1L); and maximum width of m1 (m1W). Variables were measured with hand-held digital calipers, to the nearest 0.1 mm, by the first author.
In a few cases, we found Arctonyx skins with accompanying standard external measurements (head–body length, tail length, hindfoot length and sometimes ear length) available on the tags, apparently representing flesh measurements obtained in the field by the specimens' original collectors (mainly at AMNH, BMNH, FMNH and ZRC, and mainly for specimens of A. hoevenii). Very few original measurements are available for A. collaris and A. albogularis, so we supplemented our comparisons with flesh measurements obtained from the literature (Thomas, 1922; Lönnberg, 1923; Allen, 1938; Pocock, 1941). In such cases, these previous authors estimated flesh measurements from museum study skins, and we deemed their estimates to be reliable based on our re-examinations of the specimens in question in most cases.
Principal components analyses and discriminant function analyses were computed using the combination of cranial and dental measurements indicated in tables in the text. All measurement values were transformed to natural logarithms prior to multivariate analyses, and principal components were extracted from the covariance matrix. The software program Statistica 6.0 (StatSoft Inc., Tulsa, OK, USA) was used for all analytical procedures.
In assessing whether any measured variables differ significantly (i.e. in t-test comparisons) between the sexes in Arctonyx taxa, we compared only pooled sets of adults and old adults for both sexes within the three species that we recognize here. (Within Arctonyx taxa, a number of measured variables differ significantly between young adults and adults/old adults, especially zygomatic width, braincase height and interorbital width, but not condylobasal length.) Within all three Arctonyx species, no measured variable differed significantly between sexes in these comparisons, with a single exception - fully grown female A. collaris skulls averaged significantly larger (P= 0.01) in condylobasal length than males (N= 3 for females, N = 5 for males). However, this last comparison is based on very little data, because most adult and old adult skulls of A. collaris in our dataset (N = 18) are either somewhat broken (such that condylobasal length cannot be measured) or, more frequently, are not explicitly sexed. All three intact female skulls of A. collaris in these comparisons are from Peninsular Thailand, while all five intact male skulls are from India, Myanmar and Laos; thus, the difference between these samples might be attributed as much to locality as to sex. Condylobasal length in A collaris does not differ significantly between males and females when young adults from our dataset (representing more individuals from many other localities) are also pooled in these comparisons. Because there seems to be no clear pattern of sexual dimorphism in the genus, and because world museum samples of Arctonyx are rather limited, we pooled males and females in all morphometric comparisons, but where relevant, have discriminated between young adults on the one hand and adults and old adults on the other.
RESULTS
Initially, our investigations of geographical variation were based on our study of Arctonyx skins and skulls at ANSP, BMNH, FMNH, MCZ, MNHN, RMNH, USNM and ZRC.
Direct comparisons of skulls from various localities (a wide range of localities, from China to Sumatra, is represented at both BMNH and USNM) and compilation of univariate craniodental measurements provided us with an immediate indication that Sumatran Arctonyx are craniodentally distinctive in being very small, with diagnostically small teeth relative to mainland Asian Arctonyx (Figures 2–6). Direct comparisons against mainland animals showed that Sumatran specimens were also distinctive in having relatively elongate rostra and pronounced sagittal cresting (despite their small size). These cranial distinctions are complemented by clear external distinctions: Sumatran Arctonyx are conspicuously darker than other Arctonyx, with the dorsum behind the head being mostly or entirely black, and with comparatively little white fur relative to mainland Arctonyx skins.
Figure 2.
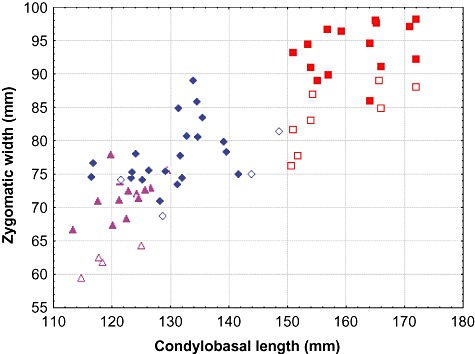
Comparative cranial size of Arctonyx taxa. Skull length (condylobasal length) versus width (zygomatic width) in 66 intact adult skulls (A. hoevenii of Sumatra, triangles; A. albogularis of China and India, diamonds; A. collaris of Indochina and eastern India, squares). Adults and old adults are denoted by solid shapes, young adults by open shapes. The clusters of adult and old adult skulls of Arctonyx collaris are widely separated from other Arctonyx taxa in this bivariate space, and do not overlap bivariately with A. hoevenii or A. albogularis even when young adults are included.
Figure 6.
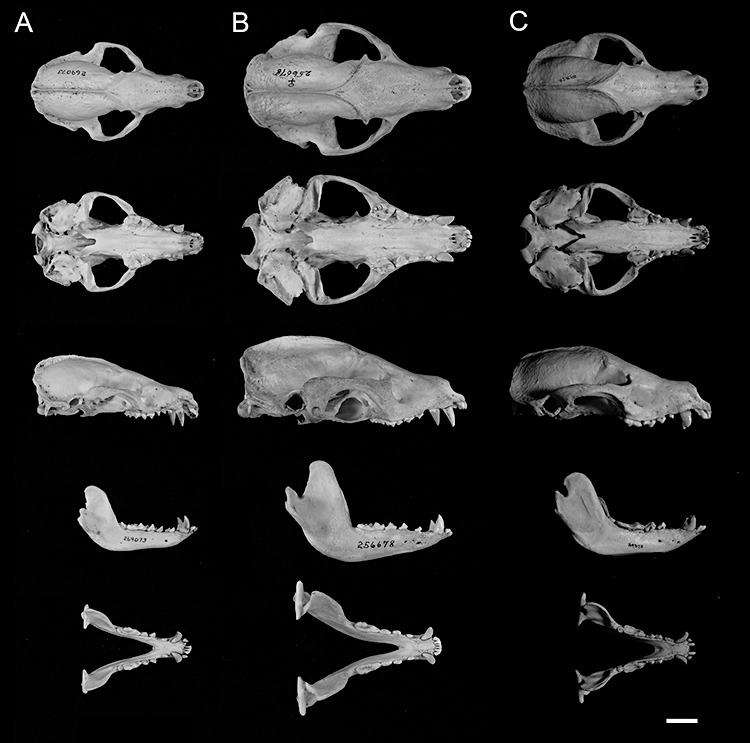
Representative skulls of the three species of Arctonyx. A, Arctonyx hoevenii, USNM 269073, adult female from ‘Atjeh’, northern Sumatra. B, A. collaris, USNM 256678, adult female, from Trang, Peninsular Thailand (topotypical). C, A. albogularis, FMNH 39373, adult female, from Hsien, Fujian Province, China. Scale bar = 20 mm.
Apart from the distinctiveness of Sumatran animals, a second surprising finding was that Arctonyx specimens from mainland Asia grouped into two sharply distinct size classes: adults with condylobasal length ≤ 142 mm (range 116–142 mm in 23 intact skulls), even in the oldest animals, on the one hand, and adults with condylobasal length > 150 mm (151–172 mm in 17 intact skulls), even in the youngest adults, on the other. None of the specimens that we measured fell into the intermediate range of lengths (i.e. 142–150 mm). All specimens from China fell into the smaller size class, and all specimens from Indochina (Myanmar, Thailand, Vietnam, Laos) fell into the larger size class, while amongst museum specimens from north-eastern India, both size classes were found. These size classes could usually be further distinguished on the basis of qualitative cranial characteristics. Animals in the larger size class usually have a large diastema between the second and third upper premolars (in both the upper and the lower jaws), conspicuously longer than the corresponding gap seen in animals in the smaller size class. Like the much smaller Sumatran animals, skulls from the larger mainland size class also possess a pronounced sagittal crest, lending the skull a more vaulted braincase in lateral profile. Close inspection indicated that the mainland Arctonyx size classes also differed in several external aspects, although these differences are more subtle than the differences in colour that distinguish Sumatran Arctonyx from other populations. Compared with the smaller size class, larger Arctonyx specimens from mainland Asia tend to have sparser and coarser pelage, with little underfur, a paler back, and a tail that tends to be longer, usually measuring about one-third as long as the head and body (averaging one-quarter as long in the smaller size class).
In combination, these differences in cranial size and qualitative craniodental and external morphology allow for diagnostic determinations of at least two distinctive kinds of hog-badgers in mainland Asia. Because these distinctions are clear-cut and because both kinds are represented amongst Arctonyx samples collected in eastern India, it seemed clear based on our initial investigations that at least two species of Arctonyx must be admitted on the Asian mainland.
We further explored and tested this hypothesis by studying metric and qualitative morphological distinctions among the comparatively sizeable holdings of Arctonyx at AMNH, not represented in our earlier comparisons, and by comparing our three perceived operational taxonomic units (Sumatran Arctonyx, large mainland Arctonyx, small mainland Arctonyx) in continuing bivariate and multivariate morphometric analyses. Skulls from specimens at AMNH could be sorted using the same metric and qualitative criteria into a larger mainland size class (specimens from India, Laos and Thailand) and a smaller mainland size class (specimens from China). However, the material at AMNH narrowed the distinction in cranial length evident between the two mainland Asian size classes. Two young adult skulls from Fujian Province (AMNH 41475, condylobasal length 148.5 mm, and AMNH 57373, 143.8 mm) are larger than all other skulls referred to the smaller Asian size class, and approach young adult specimens of the larger size class in bivariate comparisons of overall size (Fig. 2). However, these skulls and their accompanying skins possess the qualitative features of the smaller mainland Arctonyx (lack of a marked premolar diastema, little promise of a marked sagittal crest, long black guard-hairs on the dorsum, heavy white underfur), and we thus classify these with the smaller taxon, with the suggestion that these skulls perhaps approximate the upper limit of cranial size seen in that species.
In bivariate comparisons illustrating overall cranial size on the basis of skull length and width, the two mainland Arctonyx taxa do not overlap, while in bivariate comparisons of molar size, the Sumatran Arctonyx population separates clearly from all other Arctonyx (Figs 2, 3). In a principal components analysis drawing on ten craniodental measurements, these three groupings are essentially discrete, particularly when only adult and old adult skulls are compared (Fig. 4, Table 2). The three taxa diverge along the first principal component, reflecting in particular differences in overall cranial size, and the clusters of both A. hoevenii and A. collaris diverge from A. albogularis also along the second principal component, reflecting in particular their more expansive braincases (in width and height) and smaller molars, in proportional terms. Discriminant function analyses drawing on the same variables provide another means of illuminating these and other morphometric distinctions (Fig. 5, Table 2).
Figure 3.
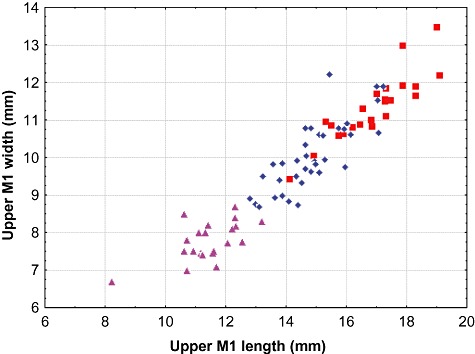
Comparative molar dimensions in Arctonyx skulls. Maximum crown length versus crown width for upper M1 (zygomatic width) in 80 intact molars, excluding excessively worn teeth (A. hoevenii, triangles; A. albogularis, diamonds; A. collaris, squares). All ages pooled. Whereas the size-variable teeth of A. collaris and A. albogularis overlap broadly in their dimensions, molar dimensions for Arctonyx hoevenii show no overlap with the other two species.
Figure 4.
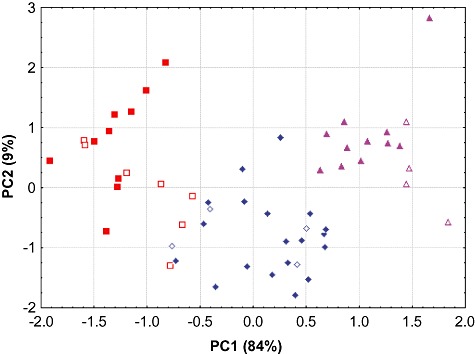
Ungrouped morphometric separation (principal components analysis) of specimens identified (on the basis of geography and qualitative features) as A. hoevenii, A. albogularis and A. collaris, drawing from ten craniodental measurements (Table 2). Symbols for taxa and ages as in Figure 3.
Table 2.
Factor loadings, eigenvalues, and cumulative variance for the principal components in the principal components analysis illustrated in Figure 4, and correlations, canonical correlations, eigenvalues and cumulative variance for the canonical variates in the discriminant function analysis illustrated in Figure 5
| PC1 | PC2 | CV1 | CV2 | ||
|---|---|---|---|---|---|
| CBL | −0.931 | 0.262 | CBL | 0.743 | 0.507 |
| CC | −0.970 | 0.164 | CC | 0.596 | 0.206 |
| ZYG | −0.907 | 0.196 | ZYG | 0.470 | 0.058 |
| BBC | −0.765 | 0.339 | BBC | 0.365 | 0.285 |
| HBC | −0.776 | 0.437 | HBC | 0.298 | 0.310 |
| ROST | −0.973 | 0.143 | ROST | 0.636 | 0.153 |
| M1L | −0.890 | −0.423 | M1L | 0.576 | −0.376 |
| M1W | −0.899 | −0.408 | M1W | 0.604 | −0.447 |
| PAL | −0.908 | 0.273 | PAL | 0.599 | 0.445 |
| MM | −0.972 | 0.036 | MM | 0.635 | 0.143 |
| Canonical correlation | 9.094 | 1.812 | |||
| Eigenvalue | 0.150 | 0.017 | Eigenvalue | 0.949 | 0.803 |
| Cumulative variance | 84.2% | 93.5% | Cumulative variance | 83.4% | 100.0% |
Figure 5.
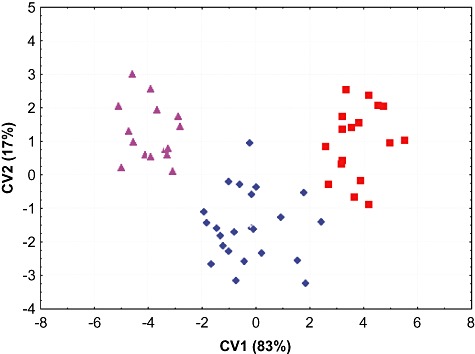
Grouped morphometric separation (discriminant function analysis) drawing from the same specimens and measurements for A. hoevenii, A. albogularis and A. collaris as in Figure 4(Table 2). All ages (young adult, adult, old adult) pooled together; A. hoevenii, triangles; A. albogularis, diamonds; A collaris, squares.
POTENTIAL CO-OCCURRENCE OF HOG-BADGER SPECIES IN ASIA AND NOMENCLATURAL ALLOCATIONS
Specimens referable to both the smaller mainland Arctonyx taxon and the larger taxon derive from eastern India. We call attention to the fact that although only one species of Arctonyx has been admitted by all recent reviewers (e.g. Ellerman & Morrison-Scott, 1966; Corbet & Hill, 1992; Wozencraft, 2005), it was well established in the 19th century naturalist literature of British India that two distinctive kinds of hog-badgers overlapped geographically in the eastern subcontinent. Blyth (1875: 29) correctly diagnosed the two and called them the ‘Large Burman sand-badger’ and the ‘Small Burman sand-badger’, noting that ‘both appear to have the same geographic range’. Likewise, Bslanford (1888) recognized two species of Arctonyx and again emphasized key diagnostic features, such as the much larger skull of one [in his key, he diagnosed the larger as having a skull length > 6 inches (152 mm), the smaller with skull length <5 inches (127 mm. - anticipating the absolute difference in skull size that we document in this paper between A. collaris and A. albogularis; see below]. Sclater (1891: 290–291) also discriminated the two distinct size classes as well, and noted that the distributions of the two kinds in India were ‘probably the same’. The possible geographical overlap of two Arctonyx taxa in eastern India was more recently posited by Rakamantha (1994), who noted during fieldwork in Manipur that ‘reliable tribal informants claim that there are two types (subspecies?) of hog-badger within Manipur; the one being the fairly large animal displayed at the Zoo, and the second variety being smaller with dark spots on its body. This needs further investigation.’ We suspect that the observations reported by Rakamantha do indeed reflect the local co-occurrence of two kinds of hog-badger in Manipur (as previously noted by Blyth, Blanford and Sclater), which lies at the juxtaposition of the recorded geographical ranges of the two taxa. The ‘dark spots’ on the smaller variety mentioned by Rakamantha probably reference the longer, darker guard hairs in the pelage of the smaller taxon, which sometimes clump and bunch, giving way to the underlying white underfur, generating the impression of dark–pale spotting.
The original description and accompanying coloured plate of Arctonyx collaris (Fig. 7) published by F. Cuvier (in Geoffroy & Cuvier, 1825) was based on an original drawing by Alfred Duvaucel, which was in turn based on two animals collected in the hill country between Bhutan and India (given as Hindustan), housed in a menagerie at Barrackpole. The two animals on which the plate is based can thus be regarded as the original syntypes; these specimens were apparently never preserved in a museum collection after their death at the menagerie. The extremely thick-set body, massive head, proportionally long tail and pale (rather than predominantly blackish) dorsum of the animal figured in the original plate (Fig. 7) provide indications that Arctonyx collaris is the original name for the larger Arctonyx species of mainland Asia. In our assessment, the measurement of ‘nine inches’ (221 mm) quoted by Cuvier for the tail length of one of the syntypes (cf. Table 4) and discussion of the animals' coarse fur (F. Cuvier, in Geoffroy & Cuvier, 1825) further confirm these animals' identity with the larger species.
Figure 7.

Plate depicting Arctonyx collaris, or the ‘Bali-Soar’, from the original description of the genus and species by Cuvier (in Geoffroy & Cuvier, 1825). Based in part on this plate, we regard Arctonyx collaris as the original name for the larger Arctonyx species of mainland Asia (see text).
Table 4.
