Abstract
To examine the relationship of impairments of the liver and kidney with viral load after nephropathogenic infectious bronchitis virus (NIBV) infection in embryonic chickens, 120 specific-pathogen-free Leghorn embryonated chicken eggs were randomly divided into two groups (infected and control), with three replicates per group and 20 eggs in each replicate. The eggs in the infected and control groups were challenged with 0.2 mL of 105.5 ELD50 NIBV and sterile saline solution, respectively. The embryonic chickens’ plasma and liver and kidney tissues were collected at 1, 3, and 5 days post-inoculation (dpi), the liver and kidney functional parameters were quantified, and the tissue viral loads were determined with real-time PCR. The results showed that plasma potassium, sodium, chlorine, magnesium, calcium, and phosphorus levels were increased. The infected group exhibited significantly higher plasma uric acid, blood urea nitrogen, and creatinine levels than the control group at 3 dpi. The plasma concentrations of aspartate aminotransferase and alanine aminotransferase were significantly increased in the infected group. The total protein, albumin, and globulin levels in the infected group were significantly lower than those in the control group. The liver-kidney viral load in the infected group peaked at 3 dpi, at which time the kidney viral load was significantly higher than that of the liver. Our results indicated that NIBV infection caused liver and kidney damage in the embryonic chickens, and the results also demonstrated that the liver and kidney damage was strongly related to the tissue viral load following NIBV infection in embryonic chickens.
Key words: nephropathogenic infectious bronchitis virus, embryonic chicken, liver, kidney, viral load
INSTRODUCTION
Avian infectious bronchitis virus (IBV) is the prototype of the Coronavirus genus (Cavanagh, 2003). IBV can cause a highly contagious disease that affects the reproductive, respiratory, and renal systems of chickens and results in great economic losses to the poultry industry worldwide (Cavanagh, 2007; Worthington, et al., 2008; Cook, et al., 2012). IBV replicates in various chicken tissues and directly or indirectly causes various lesions and clinical signs, such as watery feces, tracheal rales, nephritis, dyspnea (Cavanagh, 2003). Respiratory and urinary system symptoms are common in IBV infection, and nephropathogenic strains of IBV pathotypes cause nephritis. In young chickens, severe respiratory distress may occur, and varying degrees of respiratory distress, decreases in egg production, and the loss of the internal and shell qualities of eggs have been reported. A previous study identified nephropathogenic strains of IBV that cause infection. Some strains of the virus can cause severe kidney damage, urolithiasis and high mortality (Cook, et al., 2001; Ziegler, et al., 2002). Infectious bronchitis (IB) has become a major health problem that affects the chicken industry in most countries of the world.
In the poultry industry, many factors cause visceral gout in chickens, including high dietary calcium, vitamin A deficiency, mycotoxin toxicity, and renal insufficiency. However, nephropathogenic infectious bronchitis virus (NIBV) has become an important factor of visceral gout (Bayry, et al., 2005). Previous studies have reported that NIBV can cause kidney damage and secondary gout (Gaba et al., 2010; Ziegler, et al. 2002). Early physiological studies documented the changes in water metabolism that are observed in the excreta of young male broiler chickens, and challenge with NIBV causes significant increases in the fractional excretions of sodium (Na), calcium (Ca), potassium (P), and plasma uric acid (Afanador and Roberts, 1994). Abdel-Moneim et al. reported severe congestion of the liver, spleen and lungs in birds that had died after experimental IBV infection (Abdel-Moneim, et al., 2009).
Kinde et al. reported mature kidney pathology at 7 days post-inoculation (dpi) (Kinde, et al., 1991), and Mahmood et al. isolated the nephropathogenic strain of IBV from the kidneys of chickens at 5 dpi (Mahmood, et al., 2011). Most of the previous molecular tests for IBV have been applied to the detection of IBV in kidney, trachea, or cloacal swabs from chickens rather than embryonic chickens. In addition, embryonated chicken eggs were used to study several poultry pathogens such as viruses, bacteria, and fungi (Jacobsen, et al., 2010; Montgomery, et al., 2005) and were suggested as an alternative model for vaccine production (Abdul Hafeez, et al., 2006). The pathogenesis and virulence of clostridium perfringens and eimeriatenella was also studied after experimental infection (Alnassan, et al., 2013). At present, there is no in embryonic chicken described for the investigation of NIBV infection.
The aim of the present study was to investigate the possible pathogenesis of NIBV in the embryonic chicken. This study attempted to infect embryonic chickens with NIBV and determine the plasma liver and kidney function indices and the tissue viral loads and to explore the relationships of the impairments in the liver and kidney with viral load.
MATERIALS AND METHODS
Incubation
One hundred twenty Leghorn embryonated chicken eggs were purchased from the Shandong Institute of Poultry Science and individually numbered. The eggs were set with the blunt end up in a forced-draft incubator and housed in a Specific pathogen Free (SPF) environment at the Clinical Veterinary Laboratory of the College of Animal Science and Technology, Jiangxi Agricultural University. The eggs were incubated at 37.5 ± 0.5°C and 55% to 60% relative humidity and were automatically turned every 2 h.
Chicken Embryo Challenge and Blood Sampling
The eggs were removed from the incubator on embryonic day (ED) 13 and marked to the upper junction chamber, and the allantoic membranes were located with a pencil. Subsequently, the exposed shell was disinfected with 75% ethanol, a small hole was drilled in the shell with a whisk, and each embryo was injected with 0.2 mL of a virus solution via the allantoic cavity route. The hole was then closed with liquid paraffin, and the egg was placed back into the incubator until sampling (Slawinska, et al., 2014). For sampling (Figure 1 ), the eggs were removed from the incubator and candled to locate a distinct blood vessel of the chorioallantoic membrane (CAM). After removing a piece of the shell using ophthalmic forceps, a drop of edible oil was smeared over the small area of the outer shell membrane to render this membrane transparent and make the blood capillaries clearly visible. Next, blood (≥ 600 μL) was taken from a capillary of the CAM using a 1-mL syringe, collected in a heparinized tube and kept on ice. The blood was centrifuged (3,000 rpm, 15 min, 4°C), and the plasma was collected and stored at −20°C until analysis.
