Abstract
Paraquat (PQ) is used as a herbicide in agriculture and causes oxidative and inflammatory damage to animal tissues. The current study was conducted to investigate the positive effects of dietary propolis (PR), as a potent naturally produced antioxidant, on growth performance and immune function of turkey poults exposed to oxidative stress induced by PQ injection. Native male turkey poults (n = 120, 49-d-old) were randomly assigned into 4 groups: poults received a basal diet with a daily subcutaneous PQ injection of 5 mg/kg BW for 7 consecutive days (PQ group), an experimental diet containing 1 g/kg PR with a daily subcutaneous PQ injection for 7 days (PR+PQ group), or received the experimental PR diet with a daily subcutaneous injection of 0.5 mL sterile saline for 7 days (PR group); while the control poults received a basal diet with a daily subcutaneous saline injection for 7 consecutive days (C group). The productive performance in the PQ group showed a significant (P < 0.05) reduction in the weight gain (WG) and feed intake (FI), and impaired feed conversion ratio (FCR). Propolis supplementation in the PR+PQ group significantly ameliorated the PQ effects on WG and FCR. Turkey poults of the PQ and PR+PQ groups had a significant augmentation in the blood malondialdehyde (MDA), tumor necrosis factor-α (TNFα), and corticosterone levels, and in contrast, a significant reduction in the triiodothyronine (T3), when compared to the C group. While propolis significantly reduced the MDA and corticosterone, and increased the T3 levels in the PR+PQ group compared to the PQ group. Furthermore, the dietary PR supplementation significantly limited the PQ-suppressive effects on cell- and humoral-mediated immunity and lymphocyte proliferation of turkey poults. In addition, propolis supplementation in the PR and PR+PQ groups markedly reversed the PQ-induced DNA fragmentation and heat shock protein 70 (Hsp70) over-expression in blood cells. It can be concluded that PR could improve turkey immunity and performance, particularly under inflammation and oxidative stress induced by PQ exposure.
Keywords: propolis, paraquat, growth performance, immune function, turkey poults
INTRODUCTION
Oxidative stress develops when the generation of reactive oxygen species (ROS) in a system exceeds the system's ability to neutralize and eliminate them. The imbalance can result either from a lack of antioxidant capacity caused by disturbance in production, distribution, or by an over-abundance of ROS from an environmental or behavioral stressor (Boelsterli, 2003). ROS could be produced by several agents and stressors such as heat stress, diseases, proinflammatory cytokines, and toxins. If not regulated properly, the excess ROS can impair the cell's lipids, protein, or DNA, inhibiting its normal functions (Gupta et al., 2012). DNA damage, malondialdehyde (MDA), proteins carbonyl are examples of molecules that can be modified by excessive ROS in vivo and could be used as important and significant biomarkers of oxidative stress (Ho et al., 2013). Furthermore, oxidative stress can activate the hypothalamic-pituitary adrenal axis (HPA), resulting in the release of glucocorticoids (Colaianna et al., 2013). High levels of corticosterone induce poor performance (Virden et al., 2007) and depression of the innate immune system (Yang et al., 2015). It was found that induced-oxidative stress in broiler chickens leads to proteolysis and gluconeogenesis (Lin et al., 2004), several pathologies incidence (Fellenberg and Speisky, 2006), DNA damage (Huang et al., 2015), and depression of immune function and growth performance with a high mortality rate (Kamel et al., 2017; Mehaisen et al., 2017). Depending on the severity of the oxidative harm, the consequence of these modulations can vary from modifying cell function to cell death, which negatively affects poultry flocks.
Paraquat (PQ; 1,1-dimethyl-4,4-bipyridium dichloride) is widely used in agriculture as a non-selective contact herbicide with redox activity. PQ is known to exert its toxic effects via oxidative stress mechanisms (Ray et al., 2007). The potential mechanism of PQ toxicity is the cyclic single-electron redox reaction that depletes cellular nicotinamide adenine dinucleotide phosphate (NADPH) and generates superoxide anion. Superoxide ions may form hydrogen peroxide and hydroxyl radicals; the latter being an extremely potent oxidant that may damage nucleic acids, proteins, and polysaccharides (Tukmechi et al., 2013). Moreover, trace amounts of PQ can be detected in more than 100 crops such as corn, tomatoes, olives, field beans, and fruits (Prasad et al., 2009). PQ effects in rodent models showed that prolonged exposure leads to accumulation and persistent damage in the brain, lung, and liver tissues (Ortiz et al., 2016). A study on paraquat toxicity in turkeys reported that all injected birds were affected at the dose of 6.25 mg/kg BW while the lethal dose was about 20 mg/kg and 100 mg/kg by intravenous and intraperitoneal injection, respectively (Smalley, 1973).
Propolis (PR) is an adhesive resinous material made by honey bees and it contains a variety of chemical compounds such as poly-phenols (flavonoid aglycones, phenolic acids and their esters, phenolic aldehydes, alcohols, and ketones), terpenoids, steroids, amino acids, and inorganic compounds (Newairy and Abdou, 2013). Flavonoid and phenolic compounds in propolis have been appeared to be capable of scavenging free radicals and thereby defending lipids and other compounds from being oxidized or destroyed during oxidative damage (Seven et al., 2009). In addition, propolis is thought to be responsible for many biological and pharmacological activities including anticancer, anti-inflammatory, anti-bacterial, antifungal, antiviral, antioxidant, hepato-protective, and immuno-stimulating activities (Haščík et al., 2015). Many previous reports indicated that the inclusion of propolis in the poultry diet has a positive effect on the humoral immunity of laying hens (Cetin et al., 2010; Freitas et al., 2011), on the hatchability and performance of quail chicks (Aygun et al., 2012), and on the hemoglobin concentrations and eosinophil count of blue-fronted parrots (Silva et al., 2014).
Based on PQ actions, it is a good and well-documented agent to induce oxidative stress when there is a need to provide a better understanding of this type of stress on poultry production. On the other hand, much research is focused on the ability of propolis to improve production performance, enhance immune function, and inhibit inflammatory response, which is very critical for poultry industry. However, it is not clear whether propolis supplementation could also reverse the inflammatory status and the negative effects of oxidative stress induced by paraquat treatment. Thus, the current study was designed to investigate the effects of propolis supplementation on controlling the oxidative stress induced by paraquat injection to turkeys. The oxidative stress was determined as the level of MDA, which is an important and significant biomarker of oxidative stress, in the blood. In addition, the inflammation status and immune function were evaluated in turkey poults after propolis supplementation with or without paraquat injection. Furthermore, DNA fragmentation test and stressed-related protein expression of heat shock protein 70 (Hsp70) were analyzed in the blood. In addition, growth performance of turkey birds was obtained under propolis supplementation in order to test whether it has the ability to reverse the negative effects of oxidative stress induced by paraquat.
MATERIALS AND METHODS
Birds and Diet
One hundred and twenty, 49-d-old, male Baladi turkey poults were used in this investigation. Baladi turkey is an Egyptian native breed that is characterized by low growth performance and high immune response. The turkey poults were reared in an opened-house with feed and water ad libitum. A basal diet was formulated according to the recommendations of the Regional Center for Food and Feed in Egypt to meet the local needs and was used as a control diet in the present study. The composition and the calculation analysis of the basal diet are presented in Table 1. Propolis was mixed into the basal diet to produce experimental diet containing 0.10% propolis (1 g propolis/kg diet).
Table 1.