Standard external measurements (mm) (and proportions) for the species of Arctonyx. Summary values are means ±1 SD
| A. albogularis | A. collaris | A. hoevenii | |
|---|---|---|---|
| Head–body length (HB) | 623 ± 60.2 | 787 ± 109 | 590 ± 60.5 |
| range | 546–700 | 650–1041 | 510–710 |
| N= 7 | N= 11 | N= 14 | |
| Tail length (T) | 158 ± 36.5 | 244 ± 32.4 | 128 ± 27.5 |
| range | 114–222 | 190–290 | 80–180 |
| N= 7 | N= 11 | N= 14 | |
| T/HB | 25% | 32% | 22% |
| range | 20–37% | 23–38% | 15–28% |
| N= 7 | N= 11 | N= 14 | |
| Hindfoot length | 85.4 ± 7.69 | 125 ± 8.43 | 74.7 ± 13.7 |
| range | 76–95 | 111–135 | 50–87 |
| N= 6 | N= 11 | N= 13 | |
| Ear length | 39 | 40 | 26.7 ± 5.2 |
| range | 18–34 | ||
| N= 1 | N= 1 | N= 13 |
As noted above, some 19th-century naturalists in British India (Gray, Horsfield, Blyth and Blanford) correctly used the name A. collaris for the larger of the two Indian hog-badgers, and the name A. taxoides, erected by Blyth (1853), for the smaller badger (diagnosed based on its smaller size and longer fur; isonyx and taraiyensis, manuscript names for the smaller form, also appeared in the British Indian literature; Hodgson, in Horsfield, 1856; Gray, 1863). Pocock (1940, 1941: 429) criticized this early view, noting that ‘these authors assume, on what evidence I cannot ascertain, that collaris was the name for the larger British Indian Hog-Badger, the most common form to the east of the Bay of Bengal.’ Pocock instead used the name collaris for the smaller taxon (‘A. c. collaris’, in his revision), and introduced the new name A. c. consul for the larger form, based on specimens from Mt Toungoo in modern-day Myanmar (Pocock, 1940). Pocock's (1941: 429) argument was based on ‘geographical reasons’. Although no specimens from the vicinity of Bhutan were available to Pocock (or to us), he observed that most specimens from that general neighborhood (he cites the type of isonyx from ‘Sikkim Terai’, a specimen in the Calcutta Museum from Darjeeling and an additional specimen from ‘Assam’) represent the smaller Indian hog-badger, which, according to Pocock, ‘forcibly suggests racial identity between Cuvier's type and the Hog-Badger … of the Sikkim Terai.’ We disagree with Pocock's attributions. Based on our review of museum specimens and Cuvier's original account, we instead follow earlier authors from British India in using the name Arctonyx collaris for the larger species, noting that both the smaller and the larger forms are known historically from the vicinity of the vague type locality (the border area between Bhutan and India; see Fig. 11). Because both taxa are known in the region and there has been considerable historical confusion about the precise identity of Duvaucel's original animals, we consider it profitable to designate a neotype for A. collaris. We have examined only five specimens from the Indian subcontinent representing the larger Arctonyx species (Appendix 2). Of these, only two are adults with a relatively firm locality, represented by both a skin and a skull (BMNH 43.152, young adult female, from ‘Naga Hills’, 3500 feet [Nagaland State, India]; and AMNH 171170, old adult male, from Nongpoh, Khasi Hills, Meghalaya State, India). Both of these specimens were collected in the broad vicinity of juxtaposition between India and Bhutan, the type locality as vaguely provided in the original description. We choose the latter specimen (AMNH 171170), the more mature of the two, as the neotype of Arctonyx collaris, and accordingly restrict the type locality of collaris to Nongpoh (25°54′N, 91°53′E), Khasi Hills (Meghalaya State, India). This specimen was collected on 13 May 1949 by W. N. Koelz, and is represented by a partially broken skull and flat skin (original flesh measurements: head–body length 750, tail 190, hindfoot with claw 130, ear 40).
Figure 11.
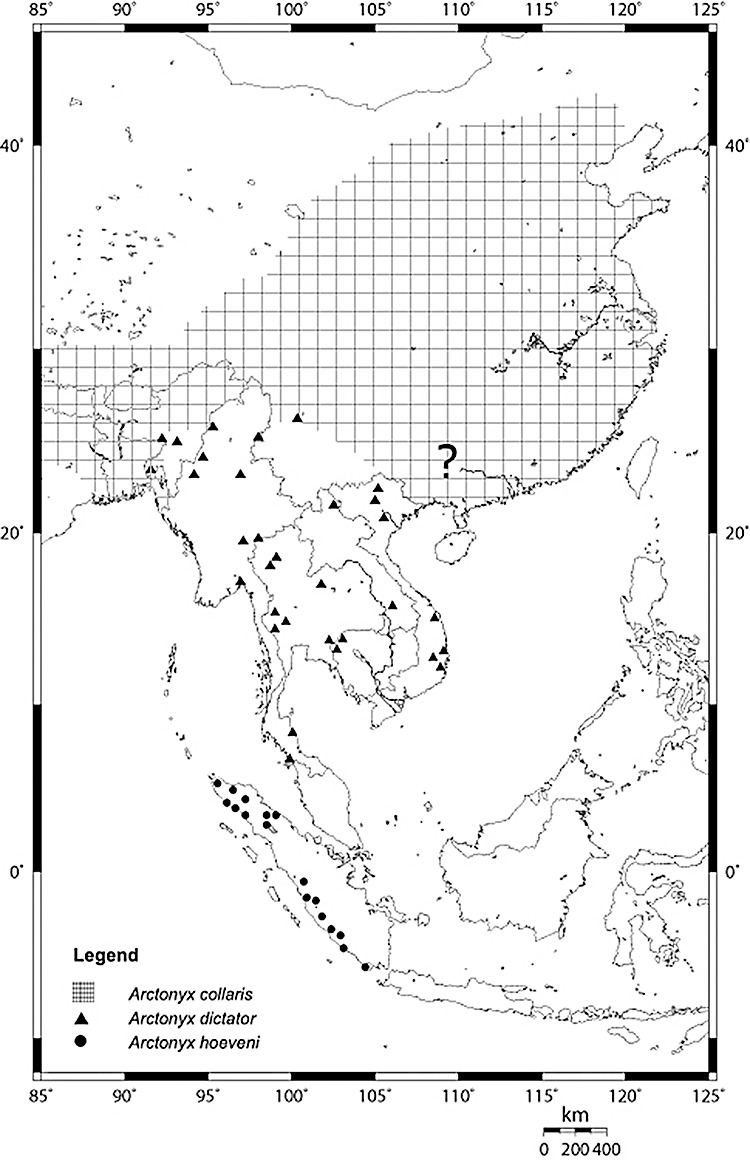
Map of the approximate distribution of Arctonyx spp. Verified point localities are figured for A. collaris (triangles) and A. hoevenii (circles). The approximate range of A. albogularis is shown by cross-hatching, mainly as documented by Pocock (1941), Zhang (1997) and the present study. We have not seen specimens of Arctonyx from far south-eastern China, and only questionably refer these populations to A. albogularis (cf. Zhang, 1997).
Pocock's (1940) name consul, originally erected as a subspecies for the larger Indian species of hog-badger, clusters in our comparisons and analyses with the neotype of A. collaris and with type specimens and samples referred to A. dictatorThomas, 1910(with type locality in peninsular Thailand), and A. annaeusThomas, 1921(with type locality in Vietnam). We argue below that these names apply to geographical populations of a single large-bodied species of hog-badger, the earliest name for which is Arctonyx collaris.
Having established A collaris as the appropriate scientific name for the largest hog-badger, this leaves Meles albogularis (type locality ‘Tibet’) and Arctonyx taxoides (type locality ‘Assam’), both erected by Blyth (1853), as the earliest names applied to the smaller Arctonyx of mainland Asia (Pocock, 1941). Both of these names, which we regard as synonyms, originally appeared in the same publication, although albogularis has page priority. We accordingly choose to fix albogularis as the appropriate name for the smaller Arctonyx of mainland Asia. The few specimens from the Indian subcontinent that we refer to albogularis (Appendix 1; including name-bearing specimens of taxoides, isonyx and taraiyensis) cluster in our comparisons and analyses with most Arctonyx specimens from China (including all of the species-level names applied to Chinese populations of Arctonyx, which we arrange within the synonymy of A. albogularis; see below).
The only epithet applied specifically to the Sumatran hog-badger is Hubrecht's original name hoevenii, as upheld via a neotype designation by Robinson & Kloss (1918). We agree with Pocock (1941) that this distinctive and disjunctly distributed montane Sundaic badger should be recognized as a separate species from other Arctonyx, as A. hoevenii.
Having determined what we regard to be the appropriate specific epithets for the three species of Arctonyx that we recognize (A. albogularis, A. collaris and A. hoevenii), we further document, diagnose and discuss these taxa below.
SYSTEMATICS
GENUSARCTONYX F. CUVIER, 1825
Type species:
Arctonyx collaris F. Cuvier, 1825.
Synonymy:
Arctonyx F. Cuvier, 1825 (type species A collaris F. Cuvier, 1825).
Arctonix Geoffroy and F. Cuvier, 1842 (misspelling of Arctonyx F. Cuvier, in index).
Trichomanis Hubrecht, 1891 (type species T. hoevenii Hubrecht, 1891).
Syarctus Gloger, 1841(renaming of Arctonyx F. Cuvier).
Synarchus Gray, 1865(misspelling of Syarctus Gloger).
Diagnosis:
The species of Arctonyx are medium- to large-bodied badgers that superficially resemble species of the closely related genus Meles (Allen, 1929, 1938; Pocock, 1941). Externally, Arctonyx can be distinguished from Meles by its naked, distinctively hog-like snout; its pale (rather than black) throat; its pale (rather than dark) front claws; and by the morphology of the subcaudal anal sac, which is situated dorsad to the anus (as opposed to Meles, in which the subcaudal anal sac incorporates the anus; Pocock, 1941: 420). The skulls of Arctonyx and Meles are very different (Fig. 8). Relative to Meles, Arctonyx skulls have (among many other differences) markedly flattened auditory bullae; a more elongate, dorso-ventrally compressed, and much narrowed rostrum; proportionally smaller and more widely spaced cheekteeth; a more strongly curved upper incisor arcade; a notched (as opposed to simply U- or V-shaped) posterior postdental palate (formed by the pterygoid bones) that extends further behind the dental arcade, essentially reaching the auditory bullae; and a reduced angular process of the mandible, which is raised above the level of the mandibular toothrow. On the basis of the close genetic relationship between Arctonyx and Meles, Marmi, López-Giráldez & Dominga-Roura (2004) recently suggested that the two could be regarded as congeneric, but the trenchant external and cranial traits that distinguish these two classically recognized genera argue for their continued distinction at the generic level (see also Bininda-Emonds, Gittleman & Purvis, 1999), especially now that both genera are no longer regarded as monotypic (see below).
Figure 8.
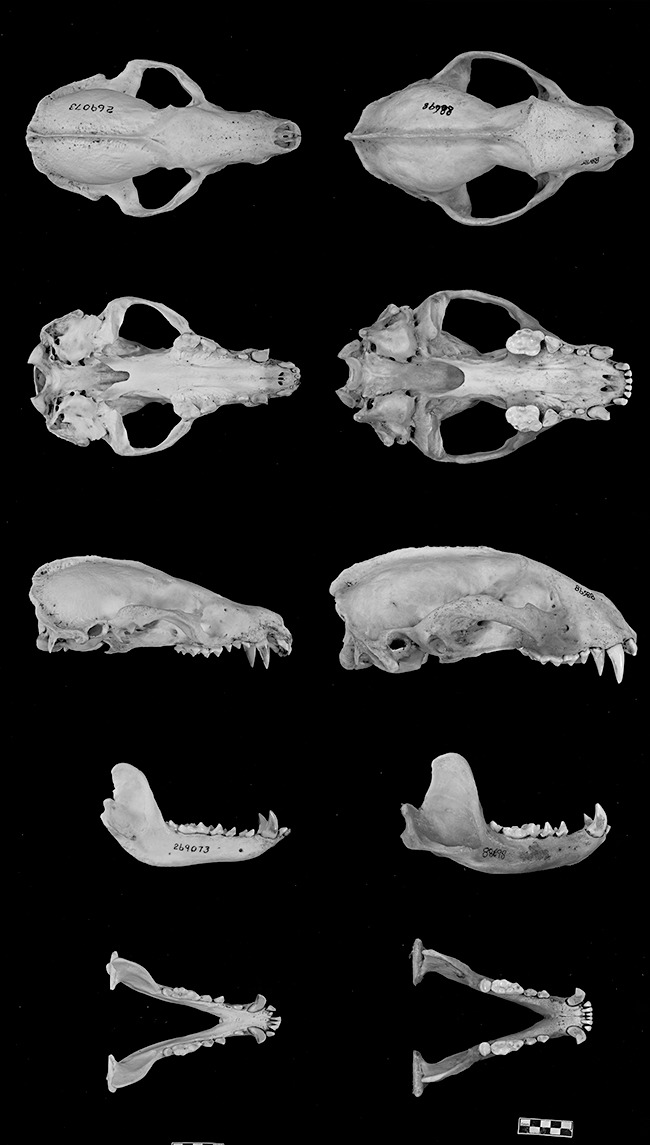
Representative skulls of Arctonyx (left, A. hoevenii, USNM 269073, adult female, from Atjeh, Sumatra) and Meles (right, M. meles, AMNH 88698, adult male, from Gouladah, Iran), of similar absolute size. Scale bar = 20 mm.
Distribution:
The modern and fossil distribution of Arctonyx is restricted to East and Southeast Asia, incorporating most of China, the eastern Indian Subcontinent and Indochina, as far south as the Isthmus of Kra; an outlying population occurs on the Sunda Shelf in the mountains of Sumatra (see below).
Relationships:
Amongst extant mustelids, Meles and Arctonyx are sister taxa, now generally regarded as the only two component genera of the mustelid subfamily Melinae (Marmi et al., 2004, 2006; Sato et al., 2004; see below). Incorporating fossil taxa, Arctonyx is considered to be the sister lineage to the extinct (Pliocene) Holarctic genus Arctomeles (Petter, 1971; Tedford & Harington, 2003). The close relationship between the Holarctic genus Arctomeles, the temperate Old World genus Meles and the temperate to tropical Old World genus Arctonyx provides a strong indication that the occurrence of Arctonyx in the tropics is a comparatively novel development in the historical biogeography of meline badgers.
Content:
We recognize three extant species of Arctonyx-A. collaris F. Cuvier, 1825; A. hoevenii (Hubrecht, 1891); and A. albogularis (Blyth, 1853) - as diagnosed and discussed below.
ARCTONYX ALBOGULARIS (BLYTH, 1853)
Taxonomic synonymy (unique names as originally proposed):
Meles albogularis Blyth, 1853.
Arctonyx taxoides Blyth, 1853.
Arctonyx isonyx Hodgson, in Horsfield, 1856.
Arctonyx collaris taraiyensis Gray, 1863. (nomen nudum).
Meles leucolaemus Milne-Edwards, 1867.
Meles (Arctonyx) obscurus Milne-Edwards, 1871.
Arctonyx leucolaemus orestes Thomas, 1911.
Arctonyx leucolaemus arestes Sowerby, 1914. (nomen nudum; lapsus for orestesThomas, 1911)
Arctonyx obscurus incultus Thomas, 1922.
Arctonyx leucolaemus milne-edwardsii Lönnberg, 1923.
?Arctonyx minorPei, 1987.
Type material and type localities:
The holotype of albogularis, from ‘Tibet’, presumably once in the collections of the Zoological Survey of India in Calcutta, was not listed among the type specimens still housed in that institution in 1977 (Khajuria, Chaturvedi & Ghoshal, 1977). (We have examined one specimen from ‘Tibet’–an adult skull, MNHN 1962-1641.) The holotype of taxoides is a subadult female, skin and skull, from ‘Assam’, deposited in the collections of the Zoological Survey of India in Calcutta (Pocock, 1941: 429; Khajuria et al., 1977: addendum) The holotype of isonyx is apparently a young adult female skin at BMNH (fidePocock, 1941), with skull lost but redrawn by Pocock (1941) from Hodgson's unpublished figures, from the ‘Terai of Nepal’ according to the original description and ‘Tibet’ according toAnderson (1879), but actually from the ‘Sikkim Terai’ (India) fidePocock (1941). The nomen nudum ‘taraiyensis’ was localized to the ‘Sikkim Terai’ in the original description. The holotype of leucolaemus is MNHN 1866-89, a mounted specimen from the vicinity of Peking (= Beijing), China (G. Veron, in litt.). The holotype of obscurus is MNHN 1870-535, a mounted skin and accompanying skull, from Moupin, Sichuan, China (G. Veron, in litt.). The holotype of orestes is BMNH 11.6.1.6, a young adult female, skin and skull, from the ‘Tsing-ling Mountains, SW Shen-si’, Shaanxi Province, 12 000 feet, China. The holotype of incultus is BMNH 2.6.10.35, an old adult male, skin and skull, from ‘Chin-teh (Tsing-tö of Stieler), about 150 km W of Hang-chow’, Anhui Province, China. The holotype of milne-edwarsii is an adult female, deposited in the Swedish Museum of Natural History, from the Minshan Mountains of southern Gansu Province, China (Lönnberg, 1923). Pei's (1987) name Arctonyx minor, applied to a Pleistocene specimen from Guangxi Province, China, seems likely to be another synonym, but we have not seen the specimen on which the name is based or the original publication (published in Chinese; cf. Corbet & Hill, 1992: 199).
Common name:
We suggest the common name ‘northern hog-badger’ for this species.
Diagnosis:
Arctonyx albogularis is a markedly smaller badger than A collaris, considerably more gracile in all aspects of cranial conformation, and differs from that species in having smaller premolars and molars, on average (Figs 2–6: Tables 1–3). It differs from both A. collaris and A. hoevenii by attaining only a moderately developed sagittal crest in the oldest animals, in typically having a less pronounced diastema between P2 and P3 in both the upper and the lower jaws, and in having a proportionally less expansive auditory meatus. Cranially, it differs further from A. hoevenii in having a larger skull on average, larger molars, a rostrum that is on average wider and proportionally less elongate, and a wider postdental palate (Figs 2–6; Tables 1–3).