Figure 1.

Embryo egg blunt end chorioallantoic membrane vascular distribution at incubation 13th day and blood sampling.
Experimental Protocol
Preliminary experiments were performed to optimize the injection days, injection doses, and post-injection (PI) sampling time points. The experiments were approved by the Ethical Committee for Experiment Use of Animals of the Jiangxi Agricultural University.
A total of 120 embryonated SPF chicken eggs were used in this experiment. All embryonated chicken eggs were randomly divided into 2 groups (infected and control), with 3 replicates per group and 20 embryonated chicken eggs in each replicate. Based on the embryo median lethal dose (ELD50), each egg (13-day-old) in the infected group was challenged with NIBV at a dose 105.5 ELD50 (0.2 mL) via the allantoic cavity route. Simultaneously, the eggs in the control group were infected with sterile saline solution (0.2 mL per chicken egg) via the same route. After the injections on ED13, blood and tissue sampling was performed at three sampling time points (1, 3, and 5 dpi). The plasma was stored at −20°C for biochemical measurements, and the liver and kidney tissues were stored at −80°C for detection of the viral load via real-time PCR. The anatomical pathology symptoms were recorded.
Measurement of the Embryo, Liver, and Kidney
The individual egg weights were recorded at 1, 3, and 5 dpi. A total of 120 eggs was weighed and the embryos were euthanized with cooling at 4°C for 4 hours, and their contents were removed from the shells to determine the embryo body, liver, and kidney weights. The values were calculated using the following formulae:
Measurement of the Plasma Liver and Kidney Functional Indices
The blood was centrifuged (3,000 rpm, 15 min, 4°C), and the supernatant was collected. The plasma samples were analyzed at the Clinical Veterinary Laboratory, College of Animal Science and Technology, Jiangxi Agricultural University. The plasma electrolyte potassium (K), sodium (Na), chlorine (Cl), magnesium (Mg), calcium (Ca), phosphorus (P) concentrations, and blood urea nitrogen (BUN), and creatinine (CR) contents were measured with an automatic biochemical analyzer (Johnson & Johnson, America). Liver indicators, such as the total protein (TP), albumin (ALB), globulin (GLB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphate (ALP), and glutamyltranspeptidase (GGT) levels were measured according to the methods of the International Federation of Clinical Chemistry (IFCC) (Santos, et al., 2002)
Determination of the Liver and Kidney Viral Loads with Reverse-Transcription Quantitative PCR (RT-qPCR)
Total RNA was extracted from the individual embryonic organs of 12 chickens (i.e., 12 livers and kidneys) per group via homogenizing with a homogenizer in RNA isolation reagent (Takara, Dalian, China) in low-temperature conditions according to the manufacturer's protocol. The RNA was diluted in nuclease-free water, and the concentration was quantitated by UV spectrophotometry at 260 nm. The integrity of the extracted RNA was confirmed by 0.8% agarose gel electrophoresis.
Before the reverse transcription reaction, all of the RNA samples were individually treated with genomic DNA elimination reagent according to the manufacturer's instructions to remove the genomic DNA (Takara, Dalian, China). For reverse transcription, 10 μL of total reaction mixture (1.0 μL of gDNA Eraser, 2.0 μL of 5× gDNA Eraser Buffer, 0.1 μg of total RNA, and 10 μL of RNase-free dH2 O) was incubated at 42°C for 2 min and stored at 4°C. The cDNA was synthesized via a reverse transcription reaction that was performed in a reaction volume of 20 μL that contained the following: the reaction solution formed from the genomic DNA elimination reaction (10 μL), RT Primer Mix (4.0 μL), 5× PrimeScript Buffer 2 (4.0 μL), and PrimeScript RT Enzyme Mix 1 (1.0 μL), RNase-free dH2 O (1.0 μL). The reaction was performed on a PCR instrumentf at 37°C for 15 min and 85°C for 15 sec and then stored at −80°C.
The nucleocapsid gene sequences from the SX9 strains of IBV were retrieved from GenBank (accession number JX273228). The sequences regarding the forward primer: 5′-TGCACCTGCACCTAAGTTTG-3′, reverse primer: 5′-AAACTGCGGGTCATCTCT-3′. The length of the constructed cDNA standards was 105 bp. The primers were synthesized by Shanghai Biological Engineering Co., Ltd. The RT-qPCR assays were performed in triplicate (Takara, Dalian, China). Total assay volume was 20 μL, which consisted of the quantitative PCR assay reaction mixtureg (10 μL SYBR Premix Ex Tap II (2×), 0.4 μL 50×ROX Reference Dye (50×), and RNase inhibitor, 1.5 mmol/L MgCl2, RNase-free water), each primer (at final concentration of 0.5 μmol/L), 2μL cDNA template. The thermal profile began with incubation at 95°C for 10 min, followed by 40 cycles of amplification alternating between 94°C for 15 s (denaturation) and 68°C for 40 s (annealing/extension). The SYBR Green I fluorescent signal was obtained once per cycle at the end of the extension step. Each RT-qPCR experiment contained triplicate no-template controls, test samples, and dilution series. The detection and quantification limit of the SYBR green real time RT-qPCR assay were determined using cycle-threshold (Ct) values obtained for each reaction containing from 109 to 103 copies of the standard RNA. Then the obtained Ct values were plotted against the amount of RNA copy number to construct standard curve. Results are expressed as viral load according to the established standard curve.