Ingredients and nutrient composition of the experimental basal diet.
| Ingredients | % | Nutrient composition | Levels |
|---|---|---|---|
| Yellow corn | 59.50 | ME (MJ/Kg) | 12.052 |
| Soybean meal (44%) | 31.80 | Crude protein (%) | 21.044 |
| Fish meal | 2.00 | Calcium (%) | 0.971 |
| Corn gluten meal | 2.00 | Phosphorus available (%) | 0.454 |
| soybean oil | 1.00 | Lysine (%) | 1.148 |
| Limestone | 1.20 | Methionine (%) | 0.504 |
| NaCl | 0.26 | ||
| CaHPO4 | 1.60 | ||
| Premix1 | 0.28 | ||
| DL-Methionine | 0.16 | ||
| Lysine | 0.10 | ||
| Coline chloride | 0.10 | ||
| Total | 100.00 |
1Premix per kg contains 48 × 106 IU vitamin A, 12 × 106 IU vitamin D3, 100 mg vitamin E, 15 mg vitamin K, 10 mg vitamin B1, 34 mg vitamin B2, 15 mg vitamin B6, 80 mg vitamin B12, 500 mg biotin, 4.5 g folic acid, 150 g niacin, 50 g pantothenic acid, 200 mg ethoxyquin, 4 g Cu, 175 mg I, 50 g Fe, 50 g Mn, 200 mg Se, and 37.5 g Zn.
Ethical Issues
The experimental protocols were approved and carried out according to the regulation and guidelines set by Cairo University Ethics Committee for the Care and Use of Experimental Animals in Education and Scientific Research (CU-IACUC).
Propolis Analysis
Propolis was collected from an apiary located at the Faculty of Agriculture, Cairo University (Giza province, Egypt). The collected propolis was kept in a clean, dark bottle at 4°C until use in the experiment. Phenolic acids and flavonoid contents were analyzed in a propolis sample using high-performance liquid chromatography (HPLC). HPLC was achieved on an Agilent 1260 Infinity HLPC Series (Agilent, Santa Clara, CA) equipped with Quaternary pump, Zorbax Eclipse plus C18 column 150 mm × 4.6 mm internal diameters, 5 μm particle (Agilent), operated at 25°C. The separation was achieved using a ternary linear elution gradient with HLPC grade water 0.2% H3PO4 (v/v), methanol and acetonitrile. The injected volume was 20 μL and the variable wavelength detector (VWD) was set at 284 nm (Ivanauskas et al., 2008). The free radical scavenging activity of propolis samples was measured according to methods described by Oktay et al. (2003). Briefly, the propolis was added to a solution of 0.1 mM of 1,1-diphenyl-2-picryl-hydrazil (DPPH) in methanol at different concentrations (25 to 75 μg/mL). The mixtures were shaken vigorously and allowed to stand at room temperature for 30 min. Then, the absorbance of reactions was measured using an automatic scanning spectrophotometer at 517 nm. The chemical characteristics of propolis used in this experiment are presented in Table 2.
Table 2.
Chemical characteristics of propolis.
| Item | Mean value |
|---|---|
| Phenolic acids (μg/mL) | 180.89 |
| Flavonoids (μg/mL) | 188.90 |
| Free radical scavenging activity (%) | 83.3 |
Experimental Design
At 49 d of age, 120 turkey poults were randomly allocated into one of 4 experimental groups (three pens per group × 10 poults in each pen). The first group of poults served as a control, received the control diet and a single dose of 0.5 mL sterile saline per day for 7 consecutive days through subcutaneous route (C group). The second group received the control diet and a single subcutaneously injected of paraquat (Sigma Aldrich, St. Louis, MO), 5 mg/kg body weight, per day for 7 consecutive days (PQ group). The third group received the experimental diet containing propolis and a daily subcutaneous injection of paraquat for a period of 7 days (PR+PQ group). The fourth group received the experimental propolis diet and a daily subcutaneous injection of saline for a period of 7 days (PR group). At 56 d of age, blood samples were collected from the brachial vein of the birds in each treatment using heparinized syringes and the physiological parameters were assayed (2 samples from each pen for each parameter; n = 6), including the MDA and tumor necrosis factor-α (TNFα) levels in the peripheral blood mononuclear cells (PBMCs) isolated from blood, as well as the corticosterone and triiodothyronine (T3) hormone concentration in the plasma. The immune function of turkey poults in each group was determined by analyzing total white blood cells (WBCs), heterophil to lymphocyte (H/L) ratio, toe web swelling in response to phytohemagglutinin-P (PHA-P) injection as an indicator for the cell-mediated immunity, antibody response to sheep red blood cells (Anti-SRBCs Ab) injection as an indicator of the humoral-mediated immunity, and the stimulation index of peripheral T-lymphocyte proliferation. Furthermore, the DNA fragmentation and blotting expression of Hsp70 were analyzed in PBMCs isolated from blood samples in each group. In addition, the productive performance was obtained for each treatment group as will be mentioned later.
Productive Performance
The initial and final body weights were recorded individually at the beginning and at the end of the experiment (49 and 56 d of age). The entire weight gain was determined for each group. Average daily feed intake was measured for each treatment group. The feed conversion ratio was calculated for each group.
Physiological Parameters
MDA and TNFα Levels in PBMCs
At 56 d of age, blood samples from different birds (n = 6) were collected from each treatment. PBMCs were isolated using histopaque-1077 (Sigma Aldrich, St. Louis, MO) as previously described (Mehaisen et al., 2017). The cells were washed twice using Roswell Park Memorial Institute (RPMI) 1640 Medium (GIBCOTM, Thermo Fisher Scientific, Waltham, MA), then re-suspended with PBS (PH 7.2), and the numbers were adjusted to be 106 cells/mL. A 1 mLcell suspension was centrifuged at 1,030 × g for 20 min at 4°C. The pellets were collected and stored at −70°C until processing. The cells were re-suspended with 1 mL PBS, kept on ice for 60 sec, and then sonicated for 1 min. The homogenates were centrifuged at 1,030 × g for 15 min at 4°C and supernatants were collected to determine MDA and TNFα levels. The level of MDA in the supernatant was determined by the thiobarbituric acid reaction method using a commercial assay (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The level of TNFα in the supernatant was measured using chicken ELISA commercial diagnostic kit (cat# WAC-016, WKEA MED SUPPLIES CORP, Changchun, China). The standard curves and calculations were performed following the kits protocol for each analysis.
Plasma Corticosterone and T3 Hormone Assay
At 56 d of age, blood samples from each group were collected (n = 6), centrifuged at 2,000 × g for 10 min at 4°C, and then plasma was separated and stored at −20°C. Plasma corticosterone and T3 concentrations were determined in duplicates using chicken ELISA kits (cat# MBS701668 for corticosterone and cat# MBS701857 for T3; MyBioSource, San Diego, CA). The intra- and inter-assay coefficient of variations was <8% and <10%, respectively for corticosterone, and was <15% for T3. The dynamic range of the assay was 0.5 to 20 and 0.5 to 8 ng/mL for corticosterone and T3, respectively.
Immunological Parameters
Total WBC Count
Total leukocytes were performed manually in 6 samples per group as described by Gehad et al. (2008). Briefly, 490 μL of brilliant cresyl blue dye was mixed with 10 μL whole blood samples, and the total leukocytes were counted using a hemocytometer.
H/L Ratio
H/L ratio was determined manually according to Zhang et al. (2009). In brief, blood smears (6 slides per group) were fixed and stained using Hema-3 (cat# 22–122,911, Fisher Scientific, Pittsburg, PA). The differential leukocyte counts were performed for a total of 200 leukocytes in each slide using light microscope and the H/L ratios were then calculated.
Toe Web Swelling
The cell-mediated immune response was assessed using the swelling of toe web that induced by intradermal mitogen injection of PHA-P. At 56 d of age, the left foot of six turkey poults from each group was injected with 100 μg of PHA-P dissolved in 0.1 mL of sterile PBS buffer into the toe web between the third and fourth digits. The thickness of toe web was measured before injection and 24 h after injection. The toe web swelling response was expressed in mm as the difference between the thickness before and after injection (Loa et al., 2001).