Table 1.
Selected qualitative and metric diagnostic features for the species of Arctonyx
| Arctonyx albogularis | Arctonyx collaris | Arctonyx hoevenii | |
|---|---|---|---|
| Dominant colour of pelage on mid-dorsum | Usually black | Usually pale | Black |
| Adult condylobasal length | 116–149 mm | 151–172 mm | 113–130 mm |
| M1 (upper) maximum length multiplied by maximum width | 124–202 mm2 | 133–233 mm2 | 55–107 mm2 |
| Major diastema between P2 and P3 (maxillary and mandibular) | Usually absent | Usually present | Usually present |
| Adult sagittal crest | Absent to moderately developed | Well developed | Well developed |
| Winter underfur | Thick and long | Short | Short |
| Tail length compared with head–body length in adults | Averaging c. 1/4 | Averaging c. 1/3 | Averaging c. 1/5 |
| Adult hindfoot length | 76–95 mm | ≥ 110 mm | 50–87 mm |
Table 3.
Selected cranial measurements (mm) for the species of Arctonyx (see Methods and Materials)
| A. albogularis | A collaris | A. hoevenii | |
|---|---|---|---|
| CBL | 130.3 ± 7.68 | 160.5 ± 7.51 | 122.0 ± 4.13 |
| 116.4–148.5 | 150.7–172.0 | 113.4–129.5 | |
| N= 30 | N= 23 | N= 22 | |
| ZYG | 77.3 ± 4.67 | 90.7 ± 6.32 | 69.5 ± 4.98 |
| 68.8–89.1 | 76.3–98.5 | 59.5–78.0 | |
| N= 27 | N= 26 | N= 18 | |
| CC | 25.2 ± 1.97 | 31.7 ± 2.61 | 22.5 ± 1.38 |
| 21.2–29.2 | 27.9–37.2 | 19.8–25.0 | |
| N= 27 | N= 21 | N= 22 | |
| ROST | 27.3 ± 2.15 | 32.6 ± 2.28 | 23.0 ± 1.20 |
| 22.9–30.2 | 28.8–36.4 | 19.5–24.9 | |
| N= 31 | N= 21 | N= 22 | |
| BBC | 48.1 ± 2.65 | 53.0 ± 1.82 | 46.8 ± 1.25 |
| 44.2–53.7 | 49.6–57.0 | 44.2–49.1 | |
| N= 31 | N= 22 | N= 22 | |
| HBC | 41.4 ± 2.78 | 48.1 ± 3.70 | 40.8 ± 2.17 |
| 37.1–50.1 | 41.3–54.1 | 37.0–44.5 | |
| N= 29 | N= 21 | N= 22 | |
| M1L | 15.0 ± 1.13 | 16.7 ± 1.28 | 11.5 ± 1.09 |
| 13.0–17.2 | 14.1–19.1 | 8.2–13.2 | |
| N= 30 | N= 25 | N= 18 | |
| M1W | 10.2 ± 0.97 | 11.2 ± 0.79 | 7.8 ± 0.54 |
| 8.8–12.2 | 9.4–13.0 | 6.7–8.7 | |
| N= 29 | N= 22 | N= 18 | |
| m1L | 15.7 ± 1.16 | 18.1 ± 1.37 | 13.3 ± 1.05 |
| 13.7–17.8 | 15.2–20.1 | 11.7–15.5 | |
| N= 29 | N= 22 | N= 20 | |
| m1W | 6.0 ± 0.57 | 6.8 ± 0.50 | 4.8 ± 0.46 |
| 5.3–7.5 | 6.1–7.7 | 3.6–5.7 | |
| N= 29 | N= 18 | N= 18 | |
| PAL | 85.9 ± 6.55 | 108.4 ± 6.83 | 80.7 ± 2.99 |
| 72.2–104.4 | 96.0–120.3 | 74.2–85.9 | |
| N= 31 | N= 19 | N= 22 | |
| MM | 38.9 ± 2.39 | 45.2 ± 2.41 | 35.1 ± 1.68 |
| 33.9–43.3 | 40.5–49.1 | 32.1–37.6 | |
| N= 31 | N= 19 | N= 21 | |
| IOB | 30.8 ± 2.46 | 37.2 ± 2.72 | 26.6 ± 1.42 |
| 26.7–35.9 | 31.2–41.5 | 24.1–29.3 | |
| N= 31 | N= 22 | N= 21 | |
| POB | 32.3 ± 3.37 | 37.1 ± 2.28 | 28.9 ± 1.49 |
| 26.8–40.0 | 32.6–41.8 | 25.6–32.3 | |
| N= 31 | N= 22 | N= 22 |
The pelage is softer and longer (the longer guard hairs in the winter pelage measuring ≥ 70 mm) than in other Arctonyx, with characteristically thick underfur in the winter months. The forequarters are blackish, and the mid-back, hindquarters and tail are white or heavily mixed with white. Although pelage coloration and marking varies somewhat geographically and individually, the back is more heavily overlaid with black guard hairs than in A. collaris, but less distinctly dark than in A. hoevenii (Figs 9, 10).
Figure 9.
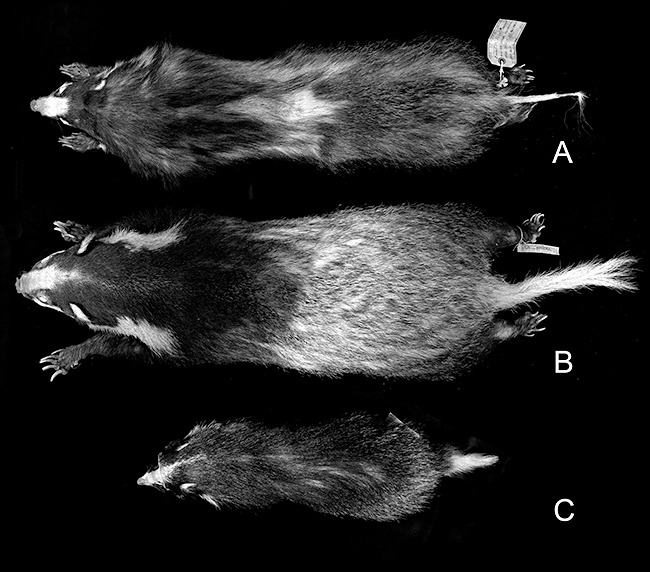
Representative study skins of the three species of Arctonyx. A, Arctonyx albogularis, USNM 258533, adult male from ‘Wen-Chuan’, Sichuan, China. B, A. collaris, USNM 256678, adult female, from Trang, Peninsular Thailand (topotypical). C, A. hoevenii, ANSP 20232, young adult female from Gunung Leuser, northern Sumatra.
Figure 10.
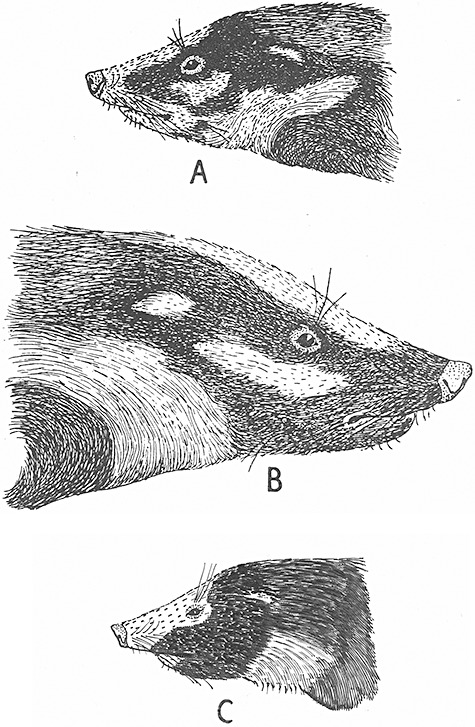
Heads of Arctonyx spp., showing colour and size. A, Arctonyx albogularis, rendered from the holotype of A. taxoides, from eastern India. B, Arctonyx collaris, based on a specimen from Mt Toungoo, Myanmar. C, Arctonyx hoevenii of Sumatra, based on specimens at ANSP and a photograph by E. Jacobsen published by Robinson & Kloss (1918) and Long & Killingley (1983). A and B drawn by Pocock (1940, 1941), C drawn by the third author (L.E.H.).
Distribution:
The recorded historical distribution of Arctonyx albogularis extends from the southern foothills of the Himalayas (Sikkim; BMNH), Assam (BMNH), Manipur (on the strength of a report by Rakamantha, 1994) and probably Bangladesh (BMNH), across the Himalayas to Tibet (Blyth, 1853; MNHN) and across the full expanse of southern and eastern China, from Gansu, Hebei, Shanxi and Liaoning Provinces in the north to Yunnan, Guangxi and Guangdong in the south (Allen, 1938; Zhang, 1997). Records of Arctonyx from Nepal (Shrestha, 1997) probably represent this species.
The distributional range of A. albogularis (Fig. 11) extensively abuts or overlaps that of A. collaris in the eastern Indian Subcontinent (e.g. in eastern India and perhaps Bangladesh), where both species are recorded (Blyth, 1875; Blanford, 1888; Pocock, 1940, 1941; Rakamantha, 1994). Recently published reports of Arctonyx from the Indian states of Assam (Choudhury, 1997a), Arunachal Pradesh (Choudhury, 1997b), North [West] Bengal (Choudhury, 1999) and Nagaland (Choudhury, 2000), and from extreme northern Myanmar (Rao et al., 2005) have not been identified to species, and could represent A. albogularis, A. collaris or both species in co-occurrence (cf. Rakamantha, 1994).
Arctonyx albogularis is widely distributed and apparently common in much of China, its distribution extending over most of the eastern half of the country (Zhang, 1997). Records of occurrence are from the provinces or municipalities of Liaoning, Hebei, Beijing, Henan, Shanxi, Shandong, Shaanxi, Ningxia, Gansu, Anhui, Hubei, Hunan, Jiangxi, Sichuan, Guizhou, Yunnan, Xizang (Tibet), Zhejiang, Fujian, Jiangsu, Guangxi, Guangdong and Hong Kong (Allen, 1929, 1938; Howell, 1929; Pocock, 1941; Zhang, 1997). Its modern or historical range is not known to extend into Mongolia or the Korean Peninsula, nor to the islands of Taiwan and Hainan. The southern limit of the species is not known. However, amongst three Arctonyx skins without accompanying skulls, all taken at Lichiang, Yunnan, in early October 1916, one (AMNH 43095) is smaller and has the black back, longer and softer fur, and thick underfur typical of A. albogularis, while two others (AMNH 43159, 43160) are larger, have paler backs, shorter, coarser fur and minimal underfur, and are thus probably referable to A. collaris. Skulls would be needed to confirm the identity of these specimens definitively, but the characteristics of this series permit the suggestion that the boundary or area of overlap between the two species in southern China lies in Yunnan Province - a hypothesis in need of clarifying field investigations.
Arctonyx albogularis seems to be a rather versatile species. Museum specimens document its occurrence across a remarkable elevational range, from sea level to at least 14 000 feet (i.e. 4300 m) in China. Although there are very few museum specimens available from the Himalayan region (Pocock, 1940), Shrestha (1997) stated that in Nepal this species inhabits ‘forest and scrub in [the] elevational range [of] 1200–4000 m.’Zheng et al.(1988) documented A. albogularis in ‘forest-bush, farm land and wasteland, [and] mountain grassland’ in Shaanxi Province. Wang & Fuller (2003) found A. albogularis to be relatively common in the vicinity of rural villages and surrounding agricultural landscapes.
Geographical variation:
There is considerable variation in cranial size and shape within and between populations referred here to Arctonyx albogularis. Much of this is individual (local) in nature, but strong geographical trends, particularly in size variation, are also apparent across the species' range. We suggest that these are the principal reasons that so many geographical forms of A. albogularis from China and India have been formally named by zoologists over the past 150 years (see above).
In his overview of the Chinese mammal fauna, Allen (1938) parsed geographical variation in A. albogularis (then called Arctonyx collaris) taxonomically into two subspecies, ‘A. c. collaris’ of north-eastern India and southern China (‘the hog-nosed badger of north-eastern India extends its range across southern China, apparently without important change of characters, not withstanding the various names that have been bestowed on it’; p. 403), and ‘A. c. leucolaemus’ of northern China, with records from Hebei, Gansu and the vicinity of Beijing. Later, Pocock (1940, 1941) elected to recognize the nominal Indian form taxoides as a subspecies distinct from Chinese races on the basis of its perceived smaller skull size. However, Pocock had very few mature specimens available for his comparisons (and indeed very few mature specimens of Indian A. albogularis remain available in museum collections today). We note that the skull of an adult Indian specimen of A. albogularis at RMNH (marked ‘Hindustan’, condylobasal length 142 mm) demonstrates that the range of size variation in Indian specimens (condylobasal length c. 110–142 mm) is broadly similar to that observed across all Chinese populations we have studied (116–149 mm). In addition to recognizing three subspecies of smaller, northern hog-badgers (i.e. A. c. collaris of the Himalayan region, A. c. albogularis of southern China and A. c. leucolaemus of northern China), Pocock (1941) drew from Allen's (1938) tables of cranial measurements for Chinese hog-badgers to suggest that an additional, somewhat larger subspecies might be recognized from Fujian Province in south-eastern China.
Allen's (1938) argument for recognition of a small northern Chinese subspecies of A. albogularis appears compelling on morphological grounds. Six adult specimens that we have examined from Beijing and Hebei (including older adults) are all very small, as is Lönnberg's (1923) holotype of milne-edwardsii from Gansu (condylobasal length <124 mm in all seven specimens). In northern animals the back of the head and forequarters tend to be white, whereas they are usually black in other populations of A. albogularis. Milne-Edward's name leucolaemus was the first to be applied to this geographical sample (Milne-Edwards, 1867), and if leucolaemus is recognized as a subspecies, Lönnberg's name milne-edwardsii can be regarded as a synonym (Allen, 1938).
Populations of A. albogularis from central China are on average larger in cranial size than those from further north (we have examined specimens or series from Szechwan, Shaanxi, Zhejiang, Yunnan and Tibet). As noted above, Allen (1938) referred these to typical ‘A. c. collaris’, while Pocock (1941) recognized them under the name ‘A. c. albogularis’. As Allen (1938) and Pocock (1941) noted, specimens from Fujian Province are on average largest of all populations of A. albogularis so far studied.
In summary, a marked size gradient is apparent in Chinese A. albogularis, with animals on average being largest in south-eastern China, somewhat smaller in most of central China and relatively very small in northern China. Interestingly, this is the opposite of what would be predicted by Bergman's Rule, a common scaling rule in mammals in which body size increases with increasing latitude (e.g.Meiri & Dayan, 2003; Meiri, Dayan & Simberloff, 2004). Pending further comparisons into the nature of this variation, we choose not to formally credit geographical variation in A. albogularis through the explicit recognition of subspecies, although both Allen's (1938) discrimination of A. c. leucolaemus and A. c. collaris (i.e. here A. a. leucolaemus and A. a. albogularis), and Pocock's (1941) suggestion of additional subdivisions appear to have some merit. We advocate a combined study of molecular phylogeography, craniometric variation and pelage patterning across the wide range of this species in India and China to evaluate more incisively the significance of geographical variation within badger populations that we refer to A. albogularis. Amassing appropriate samples will require the assistance of colleagues especially in China, whom we encourage to deposit relevant vouchers in Chinese museum collections to complement those samples, documented in part here, that are currently available in other institutions.
Natural history:
Arctonyx albogularis is a medium-sized badger (about the size of Meles meles) that occurs in temperate forests and grasslands of eastern Asia (the Himalayas and China) at all elevations up to about 4300 m. It is an opportunistic omnivore (see below) and is apparently common in many areas. It lives in burrows, dug especially along rivers and streams and under boulders, and apparently is solitary except during the mating season in April and May (Allen, 1938; Zheng et al., 1988). Young (litters apparently ranging in size from one to four) are born in February and March and weaned after about 4 months (Zheng et al., 1988). Unlike other species of Arctonyx, A. albogularis hibernates throughout the winter, from November to February or May, at least in northern China (Zheng et al., 1988). Zheng et al.(1988) reported two daily activity peaks for A. albogularis in Shaanxi, one at 03:00–05:50 h in the morning, and another at 19:00–2100 h in the evening. Northern hog-badgers are preyed upon by various large carnivores, including leopards (Panthera pardus), wolves (Canis lupus) and bears (Ursus thibetanus) (Zheng et al., 1988).
We are aware of two studies on the diet of A. albogularis. Wang & Fuller (2003) collected 45 scat samples over the course of 1992–1994, in all months, near Taohang Village in Jiangxi Province, south-eastern China. They found that A. albogularis was entirely animalivorous, feeding especially on small vertebrates (in 77% of samples, with rodents dominating), also taking a considerable proportion of gastropods (19% of samples), but no plant matter. The three other small to medium-sized sympatric carnivorans studied by Wang & Fuller (2003) (Viverricula indica, Herpestes urva and Paguma larvata) all fed on a greater proportion of plant material.
In considerable contrast, a detailed study of diet in A. albogularis at Long Xian in Shaanxi Province (based on dissections of stomachs from 57 animals) found that earthworms, roots, leaves, beetles, cicadas, lepidopteran larvae and acorns dominated in the diet, with some seasonal variability. Remains of small vertebrates (rodents, snakes, frogs and birds) were found in only 16% of stomachs. Earthworms were eaten by badgers with much greater frequency from late spring to autumn than in winter and early spring, and important earthworm taxa eaten included Allolobophora caliginosa, Pheretime hupeiensis, P. diffringens and Drawida japonica.
A few other additional, anecdotal observations on diet in A. albogularis are available. Long & Killingley (1983) noted that ‘two hog badgers in the Milwaukee Zoo ate “most anything,” both meat and vegetal.’Gao & Sun (2005) described how Arctonyx diggings (for ‘insects and amphibians’) encourage seedling recruitment in Liaodong oaks (Quercus wutaishanica) in the Dongling Mountains of China. It seems clear from these various studies and accounts that A. albogularis is an opportunistic feeder, and that its diet no doubt varies with season, location and perhaps also with individual preference and with local differences in sympatric carnivoran assemblages.
