Abstract
The chicken MHC has been associated with disease resistance, though the mechanisms are not understood. The functions of macrophages, critical to both innate and acquired immunity, were compared between the more infectious bronchitis virus-resistant B2 and the more infectious bronchitis virus-susceptible B19 lines. In vivo peripheral blood concentrations of monocytes were similar in B2 or B19 homozygous haplotypes. Peripheral blood-derived macrophages were stimulated with poly I:C, simulating an RNA virus, or IFNγ, a cytokine at the interface of innate and adaptive immunity. Not only did B2-derived peripheral monocytes differentiate into macrophages more readily than the B19 monocytes, but as determined by NO production, macrophages from B2 and B2 on B19 genetic background chicks were also significantly more responsive to either stimulant. In conclusion, the correlation with resistance to illness following viral infection may be directly linked to a more vigorous innate immune response.
Key words: macrophage, monocyte, innate immunity, disease resistance, infectious bronchitis virus
INTRODUCTION
Selective breeding of poultry has resulted in improved productivity in broilers at the cost of decreased immune competence and greater susceptibility to disease (Han and Smyth, 1972; Nestor et al., 1996; Bayyari et al., 1997). On the other hand, some lines of birds have been shown to be more resistant to a variety of pathogens (Bearse et al., 1939; Hutt and Scholes, 1941). Several studies have demonstrated an association between the chicken MHC-B haplotype and resistance to various pathogens including Marek's disease virus, avian leukosis virus, Newcastle disease virus, Rous sarcoma virus, and Salmonella (Heinzelmann et al., 1981; Briles et al., 1982; Lamont, 1989; Dunnington et al., 1992; Yoo and Sheldon, 1992; Mays et al., 2005; Kim et al., 2008; Banat et al., 2013). Described in 1950 (Briles et al., 1950), at least 51 B haplotypes of the chicken B complex have been identified using traditional serological techniques (Briles and Briles, 1987; Landesman et al., 1993; Sung et al., 1993; Miller et al., 2004) or gene sequencing, RFLP, single-strand conformational polymorphism, and SNP (Juul-Madsen et al., 1993; Fulton et al., 2006; Juul-Madsen et al., 2006).
Retrospective studies have correlated susceptibility and resistance to avian infectious bronchitis virus (IBV)-induced clinical illness, with the B21 and B15 haplotype lines, respectively (Bacon et al., 2004). More recently, infectivity studies identified B2, B5, and B8 chicken lines as being more resistant to IBV-induced illness than their B12 and B19 counterparts (Banat et al., 2013). Given that the differences identified regarding disease resistance were observed early after infection, we propose that innate immunity plays a major role in B haplotype-associated disease resistance, with the macrophage being a key player.
Monocytes/macrophages not only play a vital role within the innate immune system, but they are also important in the activation of specific adaptive immune responses acting as antigen-presenting cells. As such, these cells can affect the progression of clinical disease. Innate cellular activity depends solely on the expression of recognition molecules, such as Toll-like receptors (TLR) that bind the conserved pathogen-associated molecular patterns of common infectious organisms. The significance of the innate immune function of the macrophage in promoting disease resistance or susceptibility is not well understood. Macrophages are activated or primed upon encountering a pathogen or inflammatory cytokines, such as interferon (IFN) γ and subsequently undergo functional maturation, increasing expression of Fc-receptors on their cell surface (Dietert et al., 1991; Qureshi, 2003). Macrophages of various chicken lines congenic for MHC have been shown to differ in their chemotactic activity (Qureshi et al., 1988) and recruitment and activation (Qureshi et al., 1986). Like mammalian macrophages, avian macrophages produce nitric oxide (NO) upon activation, killing bacteria or protozoa and inhibiting viral replication (Boockvar et al., 1994; Kreil and Eibl, 1996; MacMicking et al., 1997). Expression of inducible nitric oxide synthase (iNOS) has been shown to differ among chickens of several genotypes, as a result of enhanced transcriptional activity in iNOS hyper-responder genotypes (Hussain and Qureshi, 1997, 1998).
Another indicator for the importance of macrophages in disease susceptibility is the fact that White Leghorn (Cornell K-Strain) chicken bone marrow produces more macrophage colonies than those from broiler bone marrow cells, leading to the speculation that White Leghorn chickens may be more disease resistant. It has also been observed that broiler mortality losses are double the losses of layer-type chickens through the first 6 wk of age (Liljequist et al., 1993). Taken together, these results suggest a genetic basis of macrophage activation playing an important role in the innate immune response and resistance to disease.
Using an in vitro model, we investigated functional differences in the activation of differentiated chicken macrophages from chicks with distinct haplotypes. Type II IFNγ, a potent activator of macrophages, typically secreted by natural killer cells and other cells of the immune system upon encountering various pathogens, was used to stimulate cultured macrophages including B2/B2, B19/B19, and B2/B2 bred on the B19 genetic background (B19bgB2MHC). In addition, in an effort to emulate viral infection, the TLR3 agonist and the synthetic analog of dsRNA, poly (I:C), were used to stimulate cultured macrophages. The results show differences in activation of macrophages measured by NO release among several B haplotypes.
MATERIALS AND METHODS
Experimental Birds
Bird protocols were performed under the approval of the Institutional Animal Care and Use Committee at Western University of Health Sciences, Pomona, California. Fertilized eggs (from B2/B2, B15/B15, B21/B21, B19/B19, and B2/B2 MHC on B19 background (F3, B19bgB2) parents, descended from Modified Wisconsin Line 3) were obtained from W. Elwood Briles, Northern Illinois University, and incubated and hatched under standard conditions at (38°C, 50 to 65% humidity; Banat et al., 2013) at Western University of Health Sciences. Posthatch, chicks were maintained in the incubator for 24 h, then transferred to a preheated brooder (35°C). In addition to daily health monitoring, fresh food and water were provided ad libitum. At 3 wk of age, room temperature was adjusted to and maintained at 25°C. Chicks were housed in standard poultry cages. To minimize the risk of pecking disorders, chicks were kept under restricted lighting conditions throughout the study. Experimental birds were euthanized by insufflation of isoflurane gas (Butler, Dublin, OH).