Statistical Analysis
The data were analyzed with the use of commercially available software. The viral load in each sample was quantified using the standard curve (data not shown) and the average viral genome copy number per group was calculated. Among the treatment groups (control and infected) were performed with the Student t tests multiple comparisons test to identify the significant differences. All data are presented as the mean ± the standard deviation (M ± SD), and values of P < 0.05 were considered significant, values of P < 0.01 were considered highly significant.
RESULTS
Anatomical Lesion Observations
No clinical signs or deaths were observed in the control groups, and some of the embryos in the infected group began to exhibit signs of death such as the presence of dwarf embryos (Figure 2 ). A tendency toward bleeding from the dead embryos appeared. After 3 dpi (ED16), the embryos exhibited stunting lesions, curling, leg compression upon the head, and urate deposition in the liver and kidney. Open egg shell examinations revealed that the dead embryos were atrophied, and embryonically enlarged kidneys were observed. In contrast, no visceral gout was observed in the embryos of the control groups. Conversely, some of the embryos in the infected group developed typical visceral gout. The livers were enlarged with white urate deposits, and the kidneys were swollen and exhibited pale regions in a greater proportion of the divisions. There were noticeable chalky white masses surrounding the liver and kidneys. The liver and kidneys exhibited significant granophyric characteristics.
Figure 2.
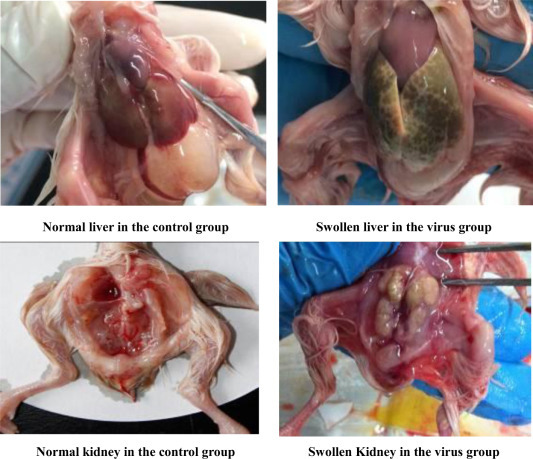
Liver and kidney were swollen in the virus group. The livers were enlarged with white urate deposits, and the kidneys were swollen and exhibited pale regions in a greater proportion of the divisions.
Liver and Kidney Weights
The embryo: egg weight (EE) ratios, liver weights, kidney weights, liver somatic indices, and kidney somatic indices (the liver and kidney parameters were normalized by the embryo weight) of the control and infected groups are given in Tables Table 1, Table 2 . The EE ratio was significantly lower in the infected group than in the control group at 5 dpi (P < 0.05). However, the liver weight was significantly higher in the infected group than the control group at 3 dpi (P < 0.05), and the kidney weight was significantly higher in the infected group than in the control group at 5 dpi (P < 0.05). The Hepatosomatic index (HSI) were significantly higher in the infected group than in the control group at 3 and 5 dpi (P < 0.05). The kidney Somatic Index (KSI) was significantly higher in the infected group than the control group at 5 dpi (P < 0.05).
Table 1.
Comparisons of the EE ratios, liver weights, kidney weights, HSI, and KSI challenged with NIBV.
| 1 dpi |
3 dpi |
5 dpi |
||||
|---|---|---|---|---|---|---|
| Control | Infected | Control | Infected | Control | Infected | |
| EE ratio (%) | 21.36 ± 3.12a | 19.41 ± 7.75a | 31.73 ± 3.62a | 28.60 ± 6.25b | 45.78 ± 6.32a | 40.16±9.49a |
| Embryo body weight (g) | 11.90 ± 1.57a | 12.48 ± 1.63a | 18.04 ± 2.09a | 16.77 ± 3.33a | 26.19 ± 3.14a | 23.88±5.50a |
| Liver weight (g) | 0.19 ± 0.03a | 0.19 ± 0.04a | 0.33 ± 0.05a | 0.36 ± 0.09a | 0.55 ± 0.08a | 0.59±0.09a |
| HSI (%) | 1.64 ± 0.18a | 1.53 ± 0.37a | 1.81 ± 0.25b | 2.22 ± 0.66a | 2.10 ± 0.28b | 2.65±0.85a |
| Kidney weight (g) | 0.057 ± 0.03a | 0.056 ± 0.01a | 0.11 ± 0.04a | 0.12 ± 0.04a | 0.15 ± 0.03b | 0.20±0.07a |
| KSI (%) | 0.47 ± 0.19a | 0.46 ± 0.11a | 0.62 ± 0.23a | 0.70 ± 0.19a | 0.59 ± 0.12b | 0.80±0.33a |
EE ratio (%) = weight of embryo (g)/weight of the respective egg (g).
HSI = hepatosomatic index = weight of liver (g)/weight of the embryo (g).
KSI = kidney somatic index = weight of kidney (g)/ weight of embryo (g).
The EE ratio, HIS and KSI in embryonic chickens infected with NIBV (n = 20). The numbers in the table indicate: mean ± standard deviation.
a,b The different letters indicate that the differences were significant (P < 0.05).
The same letters indicate that the differences were not significant (P > 0.05).
Table 2.