ARCTONYX COLLARIS F. CUVIER, 1825
Taxonomic synonymy (unique names as originally proposed):
Arctonyx collaris F. Cuvier, in Geoffroy and G. Cuvier, 1825.
Arctonyx dictator Thomas, 1910.
Arctonyx annaeus Thomas, 1921.
(?) Arctonyx rostratusMatthew and Granger, 1923.
Arctonyx collaris consul Pocock, 1940.
A. c. nemaeusPocock, 1941. (nomen nudum; lapsus for annaeusThomas, 1921)
Type material and type localities:
The original description of collaris was based on a figure drawn by Alfred Duvaucel of two animals (cf. Fig. 7, a composite impression that appeared in the original description) captured from hill country between Bhutan and India and exhibited in a menagerie at Barrackpore, West Bengal (Cuvier (in Geoffroy & Cuvier), 1825; Pocock, 1940, 1941). As noted above, the neotype of A collaris is AMNH 171170, an old adult male, skin and skull, from Nongpoh (25°54′N, 91°53′E), Khasi Hills (Meghalaya State, India). The holotype of dictator is BMNH 1910.10.1.31, an old adult female, skin and skull, from ‘Lam-ra, Trang, Northern Malay Peninsula [= peninsular Thailand]’. The holotype of annaeus is BMNH 1910.3.10.4, an immature male, skin and skull, from ‘Nha-trang’, Annam. The holotype of rostratus is no. 18393 in the AMNH Vertebrate Paleontology collection, from ‘Yen-ching-kao in the vicinity of Wan-hsien’, Sichuan (middle Pleistocene). The holotype of consul is BM(NH) 1938.10.10.2, skin and skull of an adult male, from ‘Thaundaung, near [Mt] Toungoo, 4500 feet’, Myanmar (Pocock, 1940, 1941).
Common name:
We suggest the common name ‘greater hog-badger’ for this species.
Diagnosis:
The most obvious distinguishing feature of Arctonyx collaris is its massive size; condylobasal length measures ≥ 151 mm in adults (against ≤ 149 mm in A. albogularis and <130 mm in A. hoevenii). Indeed, A. collaris is the largest extant badger; in no other modern badger does condylobasal length exceed 150 mm. Thomas (1910: 425) aptly portrayed its tremendous size in his description of Arctonyx dictator, in which he wrote that ‘the chief feature about this Arctonyx is its enormous size, as the specimen looks like a small bear, and, though a female, exceeds any example, male or female, of A. collaris[i.e. A. albogularis] in the [BMNH] collection.’ Cuvier (in Geoffroy & Cuvier, 1825) was likewise impressed with its robustness, erecting the generic name Arctonyx (meaning ‘bear-claw’) based upon this species (Palmer, 1904).
Arctonyx collaris has a massive, robustly constructed skull featuring a relatively high-domed braincase and pronounced sagittal crest, and usually a marked diastema between P2 and P3 in both the upper and the lower jaws (Figs 2–6, Tables 1–3). Although remarkably variable in size and shape (see below), the cheekteeth are on average considerably larger than in A. albogularis or A. hoevenii (Fig. 3; Tables 1–3).
Although details of the head-striping pattern and various aspects of pelage marking are individually variable (Osgood, 1932; Pocock, 1941), in general the forequarters are blackish, while the mid-back, hindquarters and tail are white or heavily grizzled with white, which renders the pelage typically paler than in the other Arctonyx species (Figs 7, 9). The pelage is characteristically shorter and coarser than in other Arctonyx. The long guard hairs of the winter coat (outer hairs measuring ≤ 80 mm) overlap in length with but tend to be considerably shorter than these hairs in Arctonyx albogularis (outer hairs ≥ 70 mm), and the winter underfur is always much less dense than in A. albogularis. Arctonyx collaris also has a proportionally longer tail than A. albogularis or A. hoevenii, and longer and more massive claws than congeners, particularly on the forelimbs.
Distribution:
Arctonyx collaris is distributed throughout the far eastern portion of the Indian Subcontinent, extending south throughout Indochina to peninsular Myanmar and Thailand (Fig. 11). Records from the subcontinent are from Nagaland (Naga Hills; BMNH), Meghalaya (Khasi and Jaintia Hills; AMNH, BMNH), ‘Bengal’ (BMNH; probably ‘some locality to the east of the Ganges and Brahmaputra, possibly from Chittagong’: Pocock, 1941), Bangladesh (‘Chittagong Hills’, BMNH; Pocock, 1941), and probably from Manipur (see Rakamantha, 1994). Arctonyx albogularis also occurs in the Indian subcontinent portion of this range. As noted above, recently published reports of Arctonyx from the Indian states of Assam (Choudhury, 1997a), Arunachal Pradesh (Choudhury, 1997b), North [West] Bengal (Choudhury, 1999) and Nagaland (Choudhury, 2000), and from extreme northern Myanmar (Rao et al., 2005) have not been identified to species, and could represent A. albogularis, A. collaris or both species in co-occurrence (cf. Rakamantha, 1994).
All Arctonyx specimens that we have examined from Myanmar (BMNH, MCZ, USNM), Thailand (AMNH, BMNH, MCZ, USNM), Vietnam (BMNH, MNHN), Cambodia (MNHN) and Laos (AMNH, ANSP, FMNH) represent A collaris (for most or all distributional records and sources and associated discussion see Thomas, 1910, 1921; Osgood, 1932; Allen & Coolidge, 1940; Pocock, 1940, 1941; Urbain & Friant, 1940; van Tien, 1966; Van Peenen, 1969; Deuve, 1972; Rabinowitz, 1990; Rabinowitz & Walker, 1991; Rozhnov, 1994a, 1994b; Bergmans, 1995; Duckworth, 1997, 1998; Duckworth, Salter & Khounboline, 1999).
The southernmost distributional extent of A. collaris lies in the far north of the Malay Peninsula, in peninsular Thailand. There is an unverified record from the Malaysian state of Upper Perak (Tate, 1947; Medway, 1978), but no vouchered specimens originate from Malaysia and it appears to be absent from most (if not all) of that country. The westernmost occurrence of A. collaris is probably defined by the Brahmaptura and Ganges drainages (see above). The exact northern boundary of occurrence of A. collaris remains to be established, but may lie in Yunnan. Apart from two skins from Lichiang, Yunnan, which we provisionally identify as A. collaris (see above, under A. albogularis), all specimens from China that we have seen (AMNH, FMNH, MCZ, MNHN, USNM) represent A. albogularis. The absolute, striking distinctions in size, pelage and qualitative cranial morphology between available adult samples of A. albogularis and A. collaris indicate no intergradation between these taxa (cf. Pocock, 1941).
Most museum records of A. collaris from India and Myanmar with associated elevational data derive from between 700 and 1500 m (BMNH). Similarly, most museum specimens from Thailand, Vietnam and Laos with associated elevational data derive from localities situated between 600 and 1300 m. There are only a handful of specimens of A. collaris in museums marked with elevations below 600 m; the lowest elevation given on a specimen label is ‘100 m’ (BMNH 15.12.1.8, Sai Yoke, Thailand). Duckworth (1997) noted that ‘all recent records [of Arctonyx in Laos] came from above 500 m, but as all were in the same catchment, it is unclear if this represents a pattern of altitude, geography, or even just chance … All records were from forest, and elsewhere it occurs mainly in forest’ (see also Duckworth, 1998; Duckworth et al., 1999). Long & Minh (2006) discussed recent records of A. collaris in central Vietnam (the Central Annamites area), where it was encountered in ‘primary lower montane forest’ at 900 m at one site, and in ‘disturbed, primary hill forest’ at 640 m at another. Based on our canvas of published accounts and specimen label data, we suggest that the seemingly patchy occurrence of A. collaris in Southeast Asia (Duckworth et al., 1999; Long & Minh, 2006) reflects its typical absence from lower-elevation habitats and preferred occurrence in little-disturbed hill and lower montane forests above about 500–600 m. Undisturbed upland forested tracts possibly offer the best combination of sites for burrowing, diversity of food and escape from predation for A. collaris. The core elevational range of this species appears to lie between about 600 and 1400 m, with all records spanning 100–1500 m, but little information is available with respect to its upper elevational limit of occurrence. In their account of the mammals of Thailand, Lekagul & McNeely (1977) quoted the upper limit of occurrence as 3500 m for ‘A. collaris’, but we suspect this elevational information is based on high-elevation localities quoted for the genus in China or Sumatra, where species other than A. collaris are found.
Geographical variation:
Aspects of geographical variation in A. collaris have been discussed by Thomas (1921), Osgood (1932), Pocock (1940, 1941); Urbain & Friant (1940) and Lekagul & McNeely (1977), but always on the basis of little comparative material, in particular with very few adult skulls available. One notion that has been presented by previous reviewers is that Indochinese Arctonyx are smaller at more northern latitudes than in the south (Pocock, 1941; Lekagul & McNeely, 1977), although Osgood (1932) observed that ‘specimens from southern Laos are fully as large as northern ones.’ Our larger sample of specimens from the region offers little evidence for a latitudinal size gradient. In any case, many fewer full-grown skulls are available from northern Myanmar and Thailand (e.g. BMNH, MCZ, USNM) than from more southerly localities, and many skulls are too imprecisely localized (e.g. skulls labelled ‘Siam’, ‘Vietnam’, or unlabelled from Indochina at MNHN and BMNH) to be useful in assessing geographical variation. We agree with Osgood (1932) that there is no clear basis for the subspecific discrimination of the nominal taxon annaeus, named by Thomas (1921) on the basis of an immature specimen, from ‘A. c. dictator’ (which we do not distinguish subspecifically from typical A. collaris). Pocock's (1940) description of consul as a somewhat smaller race linking ‘A. c. dictator’ (i.e. A. collaris) and ‘A. c. taxoides’ (i.e. A. albogularis) was prematurely conceived, in part because the majority of his specimens were immature (and see Pocock, 1941: 499). Our study of nearly all available museum material of Arctonyx identifies A. collaris as a morphologically distinctive species with no evidence for morphological intermediacy linking it to either A. albogularis or A. hoevenii (Figs 2, 4–6), and identifies no clear basis as yet for characterizing geographical variation within A. collaris at the trinomial level.
The middle Pleistocene fossil badger Arctonyx rostratusMatthew & Granger, 1923, originally described as late Pliocene in age (see Hooijer, 1947) from Sichuan Province in China (type locality ‘Yen-ching-kao in the vicinity of Wan-hsien’), differs from the modern hog-badger of Sichuan (i.e. A. albogularis) in its larger size, heavier molars, pronounced diastema between P2 and P3, and more parallel-sided rostrum. All of these are characteristic features of A. collaris. Pei (1940) discussed an additional record of ‘Arctonyx cf. rostratus’ from the Pleistocene of Jiangsu Province, further to the east. In the holotype of rostratus, condylobasal length measures 148 mm - i.e. closer to samples of modern A. albogularis (range 110–149 mm) than A. collaris (150–172 mm), although falling close to both (in the type the zygomatic arches are broken and missing, such that the skull cannot be plotted in our Fig. 2). Based on its qualitative features, we suspect that rostratus is best characterized as a synonym (or perhaps chronological subspecies) of A. collaris. If this is the case, Sichuan and Jiangsu specimens referred to rostratus indicate that the geographical range of A. collaris extended further north in the middle Pleistocene than today. However, we suggest that it is inadvisable to synonymize this fossil taxon formally before its morphometric and qualitative features are more conclusively compared against both A. collaris and A. albogularis. More recently, Long, de Vos & Ciochon (1994) referred Middle–Late Pleistocene material of A. collaris from six different cave sites in Indochina and Thailand to ‘A. cf. rostratus’.
Pocock (1941) aptly illustrated the extreme variation in cheektooth size, shape and occlusal morphology within a local population of A. collaris from Mt Toungoo in Myanmar (e.g. Fig. 12). Similarly striking is the range of dental size variation seen locally on the Bolovens Plateau of Laos and in peninsular Thailand (e.g. Figs 12, 13). Based on our museum studies of carnivores worldwide, we suspect that A. collaris shows more intrapopulational variation in molar size than any other carnivore species (Pocock, 1940), although molar variability is also striking in local series of Arctonyx hoevenii. This is an excellent avenue for further, detailed comparative ecomorphological study. We suggest that extraordinary cheektooth variability in Arctonyx may be tied to the greater comparative importance of vermivory in its diet relative to other badgers (see below). Committed vermivory in mammals is commonly correlated with evolutionary reduction or loss of molars and/or unusual molar size and shape variability (e.g. Griffiths, 1978; Rickart, Heaney & Utzurrum, 1991; Balete et al., 2007), assumedly reflecting the reduced importance of stringent genetic control in molar development in mammalian vermivores. Vermivory (and myrmecophagy) are also usually accompanied by a comparative elongation of the rostrum relative to related taxa (Anderson & Jones, 1967; Griffiths, 1978; Musser, 1982), as seen in Arctonyx relative to other badgers - assumedly an adaptation for digging through the soil with the snout.
Figure 12.
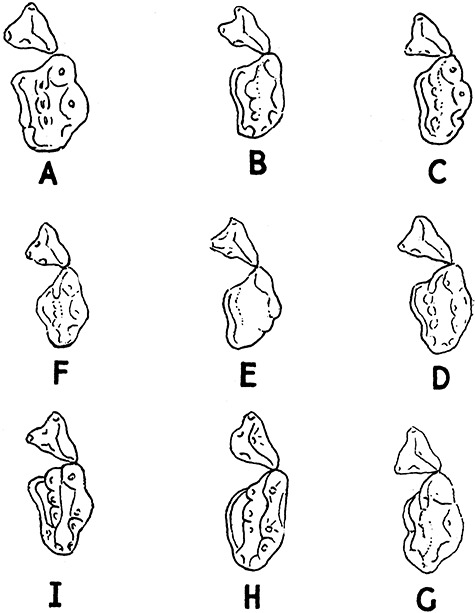
Local and regional variation in the larger cheekteeth (upper P4 and M1) of Arctonyx collaris, depicted to scale, as figured by Pocock (1941). A, adult male, eastern India (‘Bengal’). B, immature male, eastern India (Nagaland, Naga Hills). C, immature male, eastern India (Meghalaya, Jaintia Hills). D, immature male, Myanmar (Kindat area). E, old adult male, Myanmar (Mt Toungoo). F, immature female, Myanmar (Lockaw). G, immature male, Vietnam (Nhatrang). H, immature male, Myanmar (Mt Toungoo). I, immature female, Myanmar (Chin Hills). Labelled as by Pocock (1941). Based on vouchered specimens at BMNH (see Pocock, 1941).
Figure 13.

Additional examples of variation in the larger cheekteeth (upper P4 and M1) of Arctonyx collaris, depicted photographically, to scale. Above, AMNH 87394, adult male, Laos (Bolovens Plateau); middle, AMNH 87395, young adult female, Laos (Bolovens Plateau); below, AMNH 37221, old adult, unsexed, Thailand (‘Siam’). Scale bar = 10 mm.
Natural history:
Arctonyx collaris is found only in Southeast Asia, where it predominantly occurs in little-disturbed hill and lower montane forests. Entirely terrestrial, it sleeps in ‘burrows they dig for themselves or in convenient natural shelters, like rock-crevices’ (Pocock, 1941: 447). Pocock (1941: 447) characterized A. collaris as nocturnal, but Duckworth et al.(1999: 188) considered it to be diurnal. Like A. hoevenii of montane Sumatra (see below), A. collaris is probably instead best characterized as cathemeral (i.e. active sporadically in accordance with its needs, without being commitedly nocturnal or diurnal; e.g. Curtis & Rasmussen, 2006; Hill, 2006; Tattersall, 2006).
Arctonyx collaris apparently has poor eyesight, but is large, powerful and ferocious, and perhaps as a result it apparently is relatively unwary for a wild animal of its size (Pocock, 1941). It can be approached closely and killed by men and dogs (Pocock, 1941; Duckworth et al., 1999) or by large predators, such as cats. Rabinowitz (1990) and Rabinowitz & Walker (1991) found that A. collaris was a common animal in Huai Kha Khaeng Wildlife Sanctuary in Thailand, noting that ‘it was sighted on several occasions and its remains were frequently found in carnivore faeces’ (Rabinowitz & Walker, 1991). Identifiable remains of Arctonyx were found in 5% of large carnivore scats found at one site in the sanctuary.
Very little is recorded of the diet of A. collaris. Most available information was summarized by Pocock (1941) in his account of ‘Arctonyx collaris consul’, but unfortunately this is based almost entirely on observations of animals in captivity. Observations of five animals are available (Pocock, 1941: 447). Pocock (1941: 447) noted that the two animals on which Cuvier's original description of A. collaris is based ‘would eat meat, but preferred fruit, bread and milk.’ An adult male A. collaris captured in Moulmein (Myanmar) ‘ate voraciously meat, entrails, snakes and other reptiles, fish, and plantains, but was fondest of earthworms, which it greedily devoured as fast as they could be dug up.’ Another animal from Arakan (Myanmar), not identified with respect to sex or age, ‘refused to eat meat or flesh of any kind, but would take bread and milk, and was particularly partial to plantains.’ A cub from Tura in the Garo Hills, first described by Jackson (1918) (which, however, may have been either A. albogularis or A. collaris), fed on:
rice and rice-water, which it ate noisily, like a pig, holding the bowl between its paws. Later it had two large tins of earthworms daily, showing the same liking for them [as the Moulmein animal] … as well as bread and milk and pudding. It also ate centipedes and the contents presumably of reptiles' eggs, rejecting the ‘leathery’ shells, but [unlike the Moulmein animal] … it was afraid of snakes and would not touch roots, fruits, or vegetables (Pocock, 1941: 447).
In their book on Thai mammals, Lekagul & McNeely (1977) noted that Arctonyx feeds on ‘tubers, roots, earthworms, insects, and other small living creatures’, but the source of this information was not noted. Perhaps the best that can be said in summary is that A. collaris is omnivorous, and may at least partially specialize on eating earthworms.