Peripheral Blood Collection and Cell Counts
Whole blood samples were collected via jugular venipuncture in EDTA tubes from age-matched chicks 5 to 12 wk old. At no time did the amount of blood harvested from each bird exceed 3% of total blood volume [8% × BW in kilograms (BWkg)]. A 2-wk window was allowed between subsequent blood samplings. Samples were sent to Antech Diagnostics (Irvine, CA) for complete blood count and differentials.
Peripheral Blood Mononuclear Cell Isolation
Peripheral blood mononuclear cells (PBMC) from individual birds were isolated using the differential centrifugation as previously described (Seo and Collisson, 1997; Dawes et al., 2008; Drechsler et al., 2009, 2013). Briefly, 3 mL of blood was mixed with an equal volume of Alsever's solution (Sigma-Aldrich, St. Louis, MO) after which 3 mL of this mixture were layered over an equal volume of Ficoll-Hypaque (density 1.077 or 1.083; Sigma-Aldrich). Samples were centrifuged for 35 min (400 × g, 23°C; brake off) for retrieval of mononuclear cells. Isolated cells were washed twice in 5 mL of PBS (400 × g, 10 min, 23°C), counted, and viability confirmed based on the exclusion of 0.1% trypan blue dye (≥90%). The PBMC were resuspended in PBS to a final concentration of 5 × 107 cells/mL.
Macrophage Cell Culture
One milliliter of PBMC suspension (5 × 107 cells/mL) was incubated (37°C/5% CO2) for 3 h in each well of a 12-well plate containing RPMI w/o Phenol Red supplemented with 10% heat-inactivated fetal bovine serum; nonessential amino acids (0.1 mM/mL; Invitrogen, Carlsbad, CA), l-glutamine (2 mM; Sigma-Aldrich), 2-mercaptoethanol (55 μM/mL; Sigma-Aldrich), penicillin (50 U/mL; Invitrogen), and streptomycin (50 μg/mL; Invitrogen). Following removal of nonadherent cells with warm PBS, medium was replenished and cells were incubated for 24 to 48 h to facilitate monocyte adherence and complete removal of thrombocytes, nonadherent lymphocytes and other semi-adherent cells. Prior to the replacement of medium, adherent cell cultures were washed in warm PBS. Monocytes were cultured for 6 d to allow maturation and differentiation of cells, with medium changes occurring every 3 to 4 d, thus ensuring that optimal nutrient requirements were met. The morphology of adherent cells was observed daily under bright field microscopy (20× objective).
Confirming Cell Purity Using an Immunofluorescence Assay
Purity of monocyte cultures, as well as 6-d-old primary macrophages, was confirmed by immunofluorescence assay (IFA). The monoclonal antibody, KUL01, has been used for flow cytometric identification of peripherally circulating chicken cells of the mononuclear phagocyte system throughout their ontogeny (monoblasts, promonocytes, monocytes, and tissue macrophages), whereas CV1-chNL68 and the monocyte monoclonal antibody K1 cross-react with chicken lymphocytes and thrombocytes, respectively (Mast et al., 1998).
Following the removal of medium, cells were fixed in a 1:1 (vol/vol) methanol:acetone mixture. Cells were rehydrated in PBS for 15 min before incubating with blocking buffer (5% nonfat dry milk/PBS) for 20 min. Staining with the mouse anti-chicken monocyte/macrophage antibody KUL01-FITC (Southern BioTech, Birmingham, AL; 1:250) was performed for 45 min to 1 h in the dark. Cells were then washed 3 times in PBS and viewed under a Nikon Eclipse Ti fluorescence microscope (10× objective, Nikon Instruments Inc., Melville, NY).
Cell Counts and Flow Cytometry
To optimize procedures and establish macrophage counts after adherence, macrophages were counted in wells after adherence via the NIS Elements program (Nikon Instruments Inc.), with cells being counted per mm2 and calculated per well, and flow cytometry was performed to compare yields from 1.077 versus 1.083 histopaque gradients.
Briefly, mononuclear cells were washed twice in cold PBS and suspended in blocking buffer (1% NFDM, 0.1% sodium azide, and 5% heat-inactivated fetal bovine serum) for 20 min. After blocking, cells were washed and stained in the dark for 45 min with (1:250) FITC-labeled mouse anti-chicken monocyte/macrophage antibody KUL01-FITC (Southern BioTech, Birmingham, AL), again washed in cold PBS 3 times, resuspended in 1 mL of cold PBS, and analyzed on Beckman Coulter cytomics FC 500 flow cytometer (10,000 events were obtained for each sample).
IFNγ Stimulation
A 50 ρg/mL of ch-IFNγ solution (Invitrogen) was prepared in RPMI w/o Phenol Red culture medium (Invitrogen). After washing the cells twice with warm PBS, macrophage cultures were stimulated with 1 mL of RPMI-ch-IFNγ mixture.
Lipofectamine:Poly (I:C) Stimulation
Lipofectamine-mediated transfection of poly (I:C) into cells was used as a model of RNA virus infection. Five microliters each of poly (I:C) (10 mg/mL) and lipofectamine solution (Calbiochem, San Diego, CA) were incubated with 195 μL of OptiMEM medium (Invitrogen) for 5 min at room temperature. Both solutions were then combined and incubated at room temperature for 45 min before diluting the mixture 1:10 with OptimMEM to obtain a final concentration of 25 μg/mL of poly (I:C). Macrophages were incubated in 500 μL of the working solution for 5 to 6 h. Transfection was terminated by the addition of 500 μL of RPMI to each well.