Analyses of the EE ratios, liver weights, kidney weights, HSI. and KSI challenged with NIBV.
|
P-value for significance test1 |
|||
|---|---|---|---|
| 1 dpi | 3 dpi | 5 dpi | |
| EE ratio (%)2 | 0.460 | 0.018* | 0.104 |
| Embryo body weight (g) | 0.272 | 0.184 | 0.119 |
| Liver weight (g) | 0.593 | 0.136 | 0.088 |
| Liver SI (%) | 0.279 | 0.019* | 0.010* |
| Kidney weight (g) | 0.938 | 0.725 | 0.019* |
| Kidney SI (%) | 0.836 | 0.296 | 0.012* |
,*P < 0.05; **P < 0.01
EE ratio (%) = weight of embryo (g)/weight of the respective egg (g); HSI = hepatosomatic index = weight of liver (g)/weight of the embryo (g); KSI = kidney somatic index = weight of kidney (g)/ weight of embryo (g).
Liver and Kidney Function
The plasma electrolyte values are provided in Table 3 . The plasma concentrations of P and K were significantly higher in the infected group than in the control group at 3 (P < 0.05) and 5 dpi (P < 0.01). The plasma concentrations of Na, Mg, and Cl in the infected group were significantly greater than those in the control group at 3 dpi (P < 0.05). The plasma concentrations of Ca in the infected group were significantly greater than those in the control group at 3 (P < 0.05) and 5 dpi (P < 0.01). In general, the plasma concentrations of P, K, Na, Mg, Cl, and Ca in the infected group exhibited significantly increased.
Table 3.
The plasma electrolytes concentration and kidney function parameters in embryonic chickens infected with NIBV (n = 10).
| 1 dpi |
3 dpi |
5 dpi |
||||
|---|---|---|---|---|---|---|
| Control | Infected | Control | Infected | Control | Infected | |
| Na(mmol/L) | 131.47 ± 4.52a | 133.42 ± 8.58a | 117.02 ± 13.45b | 130.29 ± 6.58a | 122.57 ± 10.45a | 129.50 ± 7.33a |
| Cl(mmol/L) | 109.21 ± 5.65a | 109.02 ± 9.44a | 89.80 ± 9.61B | 110.89 ± 6.44A | 97.46 ± 6.98a | 99.62 ± 5.93a |
| K(mmol/L) | 3.20 ± 0.22a | 3.39 ± 0.95a | 3.50 ± 0.76b | 5.19 ± 1.11a | 3.54 ± 0.24B | 4.9 ± 1.10A |
| Mg(mmol/L) | 0.58 ± 0.04a | 0.57 ± 0.07a | 0.59 ± 0.10b | 0.73 ± 0.13a | 0.70 ± 0.13a | 0.74 ± 0.17a |
| Ca(mmol/L) | 1.24 ± 0.24a | 1.34 ± 0.27a | 1.28 ± 0.15b | 1.53 ± 0.19a | 1.38 ± 0.31b | 1.66 ± 0.19a |
| P(mmol/L) | 1.38 ± 0.34a | 1.40 ± 0.37a | 1.27 ± 0.17b | 1.86 ± 0.46a | 1.12 ± 0.13B | 1.50 ± 0.33A |
| BUN(mmol /L) | 1.21 ± 0.34a | 1.52 ± 0.40a | 1.17 ± 0.24B | 2.06 ± 0.29A | 1.64 ± 0.31B | 2.80 ± 0.57A |
| CR(μmol /L) | 12.52 ± 1.51B | 26.39 ± 6.61A | 15.55 ± 3.15B | 26.87 ± 7.15A | 20.20 ± 4.22a | 22.72 ± 6.79a |
| UA(μmol /L) | 89.72 ± 6.34B | 1082.37 ± 185.04A | 191.71 ± 42.81B | 1834.28 ± 234.55A | 143.01 ± 12.53B | 1625.06 ± 250.87A |
Different superscript letters within the same row means between Control and Infected at the same day. (a, b, P < 0.05; A, B, P < 0.01).
The plasma UA, CR, and BUN activity values in the embryonic chickens were compared between the control and infected groups, and these values also exhibited significant differences (Table 3). The UA levels (P < 0.01) in the control group were much lower than those in the infected group at 1, 3, and 5 dpi. The CR values (P < 0.01) in the control group were much lower than those in the infected group at 1 and 3 dpi. The BUN levels at 3 and 5 dpi in the control group were significantly lower than those in the infected group (P < 0.01). The UA, CR, and BUN values of the infected group compared with those of the control group and exhibited significantly increased.
The function liver enzyme and blood parameters of the embryonic chickens that were challenged with NIBV are illustrated in Table 4 . The embryonic chickens that were infected with NIBV exhibited significantly increased plasma concentrations of AST and GGT at 3 and 5 dpi. The plasma concentrations of ALT and ALP were significantly increased in the infected group compared with the control group across the entire experimental duration, whereas the plasma TP and GLB concentrations were significantly decreased at 1 and 5 dpi. Compared with the control group, the infected group exhibited a significantly lower plasma ALB concentration at 1 dpi. The plasma ALT, AST, ALP, and GGT concentrations of the infected group were compared with those of the control group, which revealed significantly increased. The plasma TP, GLB, and ALB concentrations of the infected group were compared with those of the control group, which revealed significantly decreased.
Table 4.