Anti-SRBCs AB. The humoral-mediated immune response was assessed by evaluating the antibody production against SRBCs. Six turkey poults from each group were injected intravenously with 1 mL of 5% saline suspension of SRBCs at 49 d of age. One wk following the injection (d 56), blood samples was collected and the antibody production against SRBCs was determined by microhemagglutination technique (Loa et al., 2001). Antibody values were expressed as log2 of the reciprocal of the highest dilution where visible agglutination was observed.
Peripheral T-Lymphocyte Proliferation
The PBMCs layer were carefully isolated from 6 heparinized blood samples obtained from each group according to the method described by Mehaisen et al. (2017). After washing in RPMI 1640 culture medium, the viable lymphocytes were detected using Trypan Blue dye and plated in triplicate wells (96-well plate) at 6 × 106 cells per well. Then, 50 μL of Concanavalin-A (Sigma Aldrich) at 45 μg/mL was added to selected wells to induce the proliferation of T lymphocyte; while control wells received 50 μL of RPMI-1640 medium. After cells’ incubation (41°C and 5% CO2 for 68 h), 15 μL of 5 mg/mL 3-[4,5-dimethylthiazol]-2,5-diphenyltetrazolium bromide (Sigma Aldrich) was added to each well and incubated for another 4 h. Subsequently, 100 μL of 10% sodium dodecyl sulfate dissolved in 0.04 M HCl solution was added to each well, then the plates were read using an automated ELISA reader (model 550 Microplate Reader, Bio-Rad Laboratories, Inc., Hercules, CA) at 570 nm. The stimulation index for T-lymphocyte proliferation was calculated as the optical density ratio of experimental group to blank control.
DNA Fragmentation Test and Hsp70 Expression in PBMC’s
The PBMCs were collected from blood samples (6 samples for each group) as mentioned previously. The DNA isolation was performed using the cells/tissue genomic DNA extraction kit (cat# GK0121, Generay Biotech Co., Ltd., Shanghai, China), according to the manufacturer's instructions. DNA fragmentation was analyzed by 2% agarose gel (Sigma-Aldrich) electrophoresis for 1 h at 80 V. Visualization of DNA was analyzed by ethidium bromide fluorescence using a UVP EC3 Imaging system (UVP Inc., Upland, CA) with VisionWorksLs Image Acquisition and Analysis software (version 5.5.3; UVP Inc., Upland, CA).
The expression of Hsp70 in the collected PBMCs (6 samples per group) was analyzed by Western blot technique. The total protein (40 μg) was loaded and separated on 12% polyacrylamide gel containing sodium dodecyl sulphate (SDS-Page). Separated proteins were then transferred to poly-vinylidene difluoride membranes using a tank transfer for 2 h at 300 mA in Tris-glycine buffer containing 20% methanol. Membranes were blocked with 5% skim milk for 1 h and incubated overnight at 4°C with diluted primary anti-rabbit IgG polyclonal antibody against Hsp70 (1:1,000; Cell Signaling Technology, Inc., Danvers, MA), followed by a horse radish peroxidase conjugated secondary antibody against rabbit IgG (1:1,500; Santa Cruz Biotechnology, Inc., Dallas, Texas). To verify equal loading of samples, the membrane was incubated with monoclonal β-actin antibody (1:1,000; Santa Cruz Biotechnology, Inc.), followed by a horse radish peroxidase conjugated goat anti-mouse IgG (1:1,000; Santa Cruz Biotechnology, Inc.). Hsp70 was detected using the ECL chemiluminescence kit (GE Healthcare Life Sciences, Amersham Place, Little Chalfont, Buckinghamshire, UK).
Statistical Analysis
A general linear model was performed using SAS software (SAS Institute Inc., 2011) to determine statistical differences between treatment groups (C, PQ, PR+PQ, and PR) for the productive traits, physiological parameters, and immunological parameters. Significant treatment effects were detected by Duncan's multiple range tests. Results are expressed as LSM ± SE and the significance level was set at P < 0.05.
RESULTS
Productive Performance
The results reveal that turkey poults in the PQ group had significantly (P < 0.05) reduced weight gain and feed intake compared to turkey poults in the C group (Table 3). In addition, the feed conversion ratio also in the PQ group was impaired significantly (P < 0.05) compared to the C group. In contrast, the dietary propolis supplementation alleviated these reductions in weight gain and feed intake in the PR+PQ group when compared to the PR group. Furthermore, administration of propolis to the diets of the PR group significantly (P < 0.05) enhanced the weight gain compared to the C group. Even though, the feed conversion ratio tended to be improved when propolis was supplemented to the diets of both PR+PQ and PR groups; the differences were not significant (P > 0.05) when compared to the C group (Table 3).
Table 3.
Effect of paraquat injection and dietary propolis supplementation on productive performance in the different turkey treatment groups.1
| Parameters | C | PQ | PQ+PR | PR |
|---|---|---|---|---|
| Weight gain (g/bird) | 354.4 ± 24.53b | 142.8 ± 11.83d | 211.6 ± 20.53c | 426.3 ± 31.23a |
| Daily feed intake (g/bird) | 145.8 ± 10.30a | 78.7 ± 6.75b | 90.7 ± 8.34b | 157.3 ± 13.86a |
| Feed conversion ratio | 2.8 ± 0.38b,c | 3.9 ± 0.15a | 3.0 ± 0.11b | 2.6 ± 0.20c |
a–dMeans within the same row with different letters are significantly different (P < 0.05).
1Treatment groups: C, control group; PQ, the group received a basal diet and a 7 consecutive day injection of paraquat; PQ+PR, the group received a 7 consecutive day injection of paraquat and a diet supplemented with propolis; and PR, the group received a basal diet supplemented with propolis.
Physiological Parameters
The change in MDA and TNFα levels in PBMCs as well as the plasma corticosterone and T3 concentrations after paraquat injection with or without dietary propolis supplementation in turkey poults are shown in Table 4. Compared with the control group, PQ injection significantly (P < 0.05) increased the MDA, TNFα, and the plasma corticosterone levels in both PQ and PR+PQ groups. However, adding propolis to the basal diet minimized (P < 0.05) the MDA level and corticosterone concentration in the PR+PQ group compared to the PQ group. In contrast, the plasma T3 concentration was significantly (P < 0.05) lower in the PQ group and higher in the PR group when compared to the C group. Moreover, dietary propolis supplementation successfully alleviated the negative effect of PQ injection and significantly (P < 0.05) raised the value of plasma T3 concentration in the PR+PQ group, however, it remained lower than that of the C group (Table 4).
Table 4.
Effect of paraquat injection and dietary propolis supplementation on some physiological parameters in the different turkey treatment groups (n = 6).1
| Parameters | C | PQ | PQ+PR | PR |
|---|---|---|---|---|
| MDA (μM/mL) | 1.1 ± 0.16c | 4.0 ± 0.88a | 2.4 ± 0.41b | 1.1 ± 0.04c |
| TNFα (pg/mL) | 98.2 ± 8.57b | 177.3 ± 21.65a | 152.3 ± 15.69a | 103.7 ± 14.28b |
| Corticosterone (ng/mL) | 2.7 ± 0.18c | 10.9 ± 0.66a | 6.2 ± 0.34b | 2.4 ± 0.13c |
| T3 (ng/mL) | 1.9 ± 0.12b | 0.5 ± 0.09d | 1.3 ± 0.19c | 2.7 ± 0.23a |
a–dMeans within the same row with different letters are significantly different (P < 0.05).
1Treatment groups (n = number of birds per group): C, control group; PQ, the group received a basal diet and a 7 consecutive day injection of paraquat; PQ+PR, the group received a 7 consecutive day injection of paraquat and a diet supplemented with propolis; and PR, the group received a basal diet supplemented with propolis.