NO Assays
Nitrite, a stable, reactive nitrogen intermediate, served as an indirect measure of NO production representing macrophage/monocyte stimulation (Crippen et al., 2003; Singh et al., 2010). Briefly, cultured macrophages were centrifuged at 200 × g for 10 min at room temperature to remove cell debris. One hundred microliters of each culture supernatant were incubated in the dark with an equal volume (1:1) of Griess reagent (1% sulfanilamide/0.1 naphthylenediamine/2.5% phosphoric acid) in individual wells of a 96-well plate for 15 min. Absorbance was measured at a wavelength of 590 nM using an ELISA plate reader (Cambrex, East Rutherford, NJ). Values were compared with concentrations derived from a standard curve prepared by serial dilution (in RPMI w/o Phenol Red) of a 2 mM sodium nitrite stock solution (Sigma-Aldrich). Assays were performed in triplicate at designated times.
Statistics
Nitric oxide concentrations were expressed as averages with SEM. Paired t-test, 2-tailed, was used to analyze differences in the kinetics between B2 and B19. Analysis of variance one-way test was used to determine statistical differences between NO groups; significance was set at P < 0.05. Differential blood cell counts of B2 and B19 haplotypes (monocytes, lymphocytes, and heterophils) were analyzed with t-test, paired, 2-tailed.
RESULTS
Concentrations of B19 and B2 Monocytes in Whole Blood Were Similar
Studies were designed to identify potential differences in the function of monocytes/macrophages purified from birds of defined haplotypes. Based on noted differences in susceptibility to avian coronavirus infection (Banat et al., 2013), potential differences in the concentrations of circulating cell populations and specifically that of monocytes in birds with distinct haplotypes had to be established. Cell counts from peripheral blood of monocytes, lymphocytes, and heterophils were not significantly different (t-test, paired, 2-tailed) between the more resistant B2 and more susceptible B19 homozygous chickens (Table 1 ). Yields of PBMC prepared from either Ficoll-Hypaque 1.077, previously used for lymphocyte studies, or 1.083, which has been recommended for mononuclear cell enrichment in mammals (Feldman and Mogelesky, 1987), were compared. Whereas the concentrations from birds of either haplotype were similar, the higher density gradient (1.083) yielded higher amounts of avian monocytes from PBMC derived from either haplotype (Table 2 ). All PBMC isolations were, therefore, performed using the higher density solution.
Table 1.
Peripheral blood cell counts1
| B haplotype | Monocytes | Lymphocytes | Heterophils | Eosinophils | Basophils |
|---|---|---|---|---|---|
| B2/B2 | 455 (±72) | 6,826 (±496) | 2,422 (±286) | 0 (±0) | 64 (±21) |
| B19/B19 | 405 (±75) | 5,736 (±541) | 2,191 (±397) | 43 (±31) | 60 (±31) |
Peripheral blood cell counts (mean cells/μL ± SD) performed by Antech Diagnostics (Irvine, CA) for B2/B2 (n = 6) and B19/B19 (n = 6) chickens. Values showed no statistically significant differences between haplotypes by a paired t-test.
Table 2.
Cell concentrations
| B haplotype | PBMC1 1.077 gradient | PBMC 1.083 gradient | MØ/well2 after adherence |
|---|---|---|---|
| B2/B2 | 6.4 × 107 | 7.3 × 107 | 2.70 (±0.33) × 105 |
| B19/B19 | 7.8 × 107 | 7.8 × 107 | 2.62 (±0.35) × 105 |
Total cell numbers after peripheral blood mononuclear cells (PBMC) isolation via gradient 1.077 and 1.083 are given for 3 mL of whole blood drawn counted by fluorescence-activated cell sorting.
Macrophage numbers given per each in well after both 6 d of adherence (n = 5/haplotype) and stimulation with IFNγ counted via the NIS Elements program, with cells being counted per mm2 and calculated per well. Macrophage counts are reported as mean (±SD).
Ex Vivo Monocytes from B2 Birds Differentiate More Readily than Those of B19 Birds
By d 4 of culture, B2 cells demonstrated distinct macrophage-like morphologies: stellate cytoplasmic processes, vacuolation, and decreased nuclear to cytoplasmic ratio (N/C; Figure 1 , panel A). In contrast, differences in N/C and number of cytoplasmic processes were not evident in B19-derived cultures until 6 d postinitiation of culture (Figure 1, panel B). Similarly, during the kinetic studies, the number of cytoplasmic processes displayed by B2 macrophages and the degree of vacuolation observed at 48 h poststimulation with IFNγ was more marked than the changes noted in B19-derived cells (Figure 2 ). To verify purity of macrophage cultures, B2/B2 and B19/B19 cells were positively stained with monoclonal antibody KUL01 after 6 d of culture (Figure 3 , panels A and B).
Figure 1.
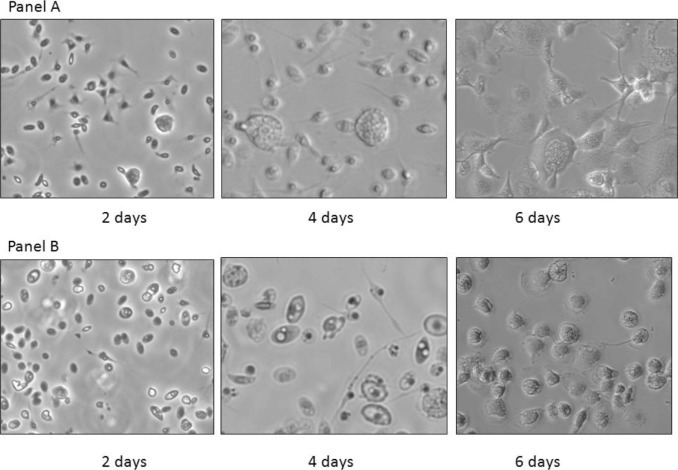
Ex vivo differentiation of monocytes prestimulation. Images demonstrate the rate of differentiation of chicken monocytes from homozygous B2 (panel A) and B19 (panel B) chicks at d 2, 4, and 6 d postadherence. Distinct vacuoles, increased numbers of stellate cytoplasmic processes, and decreased nuclear to cytoplasmic ratio are visible on d 4 with B2 cells and on d 6 with B19 cells. Pictures were taken at 20× magnification, inverted, with Nikon Eclipse Ti fluorescence microscope (Nikon Instruments Inc., Melville, NY).
Figure 2.