The liver function parameters in embryonic chickens infected with NIBV (n = 10).
| 1st day |
3rd day |
5th day |
||||
|---|---|---|---|---|---|---|
| Control | infected | Control | Infected | Control | Infected | |
| ALT(U/L) | 1.83 ± 0.53b | 2.47 ± 0.74a | 2.29 ± 0.57b | 3.16 ± 1.13a | 1.20 ± 0.32b | 1.65 ± 0.53a |
| AST(U/L) | 24.52 ± 3.52a | 25.09 ± 4.59a | 23.86 ± 5.08B | 35.95 ± 8.77A | 30.93 ± 5.41B | 41.16 ± 6.62A |
| ALP(U/L) | 539.74 ± 70.72b | 668.62 ± 151.36a | 1266.02 ± 231.34B | 1727.96 ± 251.36A | 1994.31 ± 451.77B | 2695.00 ± 314.93A |
| GGT(U/L) | 0.66 ± 0.16a | 0.65 ± 0.22a | 1.26 ± 0.52B | 2.04 ± 0.58A | 1.67 ± 0.36b | 2.62 ± 0.77a |
| TP(g/L) | 6.27 ± 0.56A | 4.01 ± 1.06B | 7.92 ± 1.12a | 7.21 ± 1.55a | 10.29 ± 1.53a | 9.06 ± 1.04b |
| ALB(g/L) | 1.82 ± 0.19A | 1.10 ± 0.38B | 2.12 ± 0.43a | 2.01 ± 0.52a | 3.07 ± 0.53a | 2.74 ± 0.52a |
| GLB(g/L) | 4.45 ± 0.42A | 2.91 ± 0.69B | 5.80 ± 0.86a | 5.20 ± 1.06a | 7.22 ± 1.13a | 6.32 ± 0.62b |
Different superscript letters within the same row means between Control and Infected at the same day. (a, b, P < 0.05; A, B, P < 0.01).
Liver and Kidney Viral Load
The detection and quantification limit of the SYBR green real time RT-qPCR assay were determined using CT values obtained for each reaction containing from 109 to 103 copies of the standard RNA. The values were plotted against the log of the number of template copies and a linear equation (y = −3.1692x + 37.672) with an R2 value = 0.9965 was generated (Figure 3 ). The assay maintained linearity for at least seven orders of magnitude. Using the slope from the linear equation, the overall efficiency of the assay was estimated to be 100.02%.
Figure 3.
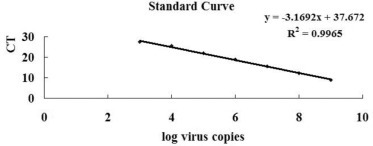
The assay standard curve was generated by plotting the Ct values and log10 of 10-fold serial dilutions (109–103) of standard RNA. An overall reaction efficiency of 100.02% was estimated using the standard curve slope as indicated by the formula.
In the infected group, the virus was detected in the livers and kidneys of the sets of 12 embryonic chickens that were killed at 1, 3, and 5 dpi. Results are expressed as viral load according to the established standard curve. The liver and kidney viral loads in the virus group peaked at 3 dpi (Figure 4 ), and the viral load was significantly higher for kidney than for liver at 3 dpi (P < 0.01). The liver and kidney samples from the embryonic chickens in the control groups were negative throughout the experiment.
Figure 4.
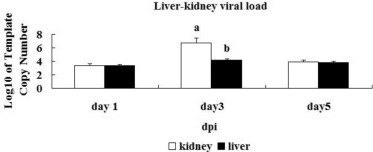
Liver and kidney viral load in embryonic chickens at 1, 3, and 5 dpi. The liver and kidney viral loads in the virus group peaked at 3 dpi, and the viral load was significantly higher for kidney than for liver at 3 dpi (P< 0.05).
DISCUSSION
The developing chicken embryo is indeed a valid and sensitive developmental research tool when used in a well-controlled experimental environment (Vorster and Lizamore, 2001). A previous study indicated that the EE ratio is an objective method for the quantification of embryo dwarfism in IBV-inoculated embryonated chicken eggs and can be used to accurately determine the endpoints of virus titration and neutralization assays (Dhinakar Raj, et al., 2004). In the studies of Wideman and Cowen (Wideman and Cowen, 1987), Gray-strain IBV decreases the relative kidney weight ratio. In our study, The EE ratios were significantly lower in the infected group than in the control group, and the HSI and KSI were significantly higher in the infected group than the control group. These findings indicate that challenge with NIBV decreased the body weight and induced liver and kidney swelling and hypertrophy. Our results also supported those of a previous study that observed nephritis in non-vaccinated flocks that was characterized by enlarged and pale kidneys, frequent urate deposits in the kidneys, severe dehydration, and weight loss, and typical signs, including embryo dwarfing and death, in embryos that were observed in different passages after challenge (Liu and Kong, 2004; Ziegler, et al., 2002). In the present study, the changes in embryo body weight, HSI and KSI reflected impaired development and liver and kidney damage following NIBV infection.
There are several electrolytes in the chicken body, and each performs a vital role. Balances of P, Cl, K, Na, Ca, and Mg are required for normal cellular metabolism and the maintenance of the physiological functions of the organs. However, neural-humoral regulation may affect the body fluids to ensure embryonic chicken health and prevent conditions that may result from fluid and electrolyte imbalances (Levy, et al., 1990; McLafferty, et al., 2014). In our study, the embryonic chickens infected with NIBV exhibited significant increases in the concentrations of Na and Cl at 3 dpi and significant increases in plasma K concentrations at 3 and 5 dpi. These results were likely due to NIBV infection-induced kidney damage and chronic glomerulonephritis in the chicken embryos (Afanador and Roberts, 1994; Cong, et al., 2013; Wideman, et al., 1983) that led to the simultaneous retention of Na, Cl, and water and acute intravascular hemolysis that caused intracellular K influx in the blood and induced hyperkalemia due to kidney tissue damage (Dote, et al., 2007; Hahn and Hahn, 2015). However, Crawford et al. reported that magnesium is the second most common intracellular cation after potassium and that approximately one-third of the magnesium in the extracellular fluid is bound to protein (albumin), while the remaining two-thirds are free (ionized). It is the ionized portion that is largely involved with the composition of bone and the metabolisms of several enzyme cofactors (Crawford and Harris, 2011). Our experiment suggests that the plasma Mg concentration in the infected group was significantly higher than that in the control group at 3 dpi. These results are likely due to the dysostosis caused by NIBV, and NIBV result in tissue cell injuries that lead to reductions in the syntheses of metabolic enzymes, which in turn leads to a decrease in the reabsorption of magnesium. The Ca and P levels in the control group were uniformly and significantly lower than those in the infected group at 3 and 5 dpi. For this reason, approximately 99% of the Ca and 80% of the P in the body exist in the skeleton (Zelenka, 2012). The dynamic distributions of the electrolytes Ca and P in the blood exhibited significant increases in the virus group likely because the maintenance of Ca and P is essential for forming the skeleton during the process of embryonic chicken growth. In the infected group, embryo development was blocked, and dysostosis resulted in the reduced absorption of Ca and P in the plasma. In conclusion, the infection of embryonic chickens with NIBV may result in the observed disturbances in plasma electrolytes.