Thomas (1910), quoting H. C. Robinson, noted that this species was known by the native name Sabima in peninsular Thailand in the early 20th century. Cuvier (in Geoffroy & Cuvier, 1825) gave the local name of this species as Bali-Saur or Bali-Soar in eastern India (apparently meaning ‘sand-pig’ or ‘bear-pig’ in Hindi). Pocock (1941: 427) provided a number of local names for Arctonyx in eastern India and Myanmar, but it is unclear whether these listed names reference A. collaris, A. albogularis or both species.
ARCTONYX HOEVENII (HUBRECHT, 1891)
Taxonomic synonymy (names as originally proposed):
Trichomanis hoevenii Hubrecht, 1891.
Arctonyx collaris hoeveni, Robinson & Kloss, 1918. (redescription and designation of neotype)
Type material and locality:
Hubrecht's (1891) unfortunate original description of hoevenii was based on a captive specimen that was lost, with only a vague type locality provided (‘in the mountainous districts that separate the Residencies of Palembang and Bencoolen in Sumatra’). Robinson & Kloss (1918: 12) preserved the usage of Hubrecht's epithet and restricted the type locality by designating a neotype for hoevenii, an adult female (skin and skull) from ‘Sungei Kumbang, Korinchi, West Sumatra’ (= Sungai Kumbang, Gunung Kerinci, West Sumatra Province, Indonesia), at 4700 feet (= 1433 m). This specimen, originally identified as ‘Federated Malay States Museums No. 440/14’, is now ZRC 4.1143.
Common name:
We suggest the common name ‘Sumatran hog-badger’ for this species.
Diagnosis:
Arctonyx hoevenii is smaller than other Arctonyx (Figs 2–6; Tables 1–4); as Hubrecht (1891) noted, it is ‘an animal of the size of a very large cat’ (apparently meaning a domesticated cat, Felis sylvestris catus). The skull can be distinguished from other Arctonyx by its small overall size, relatively narrow rostrum, pronounced sagittal crest (an indication of cranial robustness despite its small size) and proportionally small teeth. The skin can be distinguished from other Arctonyx by its sparser fur and darker dorsal pelage.
Distribution:
Arctonyx hoevenii occurs in the mountains and adjacent foothills of Sumatra, which extend from north to south across the entire length of the western portion of the island and are collectively known as the Barisan (= Barussan) Range. Corbet & Hill (1992) mapped the distribution of Arctonyx only in the mountains of southern Sumatra, but vouchered records document that it probably occurs essentially everywhere along the length of the Barisan chain, from Aceh Province in the north to South Sumatra Province in the south (Fig. 11; Appendix 3). The elevational range of A. hoevenii extends from perhaps as low as 700 m in foothill forests (Holden, 2006) to the very highest point on the island - Robinson & Kloss (1918) reported a skull of A. hoevenii picked up at 3780 m in the alpine zone on the summit of Gunung Kerinci. The core habitat of A. hoevenii would seem to be lower montane and mossy forests and subalpine meadows between 800 and 2600 m, as documented below.
Van Schaik & Griffiths (1996) obtained camera-trap photographs of A. hoevenii at two sites in Gunung Leuser National Park. The lower of these, the upper Bengkung catchment, ‘consists mainly of hilly terrain, with the southern part gently undulating. The area is almost entirely below 800 m altitude, and is clad in mixed lowland dipterocarp forest, interspersed with parts dominated by non-dipterocarps, mainly near rivers’ (Van Schaik & Griffiths, 1996: 106). In the upper Mamas River catchment they photographed A. hoevenii in a landscape situated in ‘a relatively gently sloping valley surrounded by steep ridges, covered in submontane and montane forests’ between 1200 and 1600 m.
Augeri (2005) also camera-trapped extensively in Gunung Leuser National Park, at six sites ranging across a broad elevational transect. He obtained camera-trap photographs of A. hoevenii at several montane sites, including Ketambe Atas (1217–2657 m; 2205 trap-nights, 15 Arctonyx pictures), Gunung Putar (1402–2113 m; 1792 trap-nights, 13 Arctonyx pictures), Ketambe (433–2002 m; 476 trap-nights, two Arctonyx pictures), but not at several lowland sites, including Sei Badak (50–210 m, 1848 trap-nights), Sei Birah (211–964 m, 2240 trap-nights) and Sekundur (47–107 m, 1960 trap-nights).
Arctonyx hoevenii was ‘photographed regularly’ during 25 000 h of camera trapping at Gunung Tujuh in Kerinci Seblat National Park in central Sumatra by Holden (2006: 36) in forests situated at 2000–2400 m, a habitat he characterized as ‘primary hill forest’. Holden (2006: 35–36) also discovered an Arctonyx footprint at Tandai, another site at the park situated in ‘old logged forest in a state of regeneration’ at 700 m, but noted that ‘photo-traps working in this area for 50 000 hours did not record Hog Badger, indicating that it was not a common species in this habitat.’ He further noted that ‘among local informants only those that were familiar with the high altitude forests knew the animal, referring to it as Babi Batang.’
Augeri's (2005) and Holden's (2006) observations and impressions indicate that A. hoevenii is considerably more common in higher montane forest than in lower elevational foothill forests, a result that echoes the earlier comments of Frederick A. Ulmer, Jr, who collected mammals in Sumatra for ANSP during the Vanderbilt Sumatran Expedition in 1936–1939 (quoted in Miller, 1942: 121–122):
Although two of our specimens [of Arctonyx] were collected by natives at supposedly low elevations [600 and 2900 ft (i.e. 183 and 884 m, respectively)] I saw definite evidence of hog badgers' presence in the mountains only. Their diggings were first seen at Bivouac No. 5 (7900 ft [= 2408 m]) where we found the skeleton of an aged animal. Some hair and cartilage were still adhering to the bones. Along a brook flowing through a deep ravine the badgers had dug many funnel-shaped depressions two or three inches deep. A heavy steel trap set there (baited with the carcass of a little jungle squirrel, Tomeutes) caught one; but the powerful animal pulled free, leaving only a bunch of hair. At Bivouac No. 7 (9300 ft [= 2835 m]) a badger burrow was found in the bank of a stream that flowed through a large ‘blang’ or alpine meadow. This burrow was about 10 inches in diameter; a large heap of debris had accumulated in front of it. In this area we saw the characteristic ‘grubbings’ of the badgers. Again, in a ‘blang’ between Bivouacs 8 and 9 the small funnel-shaped holes were so numerous as to pock the ground in all directions. Members of the expedition who kept the young badger ([ANSP] no. 20231) alive for a few days were in accord concerning the animal's foetid odor.
As Ulmer noted, the lowest elevational record, 600 feet, must be doubted. It is based on a mounted skin and skull apparently received from locals at Kuta Tjane (Miller, 1942: 121). Apart from this specimen, the lowest record of occurrence for A. hoevenii is Holden's (2006) footprint from secondary forest situated at 700 m, where his camera-trapping suggested it was extremely uncommon. Setting the dubious Kuta Tjane record aside, the mean elevation of 25 vouchered sites of occurrence with recorded elevational data - a compilation drawing from museum specimen data and from other elevational records discussed by Robinson & Kloss (1918, 1919), Miller (1942), Van Schaik & Griffiths (1996) and Holden (2006). - is 1748 m (SD 800 m, median 1600 m). Judging from all available museum specimens and from records and discussion in the literature, we suggest that the core elevational and habitat range of A. hoevenii embraces montane forests and adjacent mountain meadows situated between about 800 and 2400 m, if not also extending higher (where little camera-trapping work or other mammal inventory work has commenced). The skull from the summit of Kerinci mentioned by Robinson & Kloss (1918) shows that badgers extend to the very elevational limits of Sumatra, at least on occasion.
We detect no pattern of geographical morphological variation in A. hoevenii.
Natural history:
Arctonyx hoevenii is a montane badger that lives and forages in the mountain forests and upland meadows of Sumatra. It lives in terrestrial burrows, dug perhaps especially in soft soils along riverbanks (Miller, 1942). It may be active by night or by day, and has been characterized as cathemeral (Van Schaik & Griffiths, 1996). Its diet may consist almost entirely of terrestrial invertebrates. Based on observations related by van der Hoeven, Hubrecht (1891) described this badger's ‘long cylindrical tongue, which thrust out, serves the animal in the collecting of ants, which are its natural food’. Based on specimens they collected and prepared on Gunung Kerinci,Robinson & Kloss (1918) noted that ‘its food … appeared to consist chiefly of earthworms and beetle larvae.’ Jacobsen (in Robinson & Kloss, 1919: 265) wrote of a specimen he collected on Gunung Kaba:
I kept this specimen alive for a day; the only food it would take was earthworms; raw meat and insects were refused. The excrement, consisting chiefly of earth from the worms it had digested, had a very nauseous, sweetish smell, very characteristic and quite different from the pungent smell of Mydaus meliceps[= M. javanensis].
Like Arctonyx albogularis and A. collaris, A. hoevenii probably also takes other, non-invertebrate food opportunistically; Miller (1942) noted that one animal was taken in a snare baited with a squirrel carcass. However, we find the first-hand observations that A. hoevenii consumes primarily earthworms, beetle larvae and ants, provided by van der Hoeven, Robinson and Kloss, and Jacobsen to be compelling. Indeed, as noted above, the insectivorous habits of the first specimen of A. hoevenii reported in the scientific literature were so convincing that the animal was initially described within the ‘Edentata’ in the absence of clarifying specimens (Hubrecht, 1891).
Jacobsen also examined scat suggesting vermivory of Arctonyx both on Gunung Saba and on Gunung Dempo (Robinson & Kloss, 1919). The ‘funnel-shaped depressions two to three inches deep’ dug by badgers along streams on Gunung Leuser, mentioned by Ulmer (above), are likely to be places where badgers shoved their muzzles into the earth to secure earthworms with their tongues - sign and behaviour reminiscent of New Guinea's long-beaked echidna Zaglossus bartoni (Opiang, 2004), an animal of similar size and habitat requirements that is a committed earthworm-eating specialist (Griffiths, 1978; Flannery, 1995). Jacobsen, Ulmer, and Robinson and Kloss all described abundant Arctonyx diggings in Sumatran mountain meadows, probably reflecting the species' searches for earthworms (Robinson & Kloss, 1918, 1919; Miller, 1942). A greater commitment to vermivory/insectivory may offer an explanation for the relatively reduced size of its cheekteeth, even compared with A. albogularis, to which it is similar in cranial size, and the great local variability in size of the molars, unusual for a carnivore species with such a restricted distribution. Although more intensive study is desired to clarify the proportion of earthworms, other invertebrates and other elements in the diet of A. hoevenii, we suggest that Arctonyx hoevenii can join the distinctive Southeast Asian hemigaline civets (Chrotogale, Hemigalus, Diplogale), the Malagasy endemic Eupleres, the hyaenid aardwolf (Proteles cristatus), and perhaps the Sloth Bear (Melursus ursinus) and Bat-Eared Fox (Otocyon megalotis) on the short list of carnivorans for which invertebrate prey constitute the dominant part of the diet (Davis, 1962; Von Ketelhodt, 1966; Albignac, 1974; Payne & Francis, 1985; Richardson, 1987a, b; Dang, Anh & Huynh, 1992; Joshi, Garshelis & Smith, 1997; plus data from museum specimens). As in A. hoevenii, the molars in these other invertebrate-eating carnivorans are reduced in size and complexity compared with their nearest, less-specialized phylogenetic counterparts (e.g. Gregory & Hellman, 1939; Ewer, 1973; Popowics, 2003; Sacco & Van Valkenburgh, 2004; Friscia, Van Valkenburgh & Biknevicius, 2007).
Like most other badgers, Sumatran hog-badgers are undoubtedly preyed upon by humans (Schneider, 1905) and by large carnivores (perhaps, as in the case of A. collaris, especially by wild cats), and are aggressive when approached. Jacobsen (in Robinson & Kloss, 1919: 265) noted:
The animal is of very savage disposition; if excited it emits a grunting sound, exactly as if somebody was snoring, and not the rumbling or drumming sound which is peculiar to other badgers, as for instance, in Mydaus.
Long & Killingley (1983) recounted a similar anecdote of a nocturnal encounter with A. hoevenii:
In the jungles of Sumatra a group of mountain climbers encountered a hog badger at 2:00 A.M., and they ‘blinded’ it with a flashlight. It did not run, but because of its fierce snarling and growling it could not be approached. Finally it turned away and began to dig itself into the dirt.
Despite its small size, A. hoevenii is tenacious and strong. Ulmer (quoted above) noted how an animal ‘pulled free, leaving only a bunch of hair’ from a heavy steel trap; and Jacobsen (in Robinson & Kloss, 1919: 265) noted that his specimen from Gunung Kaba ‘was captured in a snare; its mate was caught the next day on the same spot but escaped by tearing the string.’
Essentially nothing is known of the social structure or reproduction in this species. Jacobsen's reference to a mated pair may suggest he observed pair bonds, but nothing definitive is reported in the literature, and most anecdotal accounts of A. hoevenii mention encounters of single animals. Like other Arctonyx, A. hoevenii has three pairs of mammae. Litter size is unknown, but one juvenile was collected in March 1939 (Miller, 1942).
Arctonyx hoevenii is probably common in many areas in the mountains of Sumatra. Van Schaik & Griffiths (1996), Augeri (2005) and Holden (2006) photographed it regularly in appropriate habitat (i.e. forests at and above 800 m) in both northern and central Sumatra. As noted above, Ulmer saw many badger ‘grubbings’ between 2600 and 2800 m on Gunung Leuser in northern Sumatra (so numerous as to ‘pock the ground in all directions’), and Jacobsen (as reported by Robinson & Kloss, 1919: 265) made similar observations on Gunung Dempo in southern Sumatra (‘I saw at an altitude of 1800–2600 m numerous traces of this badger; everywhere the soil was turned up for worms and I found there also its not-to-be-mistaken excrement’). Robinson & Kloss (1918) likewise reported that ‘at Sungei Kumbang and as high as Sungei Kring it was very common and traces of its burrowings were to be seen almost everywhere in dampish spots …’ The relatively long museum series taken between 800 and 1400 m on Gunung Merapi (ZRC, BMNH) is another potential indication of local abundance in appropriate habitat.
Holden (2006) noted that there are no specimens of A. hoevenii at the Museum Zoologicum Bogoriense (MZB, now based in Cibinong), a fact that we have confirmed. Reference to specimens from ‘the Garo (= Karo) Highlands’ at MZB (then as ‘the Buitenzorg Museum’) by Robinson & Kloss (1918: 12)–13) may be based on a mistaken impression that Schneider's (1905) Karo Highlands specimen was deposited there. In fact, Schneider (1905) mentioned that his specimen, the first of A. hoevenii to be deposited in a museum collection, is in the Zoological Museum (today the Musée Zoologique) at Strasbourg. Schneider noted that the local name for A. hoevenii in northern Sumatra was Garum, that it lived in burrows in the mountains and that its flesh was considered a local delicacy, perhaps because of its thick layer of subcutaneous fat. We do not know if Schneider's collections are still to be found in Strasbourg today.
DISCUSSION
HOG-BADGER EVOLUTION AND BIOGEOGRAPHY
All badgers, including the ferret-badgers (Melogale), ratel or honey badger (Mellivora), stink badgers (Mydaus), American Badger (Taxidea), Eurasian badgers (Meles) and hog-badgers (Arctonyx) were long classified together within the mustelid subfamily Melinae (e.g. Pocock, 1941; Ellerman & Morrison-Scott, 1951, 1966; Long & Killingley, 1983. More recently, various studies have shown Melinae, as traditionally constituted, to be a polyphyletic assemblage, with its component members sharing various traits that are either primitive amongst musteloid carnivores, or convergently derived (e.g. Radinsky, 1973; Dragoo & Honeycutt, 1997; Marmi et al., 2004; Sato et al., 2004). Mydaus is now classified with skunks in a separate carnivoran family, Mephitidae (Dragoo & Honeycutt, 1997). Melogale is now thought to be most closely related to a clade containing otters and musteline (weasel-like) mustelids, rather than other badgers, and is classified by some in the monogeneric subfamily Helictinae (Sato et al., 2004). The immediate relationships of Mellivora remain poorly established (Bininda-Emonds et al., 1999), but recent authorities have segregated the genus into its own subfamily, the Mellivorinae (McKenna & Bell, 1997). Finally, as noted above, the genera Meles and Arctonyx are closely related, and together apparently constitute the sister lineage to Taxidea. These lineages are now formally recognized by some workers at the subfamilial level as a restricted Melinae (Meles + Arctonyx) and Taxidiinae (Taxidea) (e.g. Marmi et al., 2006).
It is gradually being realized that traditional recognition of only a single species of Meles and a single species of Arctonyx (e.g. Ellerman & Morrison-Scott, 1951, 1966; Corbet & Hill, 1992; Wozencraft, 2005) has served to obscure patterns of taxonomic and ecomorphological variation in this group of mustelids. Our discrimination of three species of Arctonyx in Southeast Asia complements similar studies of morphological variation across the geographical range of Meles (Abramov, 2002; Abramov & Puzachenko, 2005, 2006), which have recently argued for the recognition of at least three distinctive, allopatric/parapatric species in the genus -M. meles of Europe and western Asia, M. leucurus of central and eastern Asia, and M. anakuma of Japan (Wozencraft, 2005), although others have argued that these might be better characterized as subspecies (Kurose et al., 2001; Marmi et al., 2006). As with the discrimination of multiple species-level taxa in Meles (cf. Abramov, 2002; Marmi et al., 2006), our arguments for the recognition of three species of Arctonyx invite active scrutiny along other avenues of investigation, including studies of molecular divergences and phylogeographical patterning, evaluation of bacular and other postcranial skeletal distinctions, and field studies of behavioural and ecological distinctions between Arctonyx taxa, especially in the geographical regions that we have identified as boundary or overlap areas between the smaller, East Asian species A. albogularis and the larger, Southeast Asian A. collaris (i.e. in the southern Himalayan foothills and southern China).