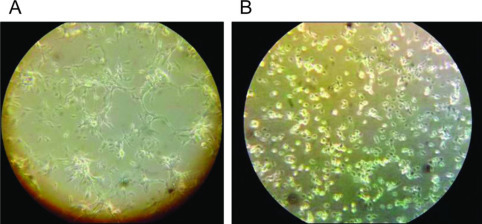
Differentiation of 48 h interferon (IFN)γ-stimulated macrophages. Macrophages cultured for 6 d from B2 and B19 haplotypes were stimulated with IFNγ for 48 h, and pictures were taken with a Nikon Eclipse Ti fluorescence microscope (Nikon Instruments Inc., Melville, NY), inverted at 10× magnification. Macrophage-like features (stellate cytoplasmic processes, vacuolation, and decreased nuclear to cytoplasmic ratio) are more pronounced in B2 cells (A) compared with their B19 counterparts (B). Color version available in the online PDF.
Figure 3.
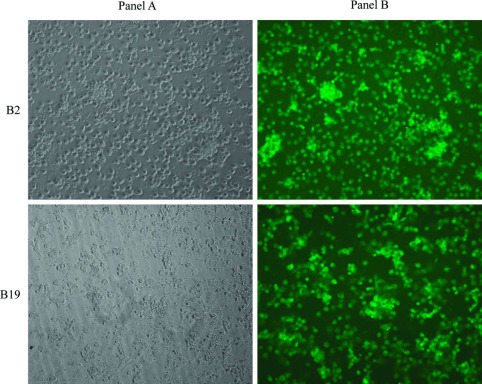
Immunofluorescent labeling of adherent macrophages. Panel A depicts the brightfield image of macrophages. The green fluorescence noted in panel B depicts positive labeling of B2 and B19 chicken cells by monoclonal antibody KUL01-FITC, confirming their monocyte/macrophage cell lineage. Pictures were taken with Nikon Eclipse Ti fluorescence microscope (Nikon Instruments Inc., Melville, NY), inverted at 10× magnification. Color version available in the online PDF.
Greater NO Production of B2-Derived Macrophages in Response to Stimulation
Potential differences in macrophage functions were examined following exposure to either IFNγ or poly (I:C). All stimulations were performed on d 6 of culture when monocytes/macrophages from both B2 and B19 birds were mature (Figure 2). Nitric oxide production served as the indicator of macrophage activation. Timed exposure (24, 48, and 72 h) of macrophages cultured 6 d to either IFNγ or poly (I:C) demonstrated the existence of functional differences between B2/B2 and B19/B19 lines of birds aged 5 to 6 wk. Beginning at 24 h postexposure to IFNγ (Figure 4A ) and poly (I:C) (Figure 4B), induced NO production by macrophages derived from B19/B19 birds was significantly (P < 0.01, t = test, paired) lower compared with that by B2/B2-derived macrophages. Additionally, the responsiveness of B2/B2 cells to either stimulant was significantly greater compared with that of unstimulated control B2/B2 cells (P < 0.01). At 48 h poststimulation with poly (I:C), there was a dramatic difference in the NO concentration of B2/B2 culture (92.76 μg/mL) supernatants than that of B19/B19 cells (17.90 μg/mL; Figure 5 ). Because background NO concentration, as well as cell death, increased at 72 h, especially in the B19 cultures, stimulation of cells in subsequent experiments with poly (I:C) (Figure 5A) or IFNγ (Figure 5B) was only characterized up to the 48 h time point using age-matched birds ages 6 to 12 wk. In addition, macrophages from B8/B8, B15/B15, and B21/21 haplotypes, as well as macrophages from B2/B2 on B19 background haplotypes (B19bgB2MHC), were stimulated with IFNγ (Table 3 ). Nitric oxide release from B19bgB2MHC macrophages was very similar (86.2 μg/mL) to that from B2/B2 haplotypes, and significantly different from NO release of B19/B19 macrophages (P < 0.01). However, stimulation of B8/B8 macrophages was very variable with repeated experiments (17.7 μg/mL). Although NO release was less than that of B2/B2 cells, B21/B21 (39.8 μg/mL) and B15/B15 (45.9 μg/mL) macrophages yielded higher levels of NO than B19/B19 cells.
Figure 4.
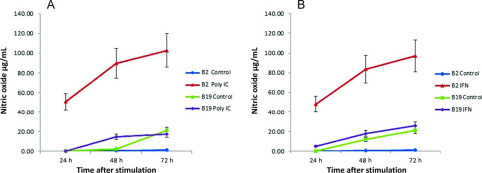
Kinetics. Nitric oxide release is shown as mean (μg/mL) ± SD by B2 and B19 macrophages at 24, 48, and 72 h poststimulation with poly (I:C); n = 8 (A) and interferon (IFN) γ; n = 6 (B). In the presence of either stimulant, NO production at all 3 time points was significantly lower in B19 than in B2 supernatants (P < 0.01, t-test, paired). Samples were taken at 6 wk of age. Color version available in the online PDF.
Figure 5.