The kidney is the most important excretory organ in terms of the elimination of drugs and their metabolites. The most common of several parameters of kidney function that are examined include the plasma concentrations of UA, BUN, and CR. Increased plasma BUN, CR, and UA levels have been reported to be markers of renal functional impairment in several previous studies (Duru, et al., 2008; Lumeij and Remple, 1991). Plasma UA levels are related to nephrotoxicity and kidney injury in broiler chickens (Kanbay, et al., 2010; Kanda, et al., 2015). We also found that plasma BUN, CR, and UA levels were significantly higher following NIBV administrations compared with the control group and resulted in kidney damage.
The liver plays a central role in the metabolism and excretion of xenobiotics, and thus the liver is highly susceptible to the adverse and toxic effects of these agents. A noticeable indication of hepatic damage is the leakage of cellular enzymes into the plasma (Giannini, et al., 2005). The estimation of plasma marker enzymes is useful for quantitative evaluations of the extent and type of hepatocellular damage in chickens (Giannini, Testa and Savarino, 2005; Kemal., et al., 2003). When the plasma membrane of a hepatocyte is injured, a variety of cytoplasmic enzymes are released into the circulation, and thus the enzyme levels in the plasma are increased. ALT, AST, and ALP in the plasma serve as indicators of liver function (Jaensch, et al., 2000). In our study, the plasma ALT, AST, and ALP levels in the infected group were significantly greater than those in the control group across the entire experimental period. We believe that NIBV infection caused liver damage in the embryonic chickens. These findings are in agreement with the severe congestion of the liver, spleen and lungs of birds that have died following experimental IBV infection (Abdel-Moneim, Zlotowski, Veits, Keil and Teifke, 2009). AST exists mainly in the hepatocellular mitochondria, and when damage or severe necrosis of the liver occurs, only the plasma AST concentration is elevated. The plasma GGT levels of the infected group did not differ significantly from those of the control group at 1 dpi but were significantly higher than the control group at 3 and 5 dpi. This pattern was observed because in the embryonic stages, GGT exists mainly in the intrahepatic bile duct epithelial cells and hepatic cytoplasm, and the development of the intrahepatic bile ducts in embryonic chickens is incomplete at 14 days old (at 1 dpi). Existing research has demonstrated that the concentrations of TP, ALB, and GLB in the plasma serve as indicators of liver function (Bernet, et al., 2001). TP includes both ALB and GLB. Changes in the levels of these parameters may explain the hypofunctioning of the hepatocytes after NIBV infections. GLB is related to immunity. ALB is made in the liver, and when the liver is damaged, ALB levels decrease (Toro, et al., 1997). Additionally, in our study, the liver TP, ALB, and GLB activities in the infected group were significantly lower than those in the control group. This pattern was observed because embryonic chickens infected with NIBV exhibit damage and functional lesions of the liver, and the synthesis of proteins is consequently reduced. In the present study, the changes in the plasma indicators reflected the liver damage that occurred after NIBV infection.
Real-time PCR is widely used to quantify viral genes; SYBR Green I-based real-time RT-PCR assay is a good method for determination of viral load (Acevedo, et al., 2013) and becomes a pivotal point for calculating the copy numbers of specific viral genes. In the infected group, NIBV was detected in the liver and kidney, which indicated that the chicken embryos had been infected with NIBV. These findings are in agreement with the IBV antigens were detected in the liver, kidney trachea, and lung of the birds tissues following experimental IBV infection. In our study, the highest detected levels occurred in the liver and kidney in the infected group. Based on separate analyses of the liver and kidney tissues, the amount of viral RNA in the liver peaked at 3 dpi, which indicated that the liver function of the embryonic chickens was most strongly influenced by NIBV infection at 3 dpi. Subsequently, the liver (along with many other vital organs) was exposed to danger. Due to stress and the lack of proper and steady nutrients, the liver can become prone to infection, malfunction, and jaundice and even cease to function. These findings agree with the above results of liver function, which revealed that the changes in liver function indicators reflected the most serious liver damage at 3 dpi. The amounts of RNA detected in the kidney were highest in the virus group, and the levels of RNA in the liver were lower than those in the kidney throughout the experiment. This pattern was observed because IBV strains vary greatly in terms of tissue tropism. NIBV peaked in the kidney at 3 dpi, and the viral load was significantly higher for kidney than for liver, which indicates that the kidney may be an important target of NIBV infection. The amounts of viral RNA in the kidney peaked at 3 dpi, which indicated that the embryonic chickens infected with NIBV exhibited the most severe kidney damage at 3 dpi. These findings are consistent with the functional kidney results. In the present study, liver and kidney damage were strongly related to the viral RNA levels in the tissues.
We conclude that embryonic chickens infected with NIBV exhibited decreased body weight, liver and kidney swelling and hypertrophy, and liver and kidney damage. Our study demonstrated that liver and kidney damage were strongly related to the viral loads of the tissues following nephropathogenic infectious bronchitis virus infection in embryonic chickens. These results suggest that embryonated chicken eggs can be used to partially elucidate the pathogenesis of NIBV in embryonic chickens.