Immunological Parameters
The effects of dietary propolis and paraquat injection on the immune function are presented in Table 5. Total WBCs were significantly (P < 0.05) lower in the PQ group than in the C group. Meanwhile, dietary propolis supplementation significantly (P < 0.05) increased the total WBCs in the PR group compared to the C group. Not only that, but it also reversed the negative effect of PQ injection by significantly (P < 0.05) raising the total WBCs in the PR+PQ group as compared to the PQ group. Furthermore, the H/L ratio was significantly (P < 0.05) increased in response to oxidative stress induced by paraquat injection in the PQ group compared to the C group. Nevertheless, propolis supplementation significantly (P < 0.05) limited the increasing rate of H/L ratio due to PQ injection in the PR+PQ group compared to the C group (Table 5). In addition, oxidative stress induced by paraquat injection significantly (P < 0.05) reduced the toe web swelling in response to PHA injection, antibody titers to SRBCs, and T-lymphocyte proliferation in the PQ group poults compared to the C group poults (Table 5). In contrast, adding propolis to the basal diet significantly (P < 0.05) alleviated the negative effect of paraquat injection on the previous immune parameters in the PR+PQ group compared to the C group. Moreover, supplementing the basal diet with propolis in the PR group significantly (P < 0.05) enhanced the T-lymphocyte proliferation compared to the C group (Table 5).
Table 5.
Effect of paraquat injection and dietary propolis supplementation on some immunological parameters in the different turkey treatment groups (n = 6).1
| Parameters | C | PQ | PQ+PR | PR |
|---|---|---|---|---|
| Total WBCs (×103/μL) | 45.3 ± 1.79b | 25.6 ± 1.51c | 51.8 ± 6.79a,b | 59.5 ± 3.02a |
| H/L ratio | 0.5 ± 0.04c | 1.7 ± 0.16a | 1.0 ± 0.08b | 0.5 ± 0.07c |
| Toe web swelling (mm) | 0.8 ± 0.03a | 0.6 ± 0.04c | 0.7 ± 0.05b | 0.8 ± 0.04a,b |
| Anti-SRBC Ab (log2) | 5.3 ± 0.56a | 3.2 ± 0.31c | 4.3 ± 0.33b | 6.2 ± 0.47a |
| Stimulation index of T-lymphocyte proliferation | 3.0 ± 0.12b | 1.2 ± 0.29d | 2.3 ± 0.18c | 3.8 ± 0.28a |
a–dMeans within the same row with different letters are significantly different (P < 0.05).
1Treatment groups (n = number of birds per group): C, control group; PQ, the group received a basal diet and a 7 consecutive day injection of paraquat; PQ+PR, the group received a 7 consecutive day injection of paraquat and a diet supplemented with propolis; and PR, the group received a basal diet supplemented with propolis.
DNA Fragmentation and Hsp70 Expression in PBMC’s
DNA fragmentation test showed appearance of short DNA fragments in response to PQ injection (Figure 1). Meanwhile, the dietary propolis supplementation to the turkey poults reduced the DNA fragmentation in either the PR+PQ or the PR group to a normal level similar to the control group. Furthermore, Hsp70 blotting level was influenced by the PQ injection and dietary propolis supplementation (Figure 2). An over-expression of Hsp70 was observed in the turkey poults of the PQ group compared to the C group. However, data of Hsp70 expression in both the PR and PR+PQ groups show that propolis supplementation to the diets of turkey poults normalized the Hsp70 expression to similar levels of the C group.
Figure 1.
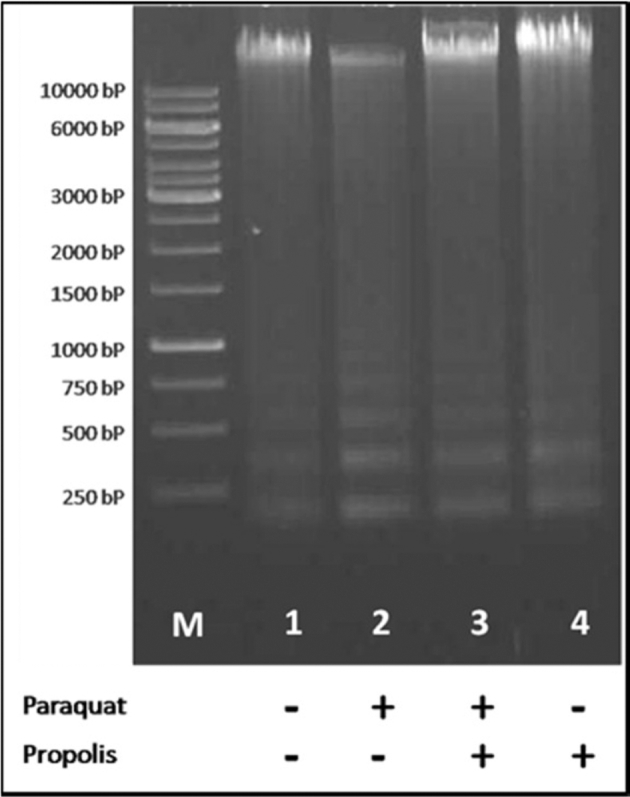
DNA fragmentation as a response to paraquat injection (5 mg/kg BW) and dietary propolis supplementation (1 g/kg diet) in the different turkey treatment groups.
Figure 2.
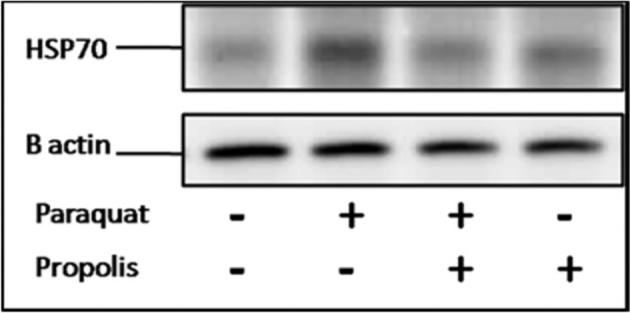
Heat shock protein 70 (Hsp70) expression as a response to paraquat injection (5 mg/kg BW) and dietary propolis supplementation (1 g/kg diet) in the different turkey treatment groups.
DISCUSSION
Paraquat is widely used as an herbicide in agriculture and may cause chronic health problems to animals and humans if it is absorbed through the skin or gastrointestinal and respiratory tracts (Ortiz et al., 2016). The deleterious effects of PQ are mainly attributed to the extreme redox activity that, in turn, induces extensive damage for several organs and tissues, leading to high mortality rates in human (Lin et al., 2006; Kang et al., 2013) and animal populations (Ray et al., 2007; Tukmechi et al., 2013). On the contrary, propolis, due to its antioxidation and anti-inflammation properties, has been recently used in poultry feeds as an alternative and practical way to alleviate deleterious effects of rearing under abnormal and stress conditions (Seven et al., 2009, 2010). So far, the research regarding PQ and PR effects on growth performance and immune function in animal science is very limited and the available discussion remains insufficient to understand such effects.