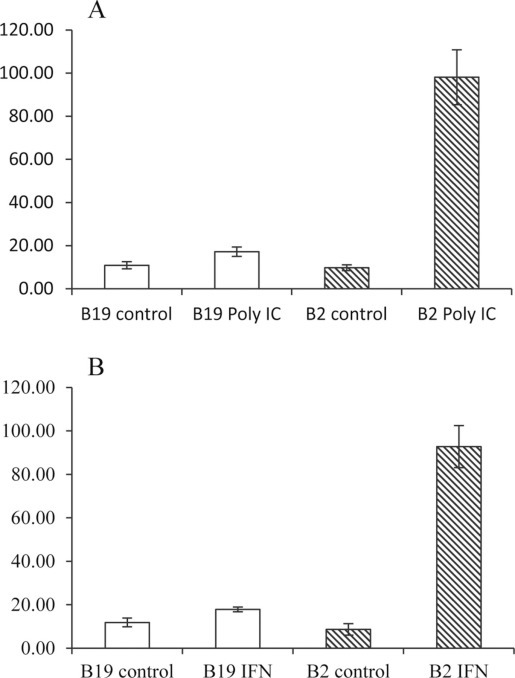
A: Nitric oxide release by poly(I:C)-stimulated B2 and B19 macrophages. Cells were stimulated for 48 h with 25 μg/mL poly (I:C). Although not significantly different, basal NO concentration was consistently greater in B19 culture supernatants (10.88 μg/mL) than in B2 supernatants (9.75 μg/mL). Following stimulation, NO production by B2 cells (98.12 μg/mL) was significantly greater than that by B19 macrophages (17.19 μg/mL; P < 0.01, one-way ANOVA). Values are reported as mean with SE, n = 28. B: Nitric oxide release by interferon (IFN)γ-stimulated B2 and B19 macrophages. Cells were stimulated for 48 h with 50 pg/mL of ch-IFNγ. Although not significantly different, basal NO concentration was consistently greater in B19 culture supernatants (11.88 μg/mL) than in B2 supernatants (8.7 μg/mL). Following stimulation, NO production was significantly greater in B2 culture supernatants (92.76 μg/mL) than in B19 culture supernatants (17.9 μg/mL; P < 0.01, one-way ANOVA). Values are reported as mean with SE, n = 21.
Table 3.
Interferon (IFN) γ stimulation of different B haplotype macrophages1
| B haplotype | Control | IFNγ |
|---|---|---|
| B2 (n = 21) | 8.7 (±2.69) | 92.76 (±9.67)a,b |
| B19 (n = 21) | 11.88 (±1.94) | 17.90 (±1.08) |
| B19bgB2MHC (n = 7) | 15.5 (±0.6) | 86.2 (±19.0)a,b |
| B8 (n = 12) | 4.8 (±1.2) | 17.7 (±16.0) |
| B15 (n = 2) | 13.6 (±2.0) | 45.9 (±9.4) |
| B21 (n = 13) | 10.0 (±1.8) | 39.8 (±4.8)a |
Significantly different compared with unstimulated sample of same haplotype by ANOVA one-way test (P < 0.01).
Significantly different compared with B19 stimulated by ANOVA one-way test (P < 0.01).
Nitric oxide release given as mean (μg/mL) ± SD from macrophages cultured 6 d from chicks with the B2, B19, B19bgB2MHC, B8, B15, B21 haplotypes (5–12 wk of age) at 48 h poststimulation with IFNγ. The B2 and B19bgB2MHC exhibit the largest nitric oxide release compared with other haplotypes.
DISCUSSION
Inbred B haplotype chicken lines provide an excellent resource for studying the genetic bases of disease resistance and susceptibility. Several clinical studies in chickens, including an in vivo IBV infectivity study of B haplotype-defined chicks (Banat et al., 2013), have shown an association between disease susceptibility and the MHC (Briles et al., 1983; Taylor, 2004; Goto et al., 2009). Because differences in IBV resistance of B2/B2 chicks to clinical illness were observed early after initial infection, innate immunity was targeted in the current study. To our knowledge, this is the first study in which differences in activation of monocytes/macrophages of B haplotype-defined birds have been investigated in the context of disease resistance and susceptibility.
The rate of differentiation of macrophages derived from the more resistant B2/B2 chickens and their subsequent activation in response to stimuli was dramatically greater than that of macrophages from the more IBV-susceptible B19 haplotype chickens. The consistent, significantly greater response of the B2 macrophages could potentially explain the differences observed early after IBV infection. A role of TLR3, which binds to dsRNA, is implicated in the macrophage response and ultimately in in vivo resistance (Malmgaard et al., 2004; Whitmore et al., 2004; Kogut et al., 2005; Nang et al., 2011). Double-stranded RNA is a replication intermediate during infection with RNA viruses, such as IBV, avian influenza virus, and Newcastle disease virus (Malmgaard et al., 2004; Nang et al., 2011). Potential functional differences related to macrophage communication with adaptive immunity were evaluated through stimulation by the lymphocyte-derived cytokine, IFNγ. The demonstrated significant differences in NO production that both poly (I:C) and IFNγ induced in the B2/B2 and the B19/B19 haplotypes may explain, at least in part, the greater resistance of birds of the homozygous B2 line to IBV-associated early onset clinical illness (Banat et al., 2013). As Dil and Qureshi (2002) showed, expression of iNOS varies among macrophages from chickens of different genetic lines and is associated with differential TLR4 expression in response to lipopolysaccharide. Further research is necessary to analyze iNOS and TLR expression on macrophages from B2 versus B19 haplotypes to investigate if similar pathways are responsible for the difference in activation.
In addition to differences in activation, even when cultured ex vivo in the absence of poly (I:C) and IFNγ, B2/B2 monocytes differentiated more readily than B19/B19 monocytes. Based on changes in morphology, B2-derived cells obtain classical features of the macrophage, such as increased cytoplasm to nuclear ratio, vacuolation, and cytoplasmic pseudopod-like projections, within 4 d as compared with the 6 d required by B19-derived cells. Although the significance of the higher rate of activation is not known, this could reflect an overall lower threshold of response of the B2 cells compared with that of the B19 homozygous macrophages.
Participation of pure cultures of monocytes/macrophages in these studies was confirmed by the 100% labeling by the KUL01 macrophage monoclonal antibody marker (Mast et al., 1998). This negates any influence by contaminating lymphocytes and their regulatory cytokines. Furthermore, the cultures were plated with equivalent concentrations of monocytes/macrophages. Hence, these studies reflect differences in cell activity and not increased B2 cell numbers. Additionally, the concentration of peripherally circulating monocytes in B19 and B2 birds was not significantly different.