Acknowledgments
This study was funded by National Natural Science Foundation of China awarded to Xiaoquan Guo (No. 31260627, 30860212), Jiangxi Young scientists target training program to Xiaoquan Guo (No. 20122BCB23022) and The Technology R&D Program of Jiangxi Province awarded to Xiaoquan Guo (No. 2010BNB00501).
Contributor Information
Xiaoquan Guo, Email: xqguo20720@aliyun.com.
Guoliang Hu, Email: hgljx3818@163.com.
REFERENCES
- Abdel-Moneim A.S., Zlotowski P., Veits J., Keil G.M., Teifke J.P. Immunohistochemistry for detection of avian infectious bronchitis virus strain M41 in the proventriculus and nervous system of experimentally infected chicken embryos. Virology journal. 2009;6:15. doi: 10.1186/1743-422X-6-15. doi 10.1186/1743–422X-6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul Hafeez M., Akhtar M., Hussain I. Protective effect of egg-propagated Eimeria tenella (local isolates) gametocytes as vaccine(s) against mixed species of coccidia in chickens. Parasitol. Res. 2006;98:539–544. doi: 10.1007/s00436-005-0113-8. [DOI] [PubMed] [Google Scholar]
- Acevedo A.M., Perera C.L., Vega A., Rios L., Coronado L., Relova D., Frias M.T., Ganges L., Nunez J.I., Perez L.J. A duplex SYBR Green I-based real-time RT-PCR assay for the simultaneous detection and differentiation of Massachusetts and non-Massachusetts serotypes of infectious bronchitis virus. Mol. Cell Probes. 2013;27:184–192. doi: 10.1016/j.mcp.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Afanador G., Roberts J.R. Effect of nephropathogenic infectious bronchitis viruses on renal function in young male broiler chickens. Br. Poult. Sci. 1994;35:445–456. doi: 10.1080/00071669408417709. [DOI] [PubMed] [Google Scholar]
- Alnassan A.A., Shehata A.A., Kotsch M., Lendner M., Daugschies A., Bangoura B. Embryonated chicken eggs as an alternative model for mixed Clostridium perfringens and Eimeria tenella infection in chickens. Parasitol. Res. 2013;112:2299–2306. doi: 10.1007/s00436-013-3392-5. [DOI] [PubMed] [Google Scholar]
- Bayry J., Goudar M.S., Nighot P.K., Kshirsagar S.G., Ladman B.S., Gelb J., Jr., Ghalsasi G.R., Kolte G.N. Emergence of a nephropathogenic avian infectious bronchitis virus with a novel genotype in India. J. Clin. Microbiol. 2005;43:916–918. doi: 10.1128/JCM.43.2.916-918.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet D., Schmidt H., Wahli T., Burkhardt-Holm P. Effluent from a sewage treatment works causes changes in serum chemistry of brown trout (Salmo trutta L.) Ecotoxicol. Environ. Saf. 2001;48:140–147. doi: 10.1006/eesa.2000.2012. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian pathology : journal of the W.V.P. A. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cong F., Liu X., Han Z., Shao Y., Kong X., Liu S. Transcriptome analysis of chicken kidney tissues following coronavirus avian infectious bronchitis virus infection. BMC genomics. 2013;14:743. doi: 10.1186/1471-2164-14-743. doi 10.1186/1471–2164-14–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K., Chesher J., Baxendale W., Greenwood N., Huggins M.B., Orbell S.J. Protection of chickens against renal damage caused by a nephropathogenic infectious bronchitis virus. Avian pathology : journal of the W.V.P. A. 2001;30:423–426. doi: 10.1080/03079450120066421. [DOI] [PubMed] [Google Scholar]
- Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian pathology : journal of the W.V.P. A. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- Crawford A., Harris H. Balancing act: hypomagnesemia & hypermagnesemia. NursingNursing. 2011;41:52–55. doi: 10.1097/01.NURSE.0000403378.71042.f0. [DOI] [PubMed] [Google Scholar]
- Dhinakar Raj G., Suresh Kumar K., Nainar A.M., Nachimuthu K. Egg:embryo weight ratio as an indicator of dwarfism induced by infectious bronchitis virus. Avian pathology : journal of the W.V.P. A. 2004;33:307–309. doi: 10.1080/0307945042000205883. [DOI] [PubMed] [Google Scholar]
- Dote E., Dote T., Shimizu H., Shimbo Y., Fujihara M., Kono K. Acute lethal toxicity, hyperkalemia associated with renal injury and hepatic damage after intravenous administration of cadmium nitrate in rats. J. Occup. Health. 2007;49:17–24. doi: 10.1539/joh.49.17. [DOI] [PubMed] [Google Scholar]
- Duru M., Nacar A., Yonden Z., Kuvandik G., Helvaci M.R., Koc A., Akaydin Y., Oksuz H., Sogut S. Protective effects of N-acetylcysteine on cyclosporine-A-induced nephrotoxicity. Ren. Fail. 2008;30:453–459. doi: 10.1080/08860220801985942. [DOI] [PubMed] [Google Scholar]
- Gaba A., Dave H., Pal J.K., Prajapati Isolation, identification and molecular characterization of IBV variant from out break of visceral gout in commercial broile. Veterinary World. 2010;3:375–377. [Google Scholar]
- Giannini E.G., Testa R., Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn L., Hahn M. Carvedilol-induced hyperkalemia in a patient with chronic kidney disease. Journal of pharmacy practice. 2015;28:107–111. doi: 10.1177/0897190014566306. [DOI] [PubMed] [Google Scholar]