In the present study, it was found that PQ significantly suppressed weight gain and feed intake, and impaired feed conversion ratio by 60%, 46%, and 39%, respectively, compared to the control poults. A similar low growth performance was obtained by feeding paraquat to fish (Babatunde and Oladimeji, 2014) or rats (Kimura et al., 1999). The low performance after paraquat treatment is attributed to the oxidative stress that may have been due to active oxygen species formed by the action of paraquat. Previous reports attributed the low performance in oxidative stressed-broilers to the reduction in associated metabolic and endocrine responses (Lin et al., 2004) or the down-regulation of all sugar, peptide, and amino acid transporter genes in the small intestine (Ebrahimi et al., 2015). On the other hand, dietary propolis ameliorated the suppression of weight gain, feed intake, and feed conversion in turkey poults that were exposed to PQ injection. Furthermore, administration of propolis to the diets of the turkey poults without PQ stress enhanced the weight gain by 20% compared to the control poults. This enhancement may probably be due to the ability of propolis to improve nutrients digestibility and absorption as a result of improving saccharase, amylase, and phosphatase activities (Mutsaers et al., 2005). Additionally, Shalmany and Shivazad (2006) reported that the increase in feed intake could be linked to the palatable substances in propolis like resin, wax, honey, and vanillin. Furthermore, Kačániová et al. (2012) found that flavonoids in propolis have antibacterial activity and prevent the ability of pathogenic bacteria to attach to the intestinal epithelium (Parkar et al., 2008); thus improving intestinal health, and consequently, enhance digestion and nutrient absorption (Denli et al., 2005).
MDA is one of the final products of lipid peroxidation and could be used as a direct indicator of oxidative stress induced by potential oxidants like paraquat in the present study. The significant increase in MDA levels upon treatment with PQ in turkey poults indicates the oxidative damages occurred to PBMCs. These results are consistent with the finding that various concentrations of PQ increase the cellular peroxidation (Zhang et al., 2010). When propolis was supplemented to the diets of turkey poults, a reduction in MDA level was obtained in the PR+PQ group compared to the PQ group. Previous studies reported that the supplemental antioxidants extracted from propolis, such as flavonoids and caffeic acid phenethyl ester, block ROS production and protect cell membranes against oxidants (Seven et al., 2010). It was also suggested that polyphenols of propolis can reduce the negative effects of oxidative stress either by chelation of iron or by free radical trapping (Thirugnanasampandan et al., 2012).
It was reported that TNFα is one of the cytokines involved in the early inflammatory phase of PQ poisoning (Amirshahrokhi, 2013) through the ROS pathway. PQ-induced ROS accumulation could promote the production of TNFα to trigger the tissue injury; and reversely, increased TNFα concentration could promote further ROS generation (Edwards et al., 2004). Results of the present study demonstrate that TNFα concentration in the PBMC’s was higher in the PQ and PR+PQ groups compared to the C group. The positive effect of propolis was not statistically significant in this study, however, it is well documented that propolis supplementation may attenuate the adverse effects of environmental stress and stress induced tissue damage by modulating the levels of cytokines such as TNFα (Fitzpatrick et al., 2001; Hu et al., 2005). In addition to the increase in the TNFα concentration in the PBMC’s, PQ injection also induced a significant high plasma corticosterone concentration. This high corticosterone levels probably decreased feed intake and reduced intestinal absorptive surface area as reported by Hu et al. (2010). Furthermore, the release of corticosterone and inflammatory cytokines exerted catabolic effects on proteins and lipids (Siegel, 1995). These findings may be responsible for the lower body weight gain in the PQ poults as previously reported in the present study.
In addition, the thyrotrophic [triiodothyronine (T3) and thyroxine (T4)] axis is considered to be prerequisites for normal growth and development (Decuypere et al., 1983). It is shown in the current study that turkey poults of PQ group had significant reduction in the plasma T3 concentration compared to the C group. Steenland et al. (1997) reported an increased hypothyroidism in the people intoxicated with paraquat herbicide and found detectable levels of paraquat in their thyroid glands. Goldner et al. (2010) observed thyroid adenomas in rats exposed to paraquat and suggested that the thyroid could be susceptible to the effects of paraquat. In contrast, the plasma T3 was significantly higher in the PR group than in the C group. The positive effects of propolis on T3/T4 ratio was previously reported in broilers reared under heat stress condition (Amen et al., 2015), reflecting improvement in thyroid hormones secretions and higher conversion rate to the active form of thyroid hormone which is responsible for metabolism. The current study also demonstrates that dietary propolis supplementation significantly increased plasma T3 concentration in the PR+PQ group compared to PQ group which, consequently, alleviated the negative effect of paraquat injection on the growth performance of turkey poults.
The immunological data obtained in the current work show that PQ injection impaired the overall immune function of turkey poults. In PQ group, the H/L ratio was increased, while the total WBCs, the toe web swelling in response to PHA injection, antibody titers to SRBCs, and T-lymphocyte proliferation were significantly decreased compared to the C group. These results are consistent with the data presented by Riahi et al. (2010) who concluded that paraquat administration at high dose (intra-peritoneal injection of 1 mg/kg BW for 21 d) in mice has an inhibitory effect on the cell-mediated and humoral immunity; including higher neutrophil cells number, and lower hemagglutination antibody titer and lymphocyte proliferation. The increase in H/L ratio in the PQ group may be due to the inflammation (Dinis-Oliveira et al., 2008), which was represented by corticosterone and TNFα elevation in the same group. In a recent study, Mehaisen et al. (2017) reported that corticosterone treatment has immunosuppressive effect in broiler chickens. In addition, free radicals are known to attack unsaturated fatty acid side chains of phospholipids, causing a substantial decrease of the membrane integrity and fluidity of immune cells (Smith and Heath, 1974). It is possible that free oxygen radicals generated by paraquat could inhibit T cell proliferation via membrane lipids peroxidation (Riahi et al., 2010). In contrast, dietary propolis supplementation enhanced the total WBCs and T-lymphocyte proliferation in the PR group compared to the C group. Not only that, but it also reversed the negative effect of paraquat injection on the immune function in the PR+PQ group. These positive effects of PR and its ability to alleviate the PQ effects on immunity may be due to the stimulation of intracellular antioxidants, which are important in lymphocyte activation and proliferation (Annunziata and Iorio, 2004).
Paraquat was found to affect the animal cell through oxidative damage in cellular macromolecules including DNA (Lascano et al., 2012). In the present study, the DNA fragmentation test showed an appearance of short DNA fragments in response to PQ injection. The paraquat toxicity may be due to the excessive release of ROS which, in turn, initiate lipid peroxidation, particularly in the polyunsaturated lipid compound of cell membranes (Hayes and Laws, 1991). Furthermore, the paraquat toxicity involves depletion of cellular reduction agent of NADPH which is used as a cofactor for lipid and nucleic acid synthesis (Kelner and Bagnell, 1989). These events induced by PQ-oxidative stress may increase the DNA damage at the cellular level (Ribas et al., 1995), and consequently, the DNA fragmentation occurred in the PBMCs lead to the impaired immune function of the turkey poults that were injected with PQ in the current study. On the other hand, Hsp70, which is classified as a constitutive protein synthesized to protect cells from stress (Al-Aqil et al., 2009), was also analyzed in the PBMCs of turkey poults in the present study. The Hsp70 was over blotted in the PQ group compared with the C group. In accordance with our results, previous reports demonstrated higher expressions of Hsp70 in rats (Crum et al., 2015) and mice (Nakanishi and Yasumoto, 1997) that received PQ compound in comparison with the controls. These findings show that PQ administration appeared to activate oxidative degradation in the cell, and consequently, increased the level of oxidized proteins, which stimulate self-defense mechanism of the cell to increase the level of Hsp70.
The dietary propolis supplementation to the turkey poults reduced the DNA fragmentation and the over expression of Hsp70 in the PR+PQ and the PR groups to normal levels similar to the C group. Phenolic acids and flavonoids are known as the basic compounds of propolis according to its quality and type (Kurek-Górecka et al., 2013), and these compounds have a powerful antioxidative activity (Gülçin, 2006). It was demonstrated that such compounds inhibit the activity of participated enzymes in the ROS creation such as cAMP phosphodiesterase, protein kinase C, ascorbic acid oxidase, lipoxygenase and Na+/K+ ATPase (Pietta, 2000). Moreover, the antioxidant compounds of propolis decrease the activity of xanthine oxidase, an oxidase of NADPH, which is responsible for the appearance of superoxide anion radical (Harborne and Williams, 2000). In addition, propolis could activate the Cu/Zn-superoxide dismutase, one of the most important antioxidant enzymes (de Sá et al., 2013). These antioxidative characteristics of propolis may participate in the mechanism of inhibiting the DNA damage and Hsp70 over-expression in PBMCs of turkey treated with PQ, and then finally these birds can express a normal immune function.