The MHC-B locus, which has been associated with resistance to tumor-associated viruses in chickens, has also been implicated in observed differences in macrophage function (Briles et al., 1983; Taylor, 2004). Qureshi et al. (1986, 1988) demonstrated that macrophages from chickens congenic for MHC also showed differences in chemotactic activity, recruitment, and activation. The congenic B2 on the B19 background demonstrated the B2 phenotype suggesting at least for B2 and B19, the genetic difference may lie within the B locus. Monocytes derived from additional homozygous MHC haplotypes were also differentially activated by IFNγ. The cells purified from B15/B15 birds, which were shown by Bacon et al. (2004) to be more resistant to IBV infection also demonstrated greater macrophage stimulation. It is possible that genes lying outside this region could also affect macrophage activation as demonstrated in other lines. Genes lying outside the B2 apparently can influence resistance to highly pathogenic avian influenza (Hunt et al., 2010).
Sequence differences between the MHC-B region of B2 and B19 haplotypes have indicated several differences in a 14-gene region between BG and BF (Hosomichi et al., 2008). Likewise, genomic regions lying outside the B region could also be implicated in monocyte differentiation and activation.
Future studies identifying differences in protein and mRNA expression following macrophage stimulation will provide important clues as to the genes and pathways responsible for greater resistance to illness. Our preliminary results from sequencing the RNA of stimulated macrophages of B2 and B19 haplotype chickens indicate differential regulation of at least the TLR pathway (manuscript in preparation), which is a critical pathway in innate immunity and vital to the host immune response to pathogens (Medzhitov and Janeway, 1997; Medzhitov, 2001).
In conclusion, the potential for using macrophage function to screen for resistance could be a valuable asset in breeding programs. Furthermore, the chicken is an exceptional resource for deciphering MHC-related mechanisms behind disease resistance. Although chickens with various defined B haplotypes have previously been shown to differ in their responses to pathogens, the cellular differences described in these studies provide a basis for future work characterizing the molecular and genetic basis of immune-mediated resistance. These studies provide a background for eventually establishing lines with resistance to infection or with enhanced immunity to vaccines.
Acknowledgments
This work was funded by grants from the USDA-National Research Institute #2006-35204-18810, USDA-National Institute of Food and Agriculture #2008-00875, USDA-Cooperative State Research Service Competitive Grants Program (2006-35204-16560), and the Avian Influenza Cooperative Agriculture Program. The authors thank Suzana Tkalcic (veterinary pathologist, College of Veterinary Medicine, Western University of Health Sciences, Pomona, CA), Miguel D. Saggese (veterinary microbiologist, College of Veterinary Medicine, Western University of Health Sciences), Renee Kopulos (Department of Biological Sciences, Northern Illinois University, DeKalb, IL), and Linda Yates (Department of Biological Sciences, Northern Illinois University) for the invaluable expertise and technical support they provided. We are very grateful to Ghida Banat (College of Veterinary Medicine, Western University of Health Sciences) and Chris Petro (California Polytechnic Institute, Pomona, CA) for their care of the chickens.
REFERENCES
- Bacon L.D., Hunter D.B., Zhang H.M., Brand K., Etches R. Retrospective evidence that the MHC (B haplotype) of chickens influences genetic resistance to attenuated infectious bronchitis vaccine strains in chickens. Avian Pathol. 2004;33:605–609. doi: 10.1080/03079450400013147. 15763730. [DOI] [PubMed] [Google Scholar]
- Banat G.R., Tkalcic S., Dzielawa J.A., Jackwood M.W., Saggese M.D., Yates L., Kopulos R., Briles W.E., Collisson E.W. Association of the chicken MHC B haplotypes with resistance to avian coronavirus. Dev. Comp. Immunol. 2013;39:430–437. doi: 10.1016/j.dci.2012.10.006. 23178407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayyari G.R., Huff W.E., Rath N.C., Balog J.M., Newberry L.A., Villines J.D., Skeeles J.K., Anthony N.B., Nestor K.E. Effect of the genetic selection of turkeys for increased body weight and egg production on immune and physiological responses. Poult. Sci. 1997;76:289–296. doi: 10.1093/ps/76.2.289. 9057208. [DOI] [PubMed] [Google Scholar]
- Bearse G.E., McClary C.F., Miller M.W. The results of eight years’ selection for disease resistance and susceptibility in White Leghorns. Poult. Sci. 1939;18:400–401. [Google Scholar]
- Boockvar K.S., Granger D.L., Poston R.M., Maybodi M., Washington M.K., Hibbs J.B., Jr., Kurlander R.L. Nitric oxide produced during murine listeriosis is protective. Infect. Immun. 1994;62:1089–1100. doi: 10.1128/iai.62.3.1089-1100.1994. 7509315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles W.E., Briles R.W. Genetics and classification of major histocompatibility complex antigens of the chicken. Poult. Sci. 1987;66:776–781. doi: 10.3382/ps.0660776. 3628161. [DOI] [PubMed] [Google Scholar]
- Briles W.E., Briles R.W., Pollock D.L., Pattison M. Marek's disease resistance of B (MHC) heterozygotes in a cross of purebred Leghorn lines. Poult. Sci. 1982;61:205–211. doi: 10.3382/ps.0610205. 7088788. [DOI] [PubMed] [Google Scholar]
- Briles W.E., Briles R.W., Taffs R.E., Stone H.A. Resistance to a malignant lymphoma in chickens is mapped to subregion of major histocompatibility (B) complex. Science. 1983;219:977–979. doi: 10.1126/science.6823560. 6823560. [DOI] [PubMed] [Google Scholar]