- Jacobsen I.D., Grosse K., Slesiona S., Hube B., Berndt A., Brock M. Embryonated eggs as an alternative infection model to investigate Aspergillus fumigatus virulence. Infect. Immun. 2010;78:2995–3006. doi: 10.1128/IAI.00268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaensch S.M., Cullen L., Raidal S.R. Assessment of liver function in chickens using galactose and indocyanine green clearances. Avian pathology: journal of the W.V.P. A. 2000;29:109–116. doi: 10.1080/03079450094135. [DOI] [PubMed] [Google Scholar]
- Kanbay M., Solak Y., Dogan E., Lanaspa M.A., Covic A. Uric acid in hypertension and renal disease: the chicken or the egg?, Blood. Purif. 2010;30:288–295. doi: 10.1159/000321074. [DOI] [PubMed] [Google Scholar]
- Kanda E., Muneyuki T., Kanno Y., Suwa K., Nakajima K. Uric acid level has a U-shaped association with loss of kidney function in healthy people: a prospective cohort study. PLoS One. 2015;10:e0118031. doi: 10.1371/journal.pone.0118031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemal C., Muzaffer D., Türker S. Reduction of toxic effects of aflatoxin B1 by using bakeryeast (Saccharomyces cerevisiae) in growing broiler chicks diets. Revista Brasileira de Zootecnia. 2003;32:615–619. [Google Scholar]
- Kinde H., Daft B.M., Castro A.E., Bickford A.A., Gelb J., Jr., Reynolds B. Viral pathogenesis of a nephrotropic infectious bronchitis virus isolated from commercial pullets. Avian. Dis. 1991;35:415–421. [PubMed] [Google Scholar]
- Levy A., Perelman B., Grevenbroek M.V., Creveld C.V., Agbaria R., Yagil R. Effect of water restriction on renal function in ostriches (Struthio camelus) Avian pathology : journal of the W.V.P. A. 1990;19:385–393. doi: 10.1080/03079459008418688. [DOI] [PubMed] [Google Scholar]
- Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian pathology : journal of the W.V.P. A. 2004;33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeij J.T., Remple J.D. Plasma urea, creatinine and uric acid concentrations in relation to feeding in peregrine falcons (Falco peregrinus) Avian pathology : journal of the W.V.P. A. 1991;20:79–83. doi: 10.1080/03079459108418743. [DOI] [PubMed] [Google Scholar]
- Mahmood Z.H., Sleman R.R., Uthman A.U. Isolation and molecular characterization of Sul/01/09 avian infectious bronchitis virus, indicates the emergence of a new genotype in the Middle East. Vet. Microbiol. 2011;150:21–27. doi: 10.1016/j.vetmic.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLafferty E., Johnstone C., Hendry C., Farley A. Fluid and electrolyte balance. Nurs. Stand. 2014;28:42–49. doi: 10.7748/ns2014.03.28.29.42.e5531. [DOI] [PubMed] [Google Scholar]
- Montgomery R.D., Jones L.S., Boyle C.R., Luo Y., Boyle J.A. The embryo lethality of Escherichia coli isolates and its relationship to various in vitro attributes. Avian. Dis. 2005;49:63–69. doi: 10.1637/7211-052004R. [DOI] [PubMed] [Google Scholar]
- Santos R.L., Tsolis R.M., Baumler A.J., Adams L.G. Hematologic and serum biochemical changes in Salmonella ser Typhimurium-infected calves. Am. J. Vet. Res. 2002;63:1145–1150. doi: 10.2460/ajvr.2002.63.1145. [DOI] [PubMed] [Google Scholar]
- Slawinska A., Siwek M.Z., Bednarczyk M.F. Effects of synbiotics injected in ovo on regulation of immune-related gene expression in adult chickens. Am. J. Vet. Res. 2014;75:997–1003. doi: 10.2460/ajvr.75.11.997. [DOI] [PubMed] [Google Scholar]
- Toro H., Pavez E.F., Gough R.E., Montes G., Kaleta E.F. Serum chemistry and antibody status to some avian pathogens of free-living and captive condors (Vultur gryphus) of central Chile. Avian pathology : journal of the W.V.P. A. 1997;26:339–345. doi: 10.1080/03079459708419216. [DOI] [PubMed] [Google Scholar]
- Vorster W., Lizamore D.J. the avian embryo a developmental research tool. Netherlands Journal of Zoology. 2001;51:135–153. [Google Scholar]
- Wideman R.F., Jr., Cowen B.S. Effect of dietary acidification on kidney damage induced in immature chickens by excess calcium and infectious bronchitis virus. Poult. Sci. 1987;66:626–633. doi: 10.3382/ps.0660626. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Mallinson E.T., Rothenbacher H. Kidney function of pullets and laying hens during outbreaks of urolithiasis. Poult. Sci. 1983;62:1954–1970. doi: 10.3382/ps.0621954. [DOI] [PubMed] [Google Scholar]
- Worthington K.J., Currie R.J., Jones R.C. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian pathology : journal of the W.V.P. A. 2008;37:247–257. doi: 10.1080/03079450801986529. [DOI] [PubMed] [Google Scholar]
- Zelenka J. Allometric growth of calcium, phosphorus, magnesium, sodium, and potassium in slow-and fast-growing young chickens. Czech Journal of Animal Science. 2012;57:557–561. [Google Scholar]
- Ziegler A.F., Ladman B.S., Dunn P.A., Schneider A., Davison S., Miller P.G., Lu H., Weinstock D., Salem M., Eckroade R.J., Gelb J., Jr. Nephropathogenic infectious bronchitis in Pennsylvania chickens 1997–2000. Avian. Dis. 2002;46:847–858. doi: 10.1637/0005-2086(2002)046[0847:NIBIPC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]