In conclusion, the PQ injection in turkey poults induces an oxidative stress status and impairs the growth performance and immune function in treated poults. The negative effects of PQ could be justified by the high levels of MDA and TNFα in PBMC’s, and the high corticosterone with low T3 concentrations in the plasma. Furthermore, the DNA fragmentation and Hsp70 expression in the PBMCs explain the malfunction of these cells and the low immunity in the PQ-treated poults. In contrast, the dietary propolis supplementation can alleviate the negative effects of PQ-induced oxidative stress on the productive, physiological, and immunological parameters of turkey poults. The positive effects of antioxidative compounds in the propolis can decrease the MDA, TNFα, corticosterone, DNA fragments, and Hsp70 levels. Therefore, the addition of propolis at a rate of 1 g/kg to the diet of turkey poults could be recommended as a potential nutritional strategy in order to improve their immunity and performance, especially under oxidative stress conditions by herbicides like paraquat.
Acknowledgements
This study was funded by the General Scientific Research Department at Cairo University (GSRD-CU) under activities carried out by the Project of Rapid Climate Change in Poultry Cellular and Molecular Physiology (RCC-PCMP). Ahmed O. Abass (Associate Professor of Poultry Physiology, Cairo University) was the principal investigator and research team leader of the project. The authors would like to thank Abdel-Rahman M. M. Atta (Professor of Poultry Immunology, Cairo University) for his technical support during this study. The authors are very grateful to all the personnel from the Poultry Biotechnology Lab and members of Poultry Services Center at Faculty of Agriculture, Cairo University, for their assistance in sample preparation and monitoring of birds throughout the experimental period.
REFERENCES
- Al-Aqil A., Zulkifli I., Sazili A. Q., Omar A. R., Rajion M. A.. 2009. The Effects of the Hot, Humid Tropical Climate and Early Age Feed Restriction on Stress and Fear Responses, and Performance in Broiler Chickens. Asian-Australasian J. Anim. Sci. 22:1581–1586.Available at http://ajas.info/journal/view.php?doi = 10.5713/ajas.2009.90021. [Google Scholar]
- Amen O., Mahmoud U. T., Abdel-Rahman M. A., Darwish M. H. A., Applegate T. J., Cheng H. W.. 2015. The effect of Brazilian propolis on serum thyroid hormones in broilers reared under chronic heat stress. Page P273 (Abstract) in International Poultry Scientific Forum. Georgia World Congress Center, Atlanta, Georgia, USA. [Google Scholar]
- Amirshahrokhi K. 2013. Anti-inflammatory effect of thalidomide in paraquat-induced pulmonary injury in mice. Int. Immunopharmacol. 17:210–215. Available at http://www.sciencedirect.com/science/article/pii/S1567576913002464. [DOI] [PubMed] [Google Scholar]
- Annunziata M., Iorio M.. 2004. The levels of glutathione and hemoglobin in sheep erythrocytes as a function of age. Ital. J. Anim. Sci. 3:283–286. Available at http://www.aspajournal.it/index.php/ijas/article/view/ijas.2004.283. [Google Scholar]
- Aygun A., Sert D., Copur G.. 2012. Effects of propolis on eggshell microbial activity, hatchability, and chick performance in Japanese quail (Coturnix coturnix japonica) eggs. Poult. Sci. 91:1018–1025. Available at https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.2011-01944. [DOI] [PubMed] [Google Scholar]
- Babatunde M. M., Oladimeji A. A.. 2014. Effects of Paraquat on the Growth and Behaviour of Oreochromis niloticus. Environ. Res. J. 8:44–47. Available at http://medwelljournals.com/abstract/?doi = erj.2014.44.47. [Google Scholar]
- Boelsterli U. A. 2003. Mechanistic toxicology: the molecular basis of how chemicals disrupt biological targets (Taylor and Francis, Eds.). CRC Press. [Google Scholar]
- Cetin E., Silici S., Cetin N., Guclu B. K.. 2010. Effects of diets containing different concentrations of propolis on hematological and immunological variables in laying hens. Poult. Sci. 89:1703–1708. Available at https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.2009-00546. [DOI] [PubMed] [Google Scholar]
- Colaianna M., Schiavone S., Zotti M., Tucci P., Morgese M. G., Bäckdahl L., Holmdahl R., Krause K., Cuomo V., Trabace L.. 2013. Neuroendocrine Profile in a Rat Model of Psychosocial Stress: Relation to Oxidative Stress. Antioxid. Redox Signal. 18:1385–1399. Available at http://www.ncbi.nlm.nih.gov/pubmed/23320850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum T. S., Gleixner A. M., Posimo J. M., Mason D. M., Broeren M. T., Heinemann S. D., Wipf P., Brodsky J. L., Leak R. K.. 2015. Heat shock protein responses to aging and proteotoxicity in the olfactory bulb. J. Neurochem. 133:780–794. Available at http://www.ncbi.nlm.nih.gov/pubmed/25640060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere E., Scanes C. G., Kühn E. R.. 1983. Effects of glucocorticoids on circulating concentrations of thyroxine (T4) and triiodothyronine (T3) and on peripheral monodeiodination in pre- and post-hatching chickens. Horm. Metab. Res. 15:233–236. Available at http://www.ncbi.nlm.nih.gov/pubmed/6307846. [DOI] [PubMed] [Google Scholar]
- Denli M., Cankaya S., Silici S., Okan F., Uluocak A. N.. 2005. Effect of Dietary Addition of Turkish Propolis on the Growth Performance, Carcass Characteristics and Serum Variables of Quail (Coturnix coturnix japonica). Asian-Australasian J. Anim. Sci. 18:848–854. Available at http://ajas.info/journal/view.php?doi = 10.5713/ajas.2005.848. [Google Scholar]
- Dinis-Oliveira R. J., Duarte J. A., Sánchez-Navarro A., Remião F., Bastos M. L., Carvalho F.. 2008. Paraquat Poisonings: Mechanisms of Lung Toxicity, Clinical Features, and Treatment. Crit. Rev. Toxicol. 38:13–71. Available at http://www.ncbi.nlm.nih.gov/pubmed/18161502. [DOI] [PubMed] [Google Scholar]
- Ebrahimi R., Jahromi M. F., Liang J. B., Farjam A. S., Shokryazdan P., Idrus Z.. 2015. Effect of Dietary Lead on Intestinal Nutrient Transporters mRNA Expression in Broiler Chickens. Biomed Res. Int. 2015:1–8. Available at http://www.hindawi.com/journals/bmri/2015/149745/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. G., Sarkar D., Klopp R., Morrow J. D., Weindruch R., Prolla T. A.. 2004. Impairment of the Transcriptional Responses to Oxidative Stress in the Heart of Aged C57BL/6 Mice. Ann. N. Y. Acad. Sci. 1019:85–95. Available at http://www.ncbi.nlm.nih.gov/pubmed/15246999. [DOI] [PubMed] [Google Scholar]
- Fellenberg M. A., Speisky H.. 2006. Antioxidants: their effects on broiler oxidative stress and its meat oxidative stability. Worlds. Poult. Sci. J. 62:53–70. Available at http://www.journals.cambridge.org/abstract_S0043933906000055. [Google Scholar]