The elucidation of Arctonyx hoevenii as a distinctive overlooked carnivore species endemic to montane Sumatra is exciting, but not necessarily surprising. The mountain chain of western Sumatra is a remarkable zone of biotic endemism, home to a highly unique biota (Stattersfield et al., 1998; Wikramanayake et al., 2002); nearly 15 bird species and many mammals are found nowhere else. Apart from the Sumatran hog-badger, mammal species found only in Sumatran highland forests (and/or grasslands) above c. 600–800 m include the erinaceid Hylomys parvus (see Ruedi, Chapuisat & Iskandar, 1994), the shrew Crocidura beccarii (see Ruedi, 1995), the murine rodents Maxomys hylomyoides, Maxomys inflatus, Mus crociduroides, Niviventer fraternus, Rattus blangorum, Rattus korinchi and Rattus hoogerwerfi (see Musser & Carleton, 2005), the flying squirrel Hylopetes winstoni, and the rabbit Nesolagus netscheri (see Corbet & Hill, 1992). The muntjac Muntiacus montanusRobinson & Kloss, 1918, recorded from 1432–2225 m on Gunung Kerinci and at 2835 m on Gunung Leuser (Robinson & Kloss, 1918; Miller, 1942), is currently regarded as a synonym of the widespread species Muntiacus muntjak (see Grubb, 2005), but may possibly be an additional, overlooked Sumatran montane endemic, and deserves further investigation. Likewise, treatment of Sundasciurus altitudinusRobinson & Kloss, 1916 as a distinctive Sumatran montane squirrel endemic distinct from and sympatric with Sundasciurus tenuis by Miller (1942), which echoed the field- and museum-based findings of Robinson & Kloss (1918, 1919), deserves renewed attention, despite comments by Corbet & Hill (1992: 296). Several additional mammal species occur in Sumatra only in montane forests, but also extend to mountain block habitats elsewhere on the Sunda Shelf. These include the serow Capricornis sumatraensis, which extends to the mountains of the Malay Peninsula (Grubb, 2005) and is rumoured to occur in montane Borneo; the large murine Sundamys infraluteus, thought to extend to montane Borneo (Musser & Newcomb, 1983); the arboreal murine Leopoldamys ciliatus, found also in the mountains of the Malay Peninsula (Miller, 1942; Musser & Carleton, 2005); the weasel Mustela lutreolina, the shrew Crocidura paradoxura and the ground squirrel Lariscus niobe, all thought to extend to the mountains of Java (van Bree & Boeadi, 1978; Corbet & Hill, 1992; Ruedi, 1995); and the small pteropodid Aethalops alecto, thought to extend to the mountains of the Malay Peninsula, Java, Bali and Lombok (Kitchener et al., 1993). In all of these cases, further clarifying comparisons are needed to establish authoritatively the taxonomic equivalence of montane Sumatran populations with those from other distant mountain blocks on the Sunda Shelf. Regardless, on current evidence Arctonyx hoevenii can be recognized as the only carnivoran endemic to Sumatra.
The presence of distinct species of Arctonyx in the hill forests of Indochina and in the mountains of Sumatra, but not in mainland Malaysia, is an interesting distributional pattern, not commonly seen in mammals. We note that a similar pattern characterizes the leporid genus Nesolagus (the striped rabbits), which comprises only two species -N. netscheri of montane Sumatra, and N. timminsi, known only from hill and montane habitats in the Annamite Range in the Vietnam/Laos border area (Surridge et al., 1999; Can et al., 2001). Another analogous example is to be found in the murine subgenus Coelomys (genus Mus), which although absent in the Malay Peninsula, is represented by an Indochinese taxon, M. pahari, occurring in hill and montane habitats in eastern India, southern China and Indochina, as well as a montane Sumatran representative (M. crociduroides) (other species of Coelomys are isolated in montane Sri Lanka and Java; Musser & Carleton, 2005).
Representatives of Nesolagus, Arctonyx and Coelomys must have crossed the Malay Peninsula to colonize Sumatra from Indochina (or, much less likely, vice versa). During cooler and drier episodes in the late Tertiary, such habitats extended to lower elevations than they do today, would have thus encompassed more expansive areas (Heaney, 1991; Janis, 1993; Brandon-Jones, 1996; Morley, 1998; Meijaard, 2003 and references therein), and may have ultimately facilitated the dispersal of temperate-adapted mammal lineages, such as badgers, to lower latitudes in Southeast Asia. Because these taxa are centred on hill and montane habitats in Southeast Asia, we envisage their former occurrence in similar habitats on the Malay Peninsula. Upland blocks in Malaya today are trivial in size compared with areas of relief in Indochina and Sumatra. During hotter and wetter climatic episodes in the Plio-Pleistocene, we assume that the small mountain areas of Peninsular Malaysia were insufficiently expansive to accommodate refugial survival of many montane faunistic elements that survived elsewhere (including Nesolagus, Arctonyx, Coelomys and Melogale–see below), producing the current pattern of distributional disjunctness between taxa thereby isolated in the larger upland areas of Indochina and the Sunda Shelf. The small land area of Malaya's upland forest habitats probably afforded these areas sufficient isolation (and perhaps insufficient area for vegetational differentiaton along elevational gradients) such that later recolonization by certain montane-associated taxa was prevented even in cooler periods. Molecular divergence estimates apparently place the split between the two species of Nesolagus and between the Indochinese and Sumatran representatives of Coelomys within the Pliocene (Surridge et al., 1999; Chevret, Veyrunes & Britton-Davidian, 2005), and we suspect that the divergence between A. hoevenii and A. collaris is of similar antiquity.
As in Sumatra, upland-restricted badgers also occur on the other very large islands of the Sunda Shelf, Borneo and Java. However, in contrast to Sumatra, where the endemic badger is an Arctonyx, on Borneo and Java (and adjacent Bali) the endemic upland badgers are species of the ferret-badger genus Melogale (M. everetti in Borneo and M. orientalis in Java and Bali). The distribution of Melogale extends from eastern and southern China to the Isthmus of Kra, absent in Malaya, with isolated representatives in montane Borneo and Java. Like Arctonyx, Melogale must have been present at some point in the past in Malaya and Sumatra. We suggest that in drier episodes during the late Tertiary, montane habitats extended to sufficiently low elevations to incorporate areas large enough to allow for the coexistence of both Arctonyx and Melogale, two temperate-adapted badger lineages. When montane forests retracted, upland areas in insular Southeast Asia were apparently not large enough to accommodate two badgers in sympatry, such that only one badger persisted in isolation in each of Sumatra, Java and Borneo, and no badgers persisted to modern times in the mountains of Malaya. Even in Pleistocene episodes of lower sea levels, when the Sunda Shelf was united with mainland Asia via an expansive land-bridge, appropriate habitats apparently remained sufficiently fragmented by non-forested habitats to prevent the Sunda Shelf's badgers from overcoming their long-term isolation by colonizing or recolonizing other Sundaic mountain blocks.
PRACTICAL CONSIDERATIONS
Our hypothesis that living hog-badgers represent three distinctive species (instead of a single morphologically variable species, A. collaris, both widely and disjunctly distributed) illuminates a number of pragmatic considerations for mammalogists and other biologists. We suggest that these implications are most important to workers in epidemiology, zoo biology, and conservation biology and management.
First, we note that distinguishing between Arctonyx species may be of considerable interest and assistance to the body of researchers studying species of Arctonyx as vectors of infectious diseases (e.g. Guan et al., 2003; Bell, Roberton & Hunter, 2004) and medically important parasites (e.g. Hoogstraal, Trapido & Kohls, 1965a, b; Hoogstraal, Dhanda & Bhat, 1970; Hoogstraal, Dhanda & El Kammah, 1971; Hoogstraal, 1971; Sprent, 1972; Daengsvang, Tingpalopong & Lichtenfels, 1975) in East and Southeast Asia.
Second, hog-badgers are and have been regularly exhibited in zoological gardens around the world, for more than a century (Sclater, 1883), and it is clear from published accounts (Parker, 1979; Jones, 1982; Long & Killingley, 1983; Hancox, 1993; Rakamantha, 1994; von Schmalz-Peixoto, 2003) and from former zoo specimens deposited in museums (ROM, USNM) that A. collaris, A. albogularis and A. hoevenii have all been exhibited in zoos at various times and places. According to Hancox (1993), zoos in six major cities had breeding programmes in place for hog-badgers in the early 1990s (Toronto, London, Milwaukee, Duisberg, Beijing and Yangon) and internet searching in May 2007 brought up many additional zoos that house Arctonyx, especially in Asia. Distinguishing between Arctonyx species in zoos will be important for educational as well as practical reasons. For example, different species should undoubtedly be housed in separate enclosures, and not bred together in captivity (i.e. if breeding between Arctonyx species is possible). Some consideration might also be made of their differential habitat associations in the wild (temperate climates for A. albogularis, mid-elevation subtropics and tropics for A. collaris, montane tropics for A. hoevenii) and possible differences in diet (perhaps more omnivorous in A. collaris and A. albogularis, and more vermivorous in A. hoevenii) in accommodating their husbandry and care.
Finally, recognition of three distinctive species of Arctonyx, occurring in different regions and different habitats, means that the status of each species must ideally be considered individually with regard to conservation concerns and wildlife management practices (Mace, 2004). Because hog-badgers are hunted, traded and sold in markets throughout eastern and Southeast Asia and because Arctonyx fur is traded internationally to make paint brushes (Domingo-Roura et al., 2006), the status of the various species is of international interest. ‘Arctonyx collaris’ (incorporating all Arctonyx taxa) is currently classified as a species of ‘Least Concern/Lower Risk’ under the Red List rankings of the IUCN (World Conservation Union), primarily because it is relatively widespread in Asia and known to be common in some regions. This ranking remains most appropriate for the northern hog-badger, i.e. Arctonyx albogularis, which clearly remains widespread and common throughout much of eastern and southern China, occurs in a great variety of habitats (including remote montane areas), and persists in agricultural landscapes and village environs (Zheng et al., 1988; Zhang, 1997; Wang & Fuller, 2003).
The conservation status of both southern species of Arctonyx, however, requires more careful reflection. Compared with A. albogularis, A. collaris is a considerably larger animal, has a smaller global geographical distribution and may be less tolerant in its habitat requirements. Its distribution throughout Southeast Asia appears to be patchy and centred on little-disturbed upland forests situated between 500 and 1400 m throughout the region, as documented above (Fig. 11). It is common in some areas, and present in a number of protected areas, but very little detailed information is available from most areas of occurrence in the region. Unsustainable hunting, indiscriminate snaring and habitat fragmentation from extensive deforestation (including deforestation in hill and lower montane forests; Pattanavibool & Dearden, 2002) are major threats to most of the larger mammals of mainland Southeast Asia (Wikramanayake et al., 1998; Duckworth et al., 1999; Marshall et al., 2006; Steinmetz, Chutipong & Seuaturien, 2006; Corlett, 2007), and these are likely to place Arctonyx under threat locally in many areas. Focused study is needed to assess the conservation outlook for A. collaris across its entire distributional range.
The conservation status of Hubrecht's forgotten edentate cum badger, Arctonyx hoevenii, is probably secure at present. Records of occurrence document that its distribution extends across the entire length of Sumatra's Barisan range (Fig. 11). It is known to be common in some areas, is present in three national parks (Gunung Leuser, Kerinci Seblat and Barisan Selatan), and ranges even into the highest and most inhospitable reaches of Sumatra's mountains. It does face some threats. It is apparently an appealing food item (Schneider, 1905) and is probably traditionally hunted for local consumption in some areas, although this has no doubt long been the case, and as a result seems likely to be sustainable. Undoubtedly much more important is the threat from logging and forest clearance, which has been widespread and severe in Sumatra in recent years (Brooks et al., 1999; Jepson et al., 2001; Linkie, Smith & Leader-Williams, 2004; Miyamoto, 2006). However, unlike other hog-badgers, A. hoevenii rarely descends below 800 m elevation, and logging and forest clearance in Sumatra remain largely, though not entirely, concentrated in lowland and hill forests up to c. 1000 m (Kinnaird & O'Brien, 1998; Kinnaird et al., 2003; Linkie et al., 2004; Gaveau, Wandono & Setiabudi, 2007). If current patterns of forest loss in Sumatra continue unabated, such that logging in montane forests is accelerated, this will probably constitute a significant threat both to populations of A. hoevenii and to the many other remarkable montane-adapted animals and plants found nowhere else.
ACKNOWLEDGEMENTS
Projects such as this are impossible without access to vouchered material deposited and carefully preserved in world museum collections. For access to specimens under their care, we thank Linda Gordon (USNM), Darrin Lunde (AMNH), Judy Chupasko (MCZ), Paula Jenkins and Daphne Hills (BMNH), Geraldine Veron and Jacque Cuisin (MNHN), Ned Gilmore (ANSP), Chris Smeenk (RMNH), Bill Stanley (FMNH), Kelvin Lim (ZRC) and Jim Patton (MVZ). We thank Heok Hee Ng (ZRC) for help with the map preparations. We especially thank Don Wilson for facilitating our research at the Smithsonian Institution and Alfred Gardner and Richard Thorington for helpful discussion. We are also grateful to Leslie Overstreet, Erin Rushing and Daria Wingreen-Mason of the Smithsonian Institution libraries for their assistance in accessing rare books and for their help in scanning the image pictured in Figure 7. Finally, we thank two anonymous reviewers and the editor of our manuscript, P. J. Hayward, for their helpful comments and assistance. Our taxonomic research has been supported by grants and fellowships to K.M.H. from the Smithsonian Institution, the National Science Foundation, the South Australian Museum and the American Society of Mammalogists.
Appendices
APPENDIX 1
Recorded occurrences of Arctonyx albogularis outside China. Zhang (1997) mapped in detail the Chinese distribution of Arctonyx with known point localities (see our Fig. 11), and we refer the reader to that source (with the caveat that documented localities of occurrence for Arctonyx in southernmost China might conceivably represent A. albogularis, A. collaris or both species). Here we document localities and samples of occurrence for A. albogularis outside China, essentially all of which are imprecisely localized.
India or Bangladesh: ‘Bengal’ (also marked ‘Chittagong’; see Pocock, 1941: 430)) (BMNH 42.4.29.54)
India: ‘Hindustan’ (RMNH cat. ost. a)
India: ‘Sikkim Terai’ (BMNH types of isonyx and taraiyensis; Pocock, 1941)
India: ‘Assam’ (type of taxoides[Zoological Survey of India]: Blyth, 1853; Pocock, 1941)
India: ‘Assam’ (BMNH unnumbered [collector's number 690])
India: ‘Darjeeling’ (Zoological Survey of India; Pocock, 1941)
Nepal: Shey-Phoksundo National Park (Shrestha, 1997; specimens not examined)
APPENDIX 2
Summary of recorded occurrences of Arctonyx collaris
Cambodia: Sissophon (ZRC 4.1142)
Cambodia: Samlaut Protected Area (http://www.badgers.org.uk/badgerpages/hog-badger.html, downloaded 8 May 2007)
Cambodia: ‘Cambodia’ (Urbain & Friant, 1940; Pocock, 1941)
China: Yunnan Province, Lichiang (AMNH 43159, 43160)
India: Nagaland, Naga Hills, 3500 feet (BMNH 43.152)
India: Nagaland, Naga Hills, Longpa, 3000 feet (BMNH 21.7.6.14)
India: Meghalaya, Khasi Hills, Nongpoh (AMNH 171170)
India: Meghalaya, Jaintia Hills, Hot Springs, 2400 feet (BMNH 21.7.8.29)
India or Bangladesh: ‘Bengal’–probably ‘some locality to the east of the Ganges and Brahmaputra, possibly from Chittagong’ (BMNH, marked 206a; see Pocock, 1941)
Laos: Phongsaly (FMNH, 31779, 31780)
Laos: Thateng, Plateau des Bolovens, 3000 feet (AMNH 87394–87396; ANSP 15178–15180; FMNH 38025-38028)
Laos: Hongsa Special Zone, Huay Tjuang (Bergmans, 1995)
Thailand: Trang (AMNH 55555; USNM 83516, 83517)
Thailand: Lamra, Trang (BMNH 1910.10.1.31)
Thailand: Seechol (= Sichon; USNM 256678)
Thailand: Sai Yoke, 100 m (BMNH 15.12.1.8)
Thailand: Klong Wang Hip (BMNH; Pocock, 1941)
Thailand: Chiang Mai (USNM, 258860)
Thailand: Mt. Angka, 4000–4300 feet [1219–1311 m] (MCZ 35894, 35932)
Thailand: Huai Kha Khaeng Wildlife Sanctuary, 400–600 m (Rabinowitz, 1990; Rabinowitz & Walker, 1991)
Thailand: Phu Khieo Wildlife Sanctuary (16°5′–16°35′N, 101°20′–101°55′E), 800–1100 m (Grassman, 2003)
Thailand: Phetchaburi Province, Kaengkrachan National Park (http://www.badgers.org.uk/badgerpages/hog-badger.html, downloaded 8 May 2007)
Thailand: ‘Siam’ (AMNH 37204, 37221)
Vietnam: ‘Vietnam’ (MNHN 1877.704)
Vietnam: Khanh Hoa Province, Nha Trang (BMNH 1910.3.10.4; Thomas, 1921)
Vietnam: Ninh Thuan Province, Phu Qui (BMNH 28.7.1.44; Pocock, 1941)
Vietnam: Quang Nam Province, Tien Phuoc District, Tien Lanh Commune (15°30′7.6″N, 108°10′57.7″E), 640 m (Long & Minh, 2006)
Vietnam: Tonkin, Bakthai Province, 10 km south of Cho Don village (22°05′N, 105°40′E), 600 m ‘bamboo forest’ (Rozhnov, 1994a)
Vietnam: Vinh Phu Province, Vinh Yen District, Tam Dao, 700–1000 m (MVZ 186562-186564)
Vietnam: Djiring Plateau (ANSP 17129)
Vietnam: Cuc Phuong Forest (S. Rosenthal, in litt.)