- Briles W.E., Mc G.W., Irwin M.R. On multiple alleles effecting cellular antigens in the chicken. Genetics. 1950;35:633–652. doi: 10.1093/genetics/35.6.633. 14793708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippen T.L., Sheffield C.L., He H., Lowry V.K., Kogut M.H. Differential nitric oxide production by chicken immune cells. Dev. Comp. Immunol. 2003;27:603–610. doi: 10.1016/s0145-305x(03)00035-1. 12697316. [DOI] [PubMed] [Google Scholar]
- Dawes M.E., Tyler J.W., Marsh A.E., Larson R.L., Steevens B.J., Lakritz J. In vitro effects of lactoferrin on lipopolysaccharide-induced proliferation, gene expression, and prostanoid production by bovine peripheral blood mononuclear cells. Am. J. Vet. Res. 2008;69:1164–1170. doi: 10.2460/ajvr.69.9.1164. 18764689. [DOI] [PubMed] [Google Scholar]
- Dietert R.R., Golembroski K.A., Bloom S.E., Qureshi M.A., Sharma J.M. Avian Cellular Immunology. CRC Press; Boca Raton, FL: 1991. The avian macrophage in cellular immunity; pp. 71–95. [Google Scholar]
- Dil N., Qureshi M.A. Differential expression of inducible nitric oxide synthase is associated with differential Toll-like receptor-4 expression in chicken macrophages from different genetic backgrounds. Vet. Immunol. Immunopathol. 2002;84:191–207. doi: 10.1016/s0165-2427(01)00402-0. 11777534. [DOI] [PubMed] [Google Scholar]
- Drechsler Y., Bohls R.L., Smith R., Silvy N., Lillehoj H., Collisson E.W. An avian, oncogenic retrovirus replicates in vivo in more than 50% of CD4+ and CD8+ T lymphocytes from an endangered grouse. Virology. 2009;386:380–386. doi: 10.1016/j.virol.2009.01.027. 19237181. [DOI] [PubMed] [Google Scholar]
- Drechsler Y., Tkalcic S., Saggese M.D., Shivaprasad H.L., Ajithdoss D.K., Collisson E.W. A DNA vaccine expressing ENV and GAG offers partial protection against reticuloendotheliosis virus in the prairie chicken (Tympanicus cupido) J. Zoo Wildl. Med. 2013;44:251–261. doi: 10.1638/2011-0229R1.1. 23805542. [DOI] [PubMed] [Google Scholar]
- Dunnington E.A., Larsen C.T., Gross W.B., Siegel P.B. Antibody responses to combinations of antigens in White Leghorn chickens of different background genomes and major histocompatibility complex genotypes. Poult. Sci. 1992;71:1801–1806. doi: 10.3382/ps.0711801. 1437966. [DOI] [PubMed] [Google Scholar]
- Feldman D.L., Mogelesky T.C. Use of Histopaque for isolating mononuclear cells from rabbit blood. J. Immunol. Methods. 1987;102:243–249. doi: 10.1016/0022-1759(87)90083-4. 3655375. [DOI] [PubMed] [Google Scholar]
- Fulton J.E., Juul-Madsen H.R., Ashwell C.M., McCarron A.M., Arthur J.A., O'Sullivan N.P., Taylor R.L., Jr. Molecular genotype identification of the Gallus gallus major histocompatibility complex. Immunogenetics. 2006;58:407–421. doi: 10.1007/s00251-006-0119-0. 16738938. [DOI] [PubMed] [Google Scholar]
- Goto R.M., Wang Y., Taylor R.L., Jr., Wakenell P.S., Hosomichi K., Shiina T., Blackmore C.S., Briles W.E., Miller M.M. BG1 has a major role in MHC-linked resistance to malignant lymphoma in the chicken. Proc. Natl. Acad. Sci. USA. 2009;106:16740–16745. doi: 10.1073/pnas.0906776106. 19805366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P.F., Smyth J.R., Jr. The influence of growth rate on the development of Marek's disease in chickens. Poult. Sci. 1972;51:975–985. doi: 10.3382/ps.0510975. 4646680. [DOI] [PubMed] [Google Scholar]
- Heinzelmann E.W., Clark K.K., Collins W.M., Briles W.E. Host age and major histocompatibility genotype influence on Rous sarcoma regression in chickens. Poult. Sci. 1981;60:2171–2175. doi: 10.3382/ps.0602171. 6276876. [DOI] [PubMed] [Google Scholar]
- Hosomichi K., Miller M.M., Goto R.M., Wang Y., Suzuki S., Kulski J.K., Nishibori M., Inoko H., Hanzawa K., Shiina T. Contribution of mutation, recombination, and gene conversion to chicken MHC-B haplotype diversity. J. Immunol. 2008;181:3393–3399. doi: 10.4049/jimmunol.181.5.3393. 18714011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt H.D., Jadhao S., Swayne D.E. Major histocompatibility complex and background genes in chickens influence susceptibility to high pathogenicity avian influenza virus. Avian Dis. 2010;54:572–575. doi: 10.1637/8888-042409-ResNote.1. 20521696. [DOI] [PubMed] [Google Scholar]
- Hussain I., Qureshi M.A. Nitric oxide synthase activity and mRNA expression in chicken macrophages. Poult. Sci. 1997;76:1524–1530. doi: 10.1093/ps/76.11.1524. 9355146. [DOI] [PubMed] [Google Scholar]
- Hussain I., Qureshi M.A. The expression and regulation of inducible nitric oxide synthase gene differ in macrophages from chickens of different genetic background. Vet. Immunol. Immunopathol. 1998;61:317–329. doi: 10.1016/s0165-2427(97)00153-0. 9613444. [DOI] [PubMed] [Google Scholar]
- Hutt F.B., Scholes J.C. Genetics of the fowl XIII breed differences in susceptibility to Salmonella pullorum. Poult. Sci. 1941;20:342–352. [Google Scholar]
- Juul-Madsen H.R., Dalgaard T.S., Rontved C.M., Jensen K.H., Bumstead N. Immune response to a killed infectious bursal disease virus vaccine in inbred chicken lines with different major histocompatibility complex haplotypes. Poult. Sci. 2006;85:986–998. doi: 10.1093/ps/85.6.986. 16776466. [DOI] [PubMed] [Google Scholar]
- Juul-Madsen H.R., Hedemand J.E., Salomonsen J., Simonsen M. Restriction fragment length polymorphism analysis of the chicken B-F and B-L genes and their association with serologically defined B haplotypes. Anim. Genet. 1993;24:243–247. doi: 10.1111/j.1365-2052.1993.tb00306.x. 7902038. [DOI] [PubMed] [Google Scholar]