- Fitzpatrick L. R., Wang J., Le T.. 2001. Caffeic acid phenethyl ester, an inhibitor of nuclear factor-kappaB, attenuates bacterial peptidoglycan polysaccharide-induced colitis in rats. J. Pharmacol. Exp. Ther. 299:915–920. Available at http://www.ncbi.nlm.nih.gov/pubmed/11714876. [PubMed] [Google Scholar]
- Freitas J. A., Vanat N., Pinheiro J. W., Balarin M. R. S., Sforcin J. M., Venancio E. J.. 2011. The effects of propolis on antibody production by laying hens. Poult. Sci. 90:1227–1233. Available at http://www.ncbi.nlm.nih.gov/pubmed/21597063. [DOI] [PubMed] [Google Scholar]
- Gehad A. E., Mehaisen G. M., Abbas A. O., Mashaly M. M.. 2008. The Role of Light Program and Melatonin on Alleviation of Inflammation Induced by Lipopolysaccharide Injection in Broiler Chickens. Int. J. Poult. Sci. 7:193–201. Available at http://www.scialert.net/abstract/?doi=ijps.2008.193.201. [Google Scholar]
- Goldner W. S., Sandler D. P., Yu F., Hoppin J. A., Kamel F., LeVan T. D.. 2010. Pesticide Use and Thyroid Disease Among Women in the Agricultural Health Study. Am. J. Epidemiol. 171:455–464. Available at https://academic.oup.com/aje/article-lookup/doi/10.1093/aje/kwp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülçin İ. 2006. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology. 217:213–220. Available at http://www.sciencedirect.com/science/article/pii/S0300483X05004786. [DOI] [PubMed] [Google Scholar]
- Gupta S. C., Hevia D., Patchva S., Park B., Koh W., Aggarwal B. B.. 2012. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid. Redox Signal. 16:1295–1322. Available at http://www.ncbi.nlm.nih.gov/pubmed/22117137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne J. B., Williams C. A.. 2000. Advances in flavonoid research since 1992. Phytochemistry. 55:481–504. Available at http://www.sciencedirect.com/science/article/pii/S0031942200002351. [DOI] [PubMed] [Google Scholar]
- Haščík P., Elimam I. O., Kročko M., Bobko M., Kačániová M., Garlík J., Šimko M., Saleh A. A.. 2015. The Influence of Propolis as Supplement Diet on Broiler Meat Growth Performance, Carcass Body Weight, Chemical Composition and Lipid Oxidation Stability. Acta Univ. Agric. Silvic. Mendelianae Brun. 63:411–418. Available at https://acta.mendelu.cz/63/2/0411/. [Google Scholar]
- Hayes W. J., Laws E. R.. 1991. Handbook of pesticide toxicology. Classes of pesticides Vol 3 Academic Press. [Google Scholar]
- Ho E., Karimi Galougahi K., Liu C., Bhindi R., Figtree G. A.. 2013. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 1:483–491. Available at http://www.ncbi.nlm.nih.gov/pubmed/24251116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. F., Guo Y. M., Huang B. Y., Zhang L. B., Bun S., Liu D., Long F. Y., Li J. H., Yang X., Jiao P.. 2010. Effect of corticosterone administration on small intestinal weight and expression of small intestinal nutrient transporter mRNA of broiler chickens. Asian-Australasian J. Anim. Sci. 23:175–181. Available at https://doi.org/2010.23.2.175. [Google Scholar]
- Hu F., Hepburn H. R., Li Y., Chen M., Radloff S. E., Daya S.. 2005. Effects of ethanol and water extracts of propolis (bee glue) on acute inflammatory animal models. J. Ethnopharmacol. 100:276–283. Available at http://www.sciencedirect.com/science/article/pii/S0378874105002151. [DOI] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H.. 2015. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 93:2144–2153. Available at https://dl.sciencesocieties.org/publications/jas/abstracts/93/5/2144. [DOI] [PubMed] [Google Scholar]
- Ivanauskas L., Jakštas V., Radušiene J., Lukošius A., Baranauskas A.. 2008. Evaluation of phenolic acids and phenylpropanoids in the crude drugs. Medicina (B. Aires). 44:48–55. [PubMed] [Google Scholar]
- Kačániová M., Rovná K., Arpášová H., Čuboň J., Hleba L., Pochop J., Kunová S., Haščík P.. 2012. In vitro and In vivo antimicrobial activity of propolis on the microbiota from gastrointestinal tract of chickens. J. Environ. Sci. Heal. Part A. 47:1665–1671. Available at http://www.ncbi.nlm.nih.gov/pubmed/22702827. [DOI] [PubMed] [Google Scholar]
- Kamel N. N., Ahmed A. M. H., Mehaisen G. M. K., Mashaly M. M., Abass A. O.. 2017. Depression of leukocyte protein synthesis, immune function and growth performance induced by high environmental temperature in broiler chickens. Int. J. Biometeorol.:1–9. Available at http://link.springer.com/10.1007/s00484-017-1342-0. [DOI] [PubMed] [Google Scholar]
- Kang C., Kim S. C., Lee S. H., Jeong J. H., Kim D. S., Kim D. H.. 2013. Absolute Lymphocyte Count as a Predictor of Mortality in Emergency Department Patients with Paraquat Poisoning (AYW Chang, Ed.). PLoS One. 8:e78160 Available at http://www.ncbi.nlm.nih.gov/pubmed/24205140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner M. J., Bagnell R.. 1989. Paraquat resistance associated with reduced NADPH reductase in an energy-dependent paraquat-accumulating cell line. Arch. Biochem. Biophys. 274:366–374. Available at http://www.ncbi.nlm.nih.gov/pubmed/2802616. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Araki Y., Takenaka A., Igarashi K.. 1999. Protective Effects of Dietary Nasunin on Paraquat-induced Oxidative Stress in Rats. Biosci. Biotechnol. Biochem. 63:799–804. Available at http://www.tandfonline.com/doi/full/10.1271/bbb.63.799. [DOI] [PubMed] [Google Scholar]
- Kurek-Górecka A., Rzepecka-Stojko A., Górecki M., Stojko J., Sosada M., Świerczek-Zięba G.. 2013. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules. 19:78–101. Available at http://www.ncbi.nlm.nih.gov/pubmed/24362627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascano R., Munoz N., Robert G., Rodriguez M., Melchiorre M., Trippi V., Quero G.. 2012. Paraquat: An Oxidative Stress Inducer. Pages 135–148 in Herbicides - Properties, Synthesis and Control of Weeds. Hasaneen Mohammed Nagib, ed. InTech. [Google Scholar]
- Lin H., Decuypere E., Buyse J.. 2004. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus): 1. Chronic exposure. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 139:737–744. Available at http://linkinghub.elsevier.com/retrieve/pii/S1096495904002866. [DOI] [PubMed] [Google Scholar]
- Lin J., Lin-Tan D., Chen K., Huang W.. 2006. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit. Care Med. 34:368–373. Available at http://www.ncbi.nlm.nih.gov/pubmed/16424716. [DOI] [PubMed] [Google Scholar]
- Loa C. C., Lin T. L., Wu C. C., Bryan T., Thacker H. L., Hooper T., Schrader D.. 2001. Humoral and cellular immune responses in turkey poults infected with turkey coronavirus. Poult. Sci. 80:1416–1424. Available at http://www.ncbi.nlm.nih.gov/pubmed/11599699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehaisen G. M. K., Eshak M. G., Elkaiaty A. M., Atta A. M. M., Mashaly M. M., Abass A. O.. 2017. Comprehensive growth performance, immune function, plasma biochemistry, gene expressions and cell death morphology responses to a daily corticosterone injection course in broiler chickens (R van den Bos, Ed.). PLoS One. 12:e0172684 Available at http://dx.plos.org/10.1371/journal.pone.0172684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaers M., van Blitterswijk H., van ’t Leven L., Kerkvliet J., van de Waerdt J.. 2005. Bee products (properties, processing and marketing) (Marieke Mutsaers, Ed.). 1 st ed. NECTAR, Netherlands Expertise Centre for (sub)Tropical Apicultural Resources, Wageningen, Netherlands. [Google Scholar]