Unknown localities in Indochina (MNHN 1938.603, 1961.154, 1961.186)
Myanmar: Kachin, 15 miles NE of Myitkyina (USNM 277852)
Myanmar: Loikaw, Karenni, 2500 feet (BMNH 94.5.10.1; Pocock, 1941)
Myanmar: Falam, Chin Hills, 5000 feet (BMNH number unclear; Pocock, 1941)
Myanmar: Mogok, Ruby Mines (BMNH number unclear; Pocock, 1941)
Myanmar: 30 miles NW of Kindat, 600 feet (BMNH 21.10.1.1; Pocock, 1941)
Myanmar: Thandaung, Mt. Toungoo, 4500 feet (BMNH, 1938.10.10.12; Pocock, 1941)
Myanmar: Moulmein (Pocock, 1940, 1941)
APPENDIX 3
Summary of recorded occurrences of Arctonyx hoevenii in the provinces of Sumatra
Aceh Province:
Gunung Leuser, Bivouac No. 5 (see Miller, 1942), 8000 ft [2438 m] (ANSP 20230)
Gunung Leuser, Bivouac No. 7 (see Miller, 1942), 9300 ft [2835 m] (Miller, 1942)
Gunung Leuser, between Bivouacs 8 and 9, 8600–9300 feet [2621–2835 m] (see Miller, 1942; de Schauensee & Ripley, 1940)
Blangkedjeren, 2900 ft [884 m] (ANSP 20231) (see Miller, 1942)
Blang Rakal, ‘±1000 m’ (RMNH 7574)
Kuta Tjane, ‘600 ft’[183 m] (ANSP 20232) (see Miller, 1942)
[Lake] Takengon, 1200 m (RMNH 5094, 5095)
Gunung Leuser National Park, upper Mamas catchment (3°20′N, 97°50′E), 1200–1600 m (van Schaik & Griffiths, 1996)
Gunung Leuser National Park, upper Bengkung catchment (3°00′N, 97°30′E), <800 m (van Schaik & Griffiths, 1996)
Gunung Leuser National Park, Ketambe Atas (1217–2657 m) (Augeri, 2005)
Gunung Leuser National Park, Gunung Putar (1402–2113 m) (Augeri, 2005)
Gunung Leuser National Park, Ketambe (433–2002 m) (Augeri, 2005)
‘Aceh’ or ‘Atjeh’: (RMNH 50992; USNM 269073, 276638)
North Sumatra Province:
Kaban Djaha (= Kabanjahe), Karo Highlands (RMNH 7575)
Gunung Sinabung (foot of the volcano) (Schneider, 1905)
‘Asahan Simeloengoen, Sumatra Ooskust’ (RMNH 7445)
West Sumatra Province:
Gunung Kerinci: Sungai Kumbang, 4500–4700 ft [1372–1433 m] (ZRC 4.1141, 4.1143; BMNH 17.8.4.6)
Gunung Kerinci, Sungai Kring, 7300 ft [2225 m] (Robinson & Kloss, 1918)
Gunung Kerinci, near the summit ‘on the edge of the crater’, 12 400 ft [=3780 m] (Robinson & Kloss, 1918)
Gunung Merapi, 2700: 2900 feet [823–884 m] and 4500 feet [1372 m] (BMNH, 1938.11.30.48–52, ZRC 4.144-1152)
Gunung Merapi, 1500 m (RMNH 7576)
Pajakombo, Padang Highlands (RMNH 7645)
‘Pad. Bovenlanden’ (= Padang Highlands) (RMNH C113)
Jambi Province:
Kerinci Seblat National Park, Gunung Tujuh, 2000–2400 m (Holden, 2006)
Kerinci Seblat National Park, Tandai, 700 m (Holden, 2006)
Bengkulu Province:
Suban Ajam, Gunung Kaba, 4000 feet [= 1219 m] (Robinson & Kloss, 1919)
‘mountainous districts [between] Palembang and Bencoolen in Sumatra’ (Hubrecht, 1891)
‘Gunung Agoeng, Pagar Alam, Benkoelen’ (RMNH 7573)
South Sumatra Province
Nina Estate, Dempo, 4000 ft [= 1219 m] (BMNH 17.8.4.6)
Gunung Dempo, north slopes, 1600 m (RMNH 7572)
Gunung Dempo, 1800–2600 m (Robinson & Kloss, 1919)
Lampung Province:
Gunung Tanggamoes, 1950 m (RMNH 7571)
REFERENCES
- Abramov AV. 2002. Variation in the baculum structure of the Palaearctic badger (Carnivora, Mustelidae, Meles). Russian Journal of Theriology 1: 57–60. [Google Scholar]
- Abramov AV, Puzachenko AY.. 2005. Sexual dimorphism of craniological characters in Eurasian badgers, Meles spp. (Carnivora, Mustelidae). Zoologischer Anzeiger 224: 11–19. [Google Scholar]
- Abramov AV, Puzachenko AY.. 2006. Geographical variability and taxonomy of Eurasian badgers (Mustelidae, Meles). Zoologichesky Zhurnal 85: 641–655. [Google Scholar]
- Albignac R. 1974. Observations éco-ethologiques sur le genre Eupleres, viverride de Madagascar. Terre Vie 28: 321–351. [Google Scholar]
- Allen GM. 1929. Mustelids from the Asiatic Expeditions. American Museum Novitates 358: 1–12. [Google Scholar]
- Allen GM. 1938. The mammals of China and Mongolia. Part I. New York: American Museum of Natural History. [Google Scholar]
- Allen GM, Coolidge HJ.. 1940. Mammal and bird collections of the Asiatic primate expedition: mammals. Bulletin of the Museum of Comparative Zoology 87: 131–166. [Google Scholar]
- Anderson J. 1879. Anatomical and zoological researches: comprising an account of the two expeditions to western Yunnan. Two volumes. London: Quaritch [Google Scholar]
- Anderson S, Jones JK Jr. 1967. Recent mammals of the world: a synopsis of families. New York: Ronald Press. [Google Scholar]
- Augeri DM. 2005. On the biogeographic ecology of the Malayan sun bear. Unpublished D. Phil. Thesis, University of Cambridge.
- Balete DS, Rickart EA, Rosell-Ambal RGB, Jansa S, Heaney LR.. 2007. Descriptions of two new species of Rhynchomys Thomas (Rodentia: Muridae: Murinae) from Luzon Island, Philippines. Journal of Mammalogy 88: 287–301. [Google Scholar]
- Bell D, Roberton S, Hunter PR.. 2004. Animal origins of SARS coronavirus: possible links with the international trade in small carnivores. Philosophical Transactions of the Royal Society of London (series B) 359: 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans W. 1995. On mammals from the People's Democratic Republic of Laos, mainly from Sekong Province and Hongsa Special Zone. Zeitschrift für Säugetierkunde 60: 286–306. [Google Scholar]
- Bininda-Emonds ORP, Gittleman JL, Purvis A.. 1999. Building large trees by combining phylogenetic information: a complete phylogeny of the extant Carnivora (Mammalia). Biological Reviews 74: 143–175. [DOI] [PubMed] [Google Scholar]
- Blanford WT. 1888. The fauna of British India, including Ceylon and Burma. Part I. London: Taylor and Francis. [Google Scholar]
- Blyth E. 1853. Report of zoological curator for September meeting. Journal of the Asiatic Society of Bengal 22: 589–594. [Google Scholar]
- Blyth E. 1875. Catalogue of mammals and birds of Burma. Journal of the Asiatic Society of Bengal 44: i–xxiv, 1-167. [Google Scholar]
- Brandon-Jones D. 1996. The Asian Colobinae (Mammalia: Cercopithecidae) as indicators of Quaternary climatic change. Biological Journal of the Linnean Society 59: 327–350. [Google Scholar]
- Brooks TM, Pimm SL, Kapos V, Ravilious C.. 1999. Threat from deforestation to montane and lowland birds and mammals in insular south-east Asia. Journal of Animal Ecology 68: 1061–1078. [Google Scholar]
- Can DN, Abramov AV, Tikhonov AN, Averianov AO.. 2001. Annamite striped rabbit Nesolagus timminsi in Vietnam. Acta Theriologica 46: 437–440. [Google Scholar]
- Chevret P, Veyrunes F, Britton-Davidian J.. 2005. Molecular phylogeny of the genus Mus (Rodentia: Murinae) based on mitochondrial and nuclear data. Biological Journal of the Linnean Society 84: 417–427. [Google Scholar]
- Choudhury A. 1997a. The distribution and status of small carnivores (mustelids, viverrids, and herpestids) in Assam, India. Small Carnivore Conservation 16: 25–26. [Google Scholar]
- Choudhury A. 1997b. Small carnivores (mustelids, viverrids, herpestids, and one ailurid) in Arunachal Pradesh, India. Small Carnivore Conservation 17: 7–9. [Google Scholar]
- Choudhury A. 1999. Conservation of small carnivores (mustelids, viverrids, herpestids, and one ailurid) in north Bengal, Small Carnivore Conservation 20: 15–17. [Google Scholar]
- Choudhury A. 2000. Some small carnivore records from Nagaland, India. Small Carnivore Conservation 23: 7–9. [Google Scholar]
- Corbet GB, Hill JE.. 1992. Mammals of the Indomalayan region. Oxford: Oxford University Press. [Google Scholar]
- Corlett RT. 2007. The impact of hunting on the mammalian fauna of tropical Asian forests. Biotropica 39: 292–303. [Google Scholar]
- Curtis DJ, Rasmussen MA.. 2006. The evolution of cathemerality in primates and other mammals: a comparative and chronoecological approach. Folia Primatologica 77: 178–193. [DOI] [PubMed] [Google Scholar]
- Daengsvang S, Tingpalopong M, Lichtenfels RJ.. 1975. A record of Tetragomphius arctonycis Jansen, 1968 (Nematoda: Ancylostomatidae) from the hog-badger, Arctonyx collaris, of Trang Province, southern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 6: 287–289. [PubMed] [Google Scholar]
- Dang NX, Anh PT, Huynh DH.. 1992. The biology and status of Owston's palm civet in Vietnam. Small Carnivore Conservation 6: 5–6. [Google Scholar]
- Davis DD. 1962. Mammals of the lowland rain-forest of north Borneo. Bulletin of the Singapore National Museum 31: 1–129. [Google Scholar]
- Deuve J. 1972. Les mammifères du Laos. Vientiane, Laos: Ministère de l'Education Nationale. [Google Scholar]
- Domingo-Roura X, Marmi J, Ferrando A, López-Giráldez JF, Macdonald DW, Jansman HAH.. 2006. Badger hair in shaving brushes comes from protected Eurasian badgers. Biological Conservation 128: 425–430. [Google Scholar]
- Dragoo JW, Honeycutt RL.. 1997. Systematics of mustelid-like carnivores. Journal of Mammalogy 78: 426–443. [Google Scholar]
- Duckworth JW. 1997. Small carnivores in Laos: a status review with notes on ecology, behaviour, and conservation. Small Carnivore Conservation 16: 1–21. [Google Scholar]
- Duckworth JW. 1998. A survey of large mammals in the central Annamite mountains of Laos. Zeitschrift für Säugetierkunde 63: 239–250. [Google Scholar]
- Duckworth JW, Salter RE, Khounboline K.. 1999. Wildlife in Lao PDR: 1999 status report. Vientiane, Lao PDR: IUCN (World Conservation Union). [Google Scholar]
- Ellerman JR, Morrison-Scott TCS.. 1951. Checklist of palaearctic and Indian mammals, 1758 to 1946. London: Trustees of the British Museum (Natural History). [Google Scholar]
- Ellerman JR, Morrison-Scott TCS.. 1966. Checklist of palaearctic and Indian mammals, 1758 to 1946, 2nd edn. London: Trustees of the British Museum (Natural History). [Google Scholar]
- Ewer RF. 1973. The carnivores. London: Weidenfeld and Nicolson. [Google Scholar]
- Flannery TF. 1995. Mammals of New Guinea, Revised edition. Sydney: Reed Books. [Google Scholar]
- Friscia AR, Van Valkenburgh B, Biknevicius AR.. 2007. An ecomorphological analysis of extant small carnivorans. Journal of Zoology (London) 272: 82–100. [Google Scholar]
- Gao X, Sun S.. 2005. Effects of the small forest carnivores on the recruitment and survival of Liaodong oak (Quercus wutaishanica) seedlings. Forest Ecology and Management 206: 283–292. [Google Scholar]
- Gaveau DLA, Wandono H, Setiabudi F.. 2007. Three decades of deforestation in southwest Sumatra: have protected areas halted forest loss and logging, and promoted re-growth? Biological Conservation 134: 495–504. [Google Scholar]
- Geoffroy E, Cuvier G.. 1819–1842. Histoire naturelle des mammiferes. Paris. [Google Scholar]
- Gloger CWL. 1841. Gemeinnutziges Hand und Hilfsbuch für Naturgänger. Breslau: Berlag von Wug Schulz & Company. [Google Scholar]
- Grassman LI., Jr 2003. Thailand cat project: final report. Kingsville: Caesar Kleberg Wildlife Research Institute, Texas A&M University-Kingsville: Feline Research Program. [Google Scholar]
- Gray JE. 1863. Catalogue of the specimens of mammals and birds of Nepal and Thibet presented by B. H. Hodgson, Esq., to the British Museum, 2nd edn. London: British Museum. [Google Scholar]
- Gray JE. 1865. Revision of the genera and species of Mustelidae contained in the British Museum. Proceedings of the Zoological Society of London 1865: 100–154. [Google Scholar]
- Gregory WK, Hellman G.. 1939. On the evolution and major classification of the civets (Viverridae) and allied fossil and Recent Carnivora: a phylogenetic study of the skull and dentition. Proceedings of the American Philosophical Society 81: 309–392. [Google Scholar]
- Griffiths M. 1978. The biology of the monotremes. New York: Academic Press. [Google Scholar]
- Grubb P. 2005. Order Artiodactyla. In: Wilson DE, Reeder DR, eds. Mammal species of the world: a taxonomic and geographic reference, 2nd edn. Baltimore, MD: Johns Hopkins University Press, 637–722. [Google Scholar]
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, Butt KM, Wong KL, Chan KW, Lim W, Shortridge KF, Yuen KY, Peiris SM, Poon LLM.. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302: 276–278. [DOI] [PubMed] [Google Scholar]
- Hancox M. 1993. Delayed implantation in badgers and mustelids: a review. Small Carnivore Conservation 8: 14–15. [Google Scholar]
- Heaney LR. 1991. A synopsis of climatic and vegetational change in Southeast Asia. Climatic Change 19: 53–61. [Google Scholar]
- Hill RA. 2006. Why be diurnal? Or, why not be cathemeral? Folia Primatologica 77: 72–86. [DOI] [PubMed] [Google Scholar]
- Holden J. 2006. Small carnivores in central Sumatra. Small Carnivore Conservation 34/35: 35–38. [Google Scholar]
- Hoogstraal H. 1971. Identity, hosts, and distribution of Haemaphysalis (Rhipistoma) canestrinii (Supino) (resurrected), the postulated Asian progenitor of the African leachi complex (Ixodoidea: Ixodidae). Journal of Parasitology 57: 161–172. [Google Scholar]
- Hoogstraal H, Dhanda V, Bhat HR.. 1970. Haemaphysalis (Kaiseriana) davisi sp. n. (Ixodoidea: Ixodidae), a parasite of domestic and wild mammals in northeastern India, Sikkim, and Burma. Journal of Parasitology 56: 588–595. [PubMed] [Google Scholar]
- Hoogstraal H, Dhanda V, El Kammah KM.. 1971. Aborphysalis, a new subgenus of Asian Haemaphysalis ticks; and identity, distribution, and hosts of H. aborensis Warburton (resurrected) (Ixodoidea: Ixodidae). Journal of Parasitology 57: 748–760. [PubMed] [Google Scholar]
- Hoogstraal H, Trapido H, Kohls GM.. 1965a. Southeast Asian Haemaphysalis ticks (Ixodoidea, Ixodidae). H. (Kaiseriana) papuana nadchatrami ssp. n. and redescription of H.(K.) semermis Neumann. Journal of Parasitology 51: 433–451. [PubMed] [Google Scholar]
- Hoogstraal H, Trapido H, Kohls GM.. 1965b. Studies on Southeast Asian Haemaphysalis ticks (Ixodoidea, Ixodidae): the identity, distribution, and hosts of H. (Kaiseriana) hystricis Supino. Journal of Parasitology 51: 467–480. [PubMed] [Google Scholar]
- Hooijer DA. 1947. Pleistocene remains of Panthera tigris (Linnaeus) subspecies from Wahnsien, Szechwan, China, compared with fossil and recent tigers from other localities. American Museum Novitates 1346: 1–17. [Google Scholar]
- Horsfield T. 1856. Catalogue of a collection of Mammalia from Nepal, Sikim, and Tibet, presented to the Hon. East India Company by B. H. Hodgson, Esq., in 1853. Proceedings of the Zoological Society of London 1856: 393–406. [Google Scholar]
- Howell AB. 1929. Mammals from China in the collections of the United States National Museum. Proceedings of the United States National Museum 75: 1–82. [Google Scholar]
- Hubrecht AAW. 1895. [Untitled description of letter to the Society. Proceedings of the Zoological Society of London 1895: 522. [Google Scholar]
- Hubrecht AAW. 1891. A new mammal from Sumatra. Notes Leyden Museum 13: 241–242. [Google Scholar]
- Jackson VA. 1918. Notes on a young hog badger Arctonyx sp. in the Garo Hills. Journal of the Bombay Natural History Society 26: 281–282. [Google Scholar]
- Janis CM. 1993. Tertiary mammal evolution in the context of changing climates, vegetation, and tectonic events. Annual Review of Ecology and Systematics 24: 467–500. [Google Scholar]
- Jepson P, Jarvie JK, MacKinnon K, Monk KA.. 2001. The end for Indonesia's lowland forests? Science 292: 859. [DOI] [PubMed] [Google Scholar]
- Jones ML. 1982. Longevity of captive mammals. Zoologische Garten 52: 113–128. [Google Scholar]
- Joshi AR, Garshelis DL, Smith JLD.. 1997. Seasonal and habitat-related diets of sloth bears in Nepal: Journal of Mammalogy 78: 584–597. [Google Scholar]
- Khajuria H, Chaturvedi Y, Ghoshal DK.. 1977. Catalogue Mammaliana. Records of the Zoological Survey of India, Miscellaneous Publication, Occasional Paper 7: 1–44, addendum. [Google Scholar]
- Kinnaird MF, O'Brien TG.. 1998. Ecological effects of wildfire on lowland rainforest in Sumatra. Conservation Biology 12: 954–956. [Google Scholar]
- Kinnaird MF, Sanderson EW, O'Brien TG, Wibisono HT, Woolmer G.. 2003. Deforestation trends in a tropical landscape and implications for endangered large mammals. Conservation Biology 17: 245–257. [Google Scholar]
- Kitchener DJ, Hisheh S, Schmitt LH, Maryanto I.. 1993. Morphological and genetic variation in Aethalops alecto (Chiroptera, Pteropodidae) from Java, Bali and Lombok Islands, Indonesia. Mammalia 57: 255–272. [Google Scholar]
- Kurose N, Kaneko Y, Abramov AV, Siriaroonrat B, Masuda R.. 2001. Low genetic diversity in Japanese populations of the Eurasian badger Meles mele s (Mustelidae, Carnivora) revealed by mitochondrial cytochrome b sequences. Zoological Science (Tokyo: ) 18: 1145–1151. [Google Scholar]
- Lekagul B, McNeely JA.. 1977. Mammals of Thailand. Bangkok: Kurusapha Ladprao Press. [Google Scholar]
- Linkie M, Smith RJ, Leader-Williams N.. 2004. Mapping and predicting deforestation patterns in the lowlands of Sumatra. Biodiversity and Conservation 13: 1809–1818. [Google Scholar]
- Long CA, Killingley CA.. 1983. The badgers of the world. Springfield, IL: Charles C. Thomas. [Google Scholar]
- Long N, Minh H.. 2006. Recent records and notes on the conservation of small carnivores in Quang Nam province, central Vietnam. Small Carnivore Conservation 34/35: 39–46. [Google Scholar]
- Long VT, De Vos J, Ciochon RL.. 1994. A fossil mammal fauna of Vietnam (Lang Trang Caves) compared with fossil and recent mammal faunas of South-East Asia; their geographical implications. Presented at the 15th IPPA Congress, Chiang Mai, Thailand, January 5–12, 1994. [Google Scholar]
- Lönnberg E. 1923. Notes on Arctonyx. Annals and Magazine of Natural History (series 9) 11: 322–326. [Google Scholar]
- Mace G. 2004. The role of taxonomy in species conservation. Philosophical Transactions of the Royal Society of London (Series B) 359: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmi J, López-Giráldez JF, Dominga-Roura X.. 2004. Phylogeny, evolutionary history, and taxonomy of the Mustelidae based on sequences of the cytochrome b gene and a complex flanking region. Zoologica Scripta 33: 481–499. [Google Scholar]
- Marmi J, López-Giráldez JF, MacDonald DW, Calafell F, Zholnerovskaya E, Dominga-Roura X.. 2006. Mitochondrial DNA reveals a strong phylogeographic structure in the b adger across Eurasia. Molecular Ecology 15: 1007–1020. [DOI] [PubMed] [Google Scholar]
- Marshall AJ, Nardiyono, Engstrom LM, Pamungkas B, Palapa J, Meijaard E, Stanley SA.. 2006. The blowgun is mightier than the chainsaw in determining population density of Bornean orangutans (Pongo pygmaeus morio) in the forests of East Kalimantan. Biological Conservation 129: 566–578. [Google Scholar]
- Matthew MD, Granger W.. 1923. New fossil mammals from the Pliocene of Sze-chuan, China. Bulletin of the American Museum of Natural History 48: 563–598. [Google Scholar]
- McKenna MC, Bell SK.. 1997. Classification of mammals above the species level. New York: Columbia University Press. [Google Scholar]
- Medway L. 1978. The wild mammals of Malaya (Peninsular Malaysia) and Singapore. Kuala Lumpur: Oxford University Press. [Google Scholar]
- Meijaard E. 2003. Solving mammalian riddles: reconstruction of the Tertiary and Quaternary distribution of mammals and their palaeoenvironments in island South-East Asia. Ph.D. thesis, Department of Anthropology and Archaeology, Australian National University, Canberra, Australia.