- Kim D.K., Lillehoj H.S., Hong Y.H., Park D.W., Lamont S.J., Han J.Y., Lillehoj E.P. Immune-related gene expression in two B-complex disparate genetically inbred Fayoumi chicken lines following Eimeria maxima infection. Poult. Sci. 2008;87:433–443. doi: 10.3382/ps.2007-00383. 18281568. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., Iqbal M., He H., Philbin V., Kaiser P., Smith A. Expression and function of Toll-like receptors in chicken heterophils. Dev. Comp. Immunol. 2005;29:791–807. doi: 10.1016/j.dci.2005.02.002. 15936435. [DOI] [PubMed] [Google Scholar]
- Kreil T.R., Eibl M.M. Nitric oxide and viral infection: NO antiviral activity against a flavivirus in vitro, and evidence for contribution to pathogenesis in experimental infection in vivo. Virology. 1996;219:304–306. doi: 10.1006/viro.1996.0252. 8623546. [DOI] [PubMed] [Google Scholar]
- Lamont S.J. The chicken major histocompatibility complex in disease resistance and poultry breeding. J. Dairy Sci. 1989;72:1328–1333. doi: 10.3168/jds.S0022-0302(89)79240-7. 2568373. [DOI] [PubMed] [Google Scholar]
- Landesman E., Uni Z., Heller E.D. Designation by restriction fragment length polymorphism of major histocompatibility complex class IV haplotypes in meat-type chickens. Anim. Genet. 1993;24:349–354. doi: 10.1111/j.1365-2052.1993.tb00339.x. 7904800. [DOI] [PubMed] [Google Scholar]
- Liljequist B., Weinstock D., Qureshi M., Brake J. Natural-killer-cell activity in male Single Comb White Leghorns and commercial broilers. Colloq. INRA. 1993;62:57–62. [Google Scholar]
- MacMicking J., Xie Q.W., Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. 9143691. [DOI] [PubMed] [Google Scholar]
- Malmgaard L., Melchjorsen J., Bowie A.G., Mogensen S.C., Paludan S.R. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 2004;173:6890–6898. doi: 10.4049/jimmunol.173.11.6890. 15557184. [DOI] [PubMed] [Google Scholar]
- Mast J., Goddeeris B.M., Peeters K., Vandesande F., Berghman L.R. Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Vet. Immunol. Immunopathol. 1998;61:343–357. doi: 10.1016/s0165-2427(97)00152-9. 9613446. [DOI] [PubMed] [Google Scholar]
- Mays J.K., Bacon L.D., Pandiri A.R., Fadly A.M. Response of White Leghorn chickens of various B haplotypes to infection at hatch with subgroup J avian leukosis virus. Avian Dis. 2005;49:214–219. doi: 10.1637/7315-120104R. 16094825. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. 11905821. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. Innate immunity: Impact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. 9039775. [DOI] [PubMed] [Google Scholar]
- Miller M.M., Bacon L.D., Hala K., Hunt H.D., Ewald S.J., Kaufman J., Zoorob R., Briles W.E. 2004 Nomenclature for the chicken major histocompatibility (B and Y) complex. Immunogenetics. 2004;56:261–279. doi: 10.1007/s00251-004-0682-1. 15257423. [DOI] [PubMed] [Google Scholar]
- Nang N.T., Lee J.S., Song B.M., Kang Y.M., Kim H.S., Seo S.H. Induction of inflammatory cytokines and toll-like receptors in chickens infected with avian H9N2 influenza virus. Vet. Res. 2011;42:64. doi: 10.1186/1297-9716-42-64. 21592354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor K.E., Saif Y.M., Zhu J., Noble D.O. Influence of growth selection in turkeys on resistance to Pasteurella multocida. Poult. Sci. 1996;75:1161–1163. doi: 10.3382/ps.0751161. 8893289. [DOI] [PubMed] [Google Scholar]
- Qureshi M.A. Avian macrophage and immune response: An overview. Poult. Sci. 2003;82:691–698. doi: 10.1093/ps/82.5.691. 12762389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi M.A., Dietert R.R., Bacon L.D. Genetic variation in the recruitment and activation of chicken peritoneal macrophages. Proceedings of the Society for Experimental Biology and Medicine Soc. Exp. Biol. Med. 1986;181:560–568. doi: 10.3181/00379727-181-42293. [DOI] [PubMed] [Google Scholar]
- Qureshi M.A., Dietert R.R., Bacon L.D. Chemotactic activity of chicken blood mononuclear leukocytes from 15I5-B-congenic lines to bacterially-derived chemoattractants. Vet. Immunol. Immunopathol. 1988;19:351–360. doi: 10.1016/0165-2427(88)90120-1. 3252620. [DOI] [PubMed] [Google Scholar]
- Seo S.H., Collisson E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 1997;71:5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. 9188584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Toro H., Tang D.C., Briles W.E., Yates L.M., Kopulos R.T., Collisson E.W. Non-replicating adenovirus vectors expressing avian influenza virus hemagglutinin and nucleocapsid proteins induce chicken specific effector, memory and effector memory CD8(+) T lymphocytes. Virology. 2010;405:62–69. doi: 10.1016/j.virol.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung A.M., Nordskog A.W., Lamont S.J., Warner C.M. Isolation and characterization of cDNA clones for chicken major histocompatibility complex class II molecules. Anim. Genet. 1993;24:227–233. doi: 10.1111/j.1365-2052.1993.tb00304.x. 8239067. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Jr. Major histocompatibility (B) complex control of responses against Rous sarcomas. Poult. Sci. 2004;83:638–649. doi: 10.1093/ps/83.4.638. 15109061. [DOI] [PubMed] [Google Scholar]
- Whitmore M.M., DeVeer M.J., Edling A., Oates R.K., Simons B., Lindner D., Williams B.R. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res. 2004;64:5850–5860. doi: 10.1158/0008-5472.CAN-04-0063. 15313929. [DOI] [PubMed] [Google Scholar]
- Yoo B.H., Sheldon B.L. Association of the major histocompatibility complex with avian leukosis virus infection in chickens. Br. Poult. Sci. 1992;33:613–620. doi: 10.1080/00071669208417500. 1322760. [DOI] [PubMed] [Google Scholar]