- Meiri S, Dayan T.. 2003. On the validity of Bergmann's rule. Journal of Biogeography 30: 331–351. [Google Scholar]
- Meiri S, Dayan T, Simberloff D.. 2004. Carnivores, biases and Bergmann's rule. Biological Journal of the Linnean Society 81: 579–588. [Google Scholar]
- Miller GS., Jr 1942. Zoological results of the George Vanderbilt Sumatran Expedition, 1936–1939. Part V. Mammals collected by Frederick A. Ulmer, Jr. on Sumatra and Nias. Proceedings of the Academy of Natural Sciences of Philadelphia 94: 107–165, pls. 3–6. [Google Scholar]
- Milne-Edwards A. 1867. Observations sur quelques mammifères du nord de la Chine. Annales des Sciences Naturelles (comprenant la zoologie) (series 5) 7: 375–377; 8: 374–376. [Google Scholar]
- Milne-Edwards A. 1871. Etude pour servir à l'histoire de la fauna mammalogique de la Chine. In: Milne-Edwards A, ed. Recherches pour servir à l'histoire des mammifères. Paris: Masson, 67–229. [Google Scholar]
- Miyamoto M. 2006. Forest conversion to rubber around Sumatran villages in Indonesia: comparing the impacts of road construction, transmigration projects and population. Forest Policy and Economics 9: 1–12. [Google Scholar]
- Morley RJ. 1998. Palynological evidence for Tertiary plant dispersals in the Southeast Asian region in relation to plate tectonics and climates. In: Hall R, Holloway JD, eds. Biogeography and geological evolution of Southeast Asia. Leiden: Backhuys Publishing, 211–234. [Google Scholar]
- Musser GG. 1982. Results of the Archbold Expeditions. No. 110. Crunomys and the small-bodied shrew rats native to the Philippine Islands and Sulawesi (Celebes). Bulletin of the American Museum of Natural History 174: 1–95. [Google Scholar]
- Musser GG, Carleton MD.. 2005. Superfamily Muroidea. In: Wilson DE, Reeder DR, eds. Mammal species of the world: a taxonomic and geographic reference, 2nd edn. Baltimore, MD: Johns Hopkins University Press, 894–1531. [Google Scholar]
- Musser GG, Newcomb C.. 1983. Malaysian murids and the giant rat of Sumatra. Bulletin of the American Museum of Natural History 174: 327–598. [Google Scholar]
- Opiang M. 2004. A study of home range, movement and den use in long-beaked echidnas, Zaglossus bartoni, in the Crater Mountain Wildlife Management Area, Papua New Guinea. Unpublished Honours thesis, University of Papua New Guinea.
- Osgood WH. 1932. Mammals of the Kelley-Roosevelts and Delacour Asiatic expeditions. Field Museum of Natural History Publications Zoological Series 18: 193–339. [Google Scholar]
- Palmer TS. 1904. Index generum mammalium: a list of the genera and families of mammals. North American Fauna 23: 1–984. [Google Scholar]
- Parker C. 1979. Birth, care, and development of Chinese hog badgers Arctonyx collaris albogularis at Metro Toronto Zoo. International Zoo Yearbook 19: 182–185. [Google Scholar]
- Pattanavibool A, Dearden P.. 2002. Fragmentation and wildlife in montane evergreen forests, northern Thailand. Biological Conservation 107: 155–164. [Google Scholar]
- Payne J, Francis CM.. 1985. A field guide to the mammals of Borneo. Kota Kinabalu: The Sabah Society. [Google Scholar]
- Pei W. 1987. Carnivora, Proboscidea and Rodentia from Liucheng Gigantopithecus cave and other caves in Guangxi. Memoirs of the Institute of Vertebrate Palaeontology and Palaeoanthropology, Academia Sinica 18: 1–134. [Google Scholar]
- Pei WC. 1940. Note on a collection of mammal fossils from Tanyang in Kiangsu Province. Bulletin of the Geological Society of China (Peking: ) 19: 379–392. [Google Scholar]
- Petter G. 1971. Origine, phylogénie et systématique des blaireaux. Mammalia 35: 567–597. [Google Scholar]
- Pocock RI. 1940. The hog-badgers (Arctonyx) of British India. Journal of the Bombay Natural History Society 41: 461–469. [Google Scholar]
- Pocock RI. 1941. The fauna of British India, including Ceylon and. Burma. Mammalia. Vol. II: Carnivora [suborders Aeluroida (part) and Arctoida]. London: Taylor and Francis. [Google Scholar]
- Popowics TE. 2003. Postcanine dental form in the Mustelidae and Viverridae (Carnivora: Mammalia). Journal of Morphology 256: 322–341. [DOI] [PubMed] [Google Scholar]
- Rabinowitz A. 1990. Notes on the behavior and movements of Leopard Cats, Felis bengalensis, in a dry tropical forest mosaic in Thailand. Biotropica 22: 397–403. [Google Scholar]
- Rabinowitz A, Walker SR.. 1991. The carnivore community in a dry tropical forest mosaic in Huai Kha Khaeng Wildlife Sanctuary, Thailand. Journal of Tropical Ecology 7: 37–47. [Google Scholar]
- Radinsky L. 1973. Are stink badgers skunks? Implications of neuroanatomy for mustelid phylogeny. Journal of Mammalogy 54: 585–593. [Google Scholar]
- Rakamantha V. 1994. Natural distribution and ecology of mustelids and viverrids in Manipur, north-eastern India. Small Carnivore Conservation 11: 16–17. [Google Scholar]
- Rao M, Myint T, Zaw T, Htun S.. 2005. Hunting patterns in tropical forests adjoining the Hkakaborazi National Park, north Myanmar. Oryx 39: 292–300. [Google Scholar]
- Richardson PRK. 1987a. Aardwolf: the most specialized myrmecophagous mammal? South African Journal of Science 83: 643–646. [Google Scholar]
- Richardson PRK. 1987b. Food consumption and seasonal variation in the diet of the aardwolf Proteles cristatus in Southern Africa. Zeitschrift für Säugetierkunde 52: 307–325. [Google Scholar]
- Rickart EA, Heaney LR, Utzurrum RB.. 1991. Distribution and ecology of small mammals along an elevational transect in southeastern Luzon, Philippines. Journal of Mammalogy 72: 458–469. [Google Scholar]
- Robinson HC, Kloss CB.. 1918. Results of an Expedition to Korinchi Peak, Sumatra. I. Mammals. Journal of the Federated Malay States Museum 8: 1–80. [Google Scholar]
- Robinson HC, Kloss CB. 1916. Preliminary diagnoses of some new species and subspecies of mammals and birds obtained in Korinchi, West Sumatra, Feb.–June 1914. Journal of the Straits Branch of the Royal Asiatic Society 73: 269–278. [Google Scholar]
- Robinson HC, Kloss CB.. 1919. On a collection of mammals from the Bencoolen and Palembang Residencies, south west Sumatra. Journal of the Federated Malay States Museum 7: 257–291. [Google Scholar]
- Rozhnov VV. 1994a. Milk teeth of Melogale personata and Arctonyx collaris and some notes on the evolution of the badgers. Small Carnivore Conservation 10: 14. [Google Scholar]
- Rozhnov VV. 1994b. Materials on the biology of the badgers of Vietnam. Zoologichesky Zhurnal 73: 227–232. [Google Scholar]
- Ruedi M. 1995. Taxonomic revision of shrews of the genus Crocidura from the Sunda Shelf and Sulawesi with description of two new species (Mammalia: Soricidae). Zoological Journal of the Linnean Society 115: 211–265. [Google Scholar]
- Ruedi M, Chapuisat M, Iskandar D.. 1994. Taxonomic status of Hylomys parvus and Hylomys suillus (Insectivora: Erinaceidae): biochemical and morphological analyses. Journal of Mammalogy 75: 965–978. [Google Scholar]
- Sacco T, Van Valkenburgh B.. 2004. Ecomorphological indicators of feeding behaviour in the bears (Carnivora: Ursidae). Journal of Zoology (London) 263: 41–54. [Google Scholar]
- Sato JJ, Hosoda T, Wolsan M, Suzuki H.. 2004. Molecular phylogeny of artoids (Mammalia: Carnivora) with emphasis on phylogenetic and taxonomic positions of ferret-badgers and skunks. Zoological Science (Tokyo) 21: 111–118. [DOI] [PubMed] [Google Scholar]
- De Schauensee RM, Ripley SD.. 1940. Zoological results of the George Vanderbilt sumatran expedition, 1936–1939. Part I. - birds from Atjeh. Proceedings of the Academy of Natural Sciences of Philadelphia 91: 311–368, pls. 14-22. [Google Scholar]
- Von Schmalz-Peixoto KE. 2003. Factors affecting breeding in captive Carnivora. Unpublished D. Phil. Thesis, Oxford University.
- Schneider G. 1905. Ergebnisse zoologischer Forschungsreisen in Sumatra. Erster Teil. Säugetiere (Mammalia). Zoologische Jahrbücher, Abteilung für Systematik, Geographie und Biologie der Tiere 23: 1–172. [Google Scholar]
- Sclater PL. 1883. List of the vertebrated animals now or lately living in the gardens of the Zoological Society of London. London: Zoological Society of London. [Google Scholar]
- Sclater PL. 1891. Catalogue of Mammalia in the Indian Museum, Calcutta. Part II. Rodentia, Ungulata, Proboscidea, Hyracoidea, Carnivora, Cetacea, Sirenia, Marsupialia, Monotremata. Calcutta: Indian Museum. [Google Scholar]
- Shrestha TK. 1997. Mammals of Nepal, with reference to those of India, Bangladesh, Bhutan, and Pakistan. Kathmandu, Nepal: R. K. Printers. [Google Scholar]
- Sowerby AdeC. 1914. Fur and feather in North China. Tientsin, China: Tientsin Press. [Google Scholar]
- Sprent JF. 1972. Toxocara vajrasrthirae sp. nov. from the hog-badger (Arctonyx collaris) of Thailand. Parasitology 65: 491–498. [DOI] [PubMed] [Google Scholar]
- Stattersfield AJ, Crosby MJ, Long AJ, Wege DC. 1998. Endemic bird areas of the world: priorities for biodiversity conservation. Cambridge: BirdLife International. [Google Scholar]
- Steinmetz R, Chutipong W, Seuaturien N.. 2006. Collaborating to conserve large mammals in Southeast Asia. Conservation Biology 20: 1391–1401. [DOI] [PubMed] [Google Scholar]
- Surridge AK, Timmins RJ, Hewitt GM, Bell DJ.. 1999. Striped rabbits in Southeast Asia. Nature 400: 726. [Google Scholar]
- Tate GHH. 1947. Mammals of Eastern Asia. New York: Macmillan [Google Scholar]
- Tattersall I. 2006. The concept of cathemerality: history and definition. Folia Primatologica 77: 7–14. [DOI] [PubMed] [Google Scholar]
- Tedford RH, Harington CR.. 2003. An Arctic mammal fauna from the Early Pliocene of North America. Nature 425: 388–390. [DOI] [PubMed] [Google Scholar]
- Thomas O. 1911. The Duke of Bedford's Zoological Exploration of Eastern Asia. XIV. On mammals from southern Shen-si, Central China. Proceedings of the Zoological Society of London 1911: 687–695. [Google Scholar]
- Thomas O. 1910. Two new mammals from the Malay Peninsula. Annals and Magazine of Natural History (series 8) 5: 424–426. [Google Scholar]
- Thomas O. 1921. The Arctonyx of Annam. Annals and Magazine of Natural History (series 9) 7: 524. [Google Scholar]
- Thomas O. 1922. On mammals from the Yunnan Highlands collected by Mr. George Forrest and presented to the British Museum by Col. Stephenson R. Clarke. D.S.O. Annals and Magazine of Natural History (series 9) 10: 391–406. [Google Scholar]
- Van Tien D. 1966. Sur une deuxieme collection des mammiferes de la region de Yen-Bai (Nord-Vietnam). Abhandlungen und Berichte aus dem Staatlichen Museum für Tierkunde in Dresden 28: 285–292. [Google Scholar]
- Urbain A, Friant M.. 1940. Recherches sur l'Arctonyx dictator Thomas. Archives du Muséum national d'histoire naturelle Paris (6) 16: 91–107. [Google Scholar]
- Van Bree PHJ, Boeadi MSC.. 1978. Notes on the Indonesian Mountain Weasel Mustela lutreolina Robinson & Thomas, 1917. Zeitschrift für Säugetierkunde 43: 166–171. [Google Scholar]
- Van Peenen PFD. 1969. Preliminary identification manual for mammals of South Vietnam. Washington, DC: United States National Museum, Smithsonian Institution. [Google Scholar]
- Van Schaik CP, Griffiths M.. 1996. Activity periods of Indonesian rain forest mammals. Biotropica 128: 105–112. [Google Scholar]
- Von Ketelhodt HF. 1966. Proteles. Zeitschrift für Säugetierkunde 31: 300–306. [Google Scholar]
- Wang H, Fuller TK.. 2003. Food habits of four sympatric carnivores in southeastern China. Mammalia 67: 513–519. [Google Scholar]
- Wikramanayake ED, Dinerstein E, Loucks CJ, Olson D, Morrison J, Lamoreux J, McKnight M, Hedao P.. 2002. Terrestrial eco-regions of the Indo-Pacific. Washington, DC: Island Press [Google Scholar]
- Wikramanayake ED, Dinerstein E, Robinson JG, Karanth U, Rabinowitz A, Olson D, Mathew T, Hedao P, Conner M, Hemley G, Bolze D.. 1998. An ecology-based method for defining priorities for large mammal conservation: the tiger as case study. Conservation Biology 12: 865–878. [Google Scholar]
- Wozencraft WC. 2005. Order Carnivora. In: Wilson DE, Reeder DR, eds. Mammal species of the world: a taxonomic and geographic reference, 2nd edn. Baltimore, MD: Johns Hopkins University Press, 532–628. [Google Scholar]
- Zhang Y. 1997. Distribution of mammalian species in China. Beijing: China Forestry Publishing House. [Google Scholar]
- Zheng S, Li G, Song S, Han Y.. 1988. Study on the ecology of sand badger. Acta Theriologica Sinica 3: 65–72. [Google Scholar]