- Nakanishi Y., Yasumoto K.. 1997. Induction after Administering Paraquat of Heme Oxygenase-1 and Heat Shock Protein 70 in the Liver of Senescence-accelerated Mice. Biosci. Biotechnol. Biochem. 61:1302–1306. Available at http://www.tandfonline.com/doi/abs/10.1271/bbb.61.1302. [DOI] [PubMed] [Google Scholar]
- Newairy A. A., Abdou H. M.. 2013. Effect of propolis consumption on hepatotoxicity and brain damage in male rats exposed to chlorpyrifos. African J. Biotechnol. 12:5232–5243. Available at http://academicjournals.org/journal/AJB/article-abstract/8C2484227776. [Google Scholar]
- Oktay M., Gülçin İ., Küfrevioğlu Ö. İ.. 2003. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT - Food Sci. Technol. 36:263–271. Available at http://linkinghub.elsevier.com/retrieve/pii/S0023643802002268. [Google Scholar]
- Ortiz M. S., Forti K. M., Suárez Martinez E. B., Muñoz L. G., Husain K., Muñiz W. H.. 2016. Effects of Antioxidant N-acetylcysteine Against Paraquat-Induced Oxidative Stress in Vital Tissues of Mice. Int. J. Sci. basic Appl. Res. 26:26–46. Available at http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4936834&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- Parkar S. G., Stevenson D. E., Skinner M. A.. 2008. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 124:295–298. Available at http://www.ncbi.nlm.nih.gov/pubmed/18456359. [DOI] [PubMed] [Google Scholar]
- Pietta P. G. 2000. Flavonoids as antioxidants. J. Nat. Prod. 63:1035–1042. Available at http://www.ncbi.nlm.nih.gov/pubmed/10924197. [DOI] [PubMed] [Google Scholar]
- Prasad K., Tarasewicz E., Mathew J., Strickland P. A. O., Buckley B., Richardson J. R., Richfield E. K.. 2009. Toxicokinetics and toxicodynamics of paraquat accumulation in mouse brain. Exp. Neurol. 215:358–367. Available at http://www.ncbi.nlm.nih.gov/pubmed/19084006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Sengupta A., Ray A.. 2007. Effects of paraquat on anti-oxidant system in rats. Indian J. Exp. Biol. 45:432–438. Available at http://www.ncbi.nlm.nih.gov/pubmed/17569284. [PubMed] [Google Scholar]
- Riahi B., Rafatpanah H., Mahmoudi M., Memar B., Brook A., Tabasi N., Karimi G.. 2010. Immunotoxicity of paraquat after subacute exposure to mice. Food Chem. Toxicol. 48:1627–1631. Available at 10.1016/j.fct.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Ribas G., Frenzilli G., Barale R., Marcos R.. 1995. Herbicide-induced DNA damage in human lymphocytes evaluated by the single-cell gel electrophoresis (SCGE) assay. Mutat. Res. Toxicol. 344:41–54. Available at http://linkinghub.elsevier.com/retrieve/pii/0165121895900373. [DOI] [PubMed] [Google Scholar]
- de Sá R. A., de Castro F. A. V., Eleutherio E. C. A., de Souza R. M., da Silva J. F. M., Pereira M. D.. 2013. Brazilian propolis protects Saccharomyces cerevisiae cells against oxidative stress. Brazilian J. Microbiol. 44:993–1000. Available at http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1517-83822013000300051&lng=en&nrm=iso&tlng=en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc 2011. SAS/STAT® 9.3 User's Guide. SAS Inst. Inc., Cary, NC, USA. [Google Scholar]
- Seven I., Aksu T., Seven P. T.. 2010. The Effects of Propolis on Biochemical Parameters and Activity of Antioxidant Enzymes in Broilers Exposed to Lead-Induced Oxidative Stress. Asian-Australasian J. Anim. Sci. 23:1482–1489. Available at http://ajas.info/journal/view.php?doi=10.5713/ajas.2010.10009. [Google Scholar]
- Seven P. T., Yılmaz S., Seven I., Cercı I. H., Azman M. A., Yılmaz M.. 2009. Effects of Propolis on Selected Blood Indicators and Antioxidant Enzyme Activities in Broilers under Heat Stress. Acta Vet. Brno. 78:75–83. Available at http://actavet.vfu.cz/78/1/0075/. [Google Scholar]
- Shalmany S. K., Shivazad M.. 2006. The effect of diet propolis supplementation on Ross broiler chicks performance. Int. J. Poult. Sci.:84–88. Available at http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.540.9523. [Google Scholar]
- Siegel H. S. 1995. Stress, strains and resistance. Br. Poult. Sci. 36:3–22. Available at http://www.tandfonline.com/doi/abs/10.1080/00071669508417748. [DOI] [PubMed] [Google Scholar]
- Silva C. R. B., Putarov T. C., Fruhvald E., Destro F. C., Filho W. C. M., Thomazini C. M., Barbosa T. S., Orsi R. O., Siqueira E. R.. 2014. Action of Brazilian propolis on hematological and serum biochemical parameters of Blue-fronted Amazons (Amazona aestiva, Linnaeus, 1758) in captivity. Poult. Sci. 93:1688–1694. Available at https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.2013-03738. [DOI] [PubMed] [Google Scholar]
- Smalley H. E. 1973. Toxicity and Hazard of the Herbicide, Paraquat, in Turkeys. Poult. Sci. 52:1625–1628. [DOI] [PubMed] [Google Scholar]
- Smith P., Heath D.. 1974. Paraquat lung: a reappraisal. Thorax. 29:643–653. Available at http://www.ncbi.nlm.nih.gov/pubmed/4480097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K., Cedillo L., Tucker J., Hines C., Sorensen K., Deddens J., Cruz V.. 1997. Thyroid hormones and cytogenetic outcomes in backpack sprayers using ethylenebis(dithiocarbamate) (EBDC) fungicides in Mexico. Environ. Health Perspect. 105:1126–1130. Available at http://www.ncbi.nlm.nih.gov/pubmed/9349837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirugnanasampandan R., Raveendran S. B., Jayakumar R.. 2012. Analysis of chemical composition and bioactive property evaluation of Indian propolis. Asian Pac. J. Trop. Biomed. 2:651–654. Available at http://www.ncbi.nlm.nih.gov/pubmed/23569988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukmechi A., Rezaee J., Nejati V., Sheikhzadeh N.. 2013. Effect of acute and chronic toxicity of paraquat on immune system and growth performance in rainbow trout, Oncorhynchus mykiss. Aquac. Res.:1737–1743. Available at http://doi.wiley.com/10.1111/are.12118.
- Virden W. S., Thaxton J. P., Corzo A., Dozier W. A., Kidd M. T.. 2007. Evaluation of Models Using Corticosterone and Adrenocorticotropin to Induce Conditions Mimicking Physiological Stress in Commercial Broilers. Poult. Sci. 86:2485–2491. Available at http://ps.oxfordjournals.org/content/86/12/2485.abstract. [DOI] [PubMed] [Google Scholar]
- Yang J., Liu L., Sheikhahmadi A., Wang Y., Li C., Jiao H., Lin H., Song Z.. 2015. Effects of Corticosterone and Dietary Energy on Immune Function of Broiler Chickens (S-B Wu, Ed.). PLoS One. 10:e0119750 Available at http://dx.plos.org/10.1371/journal.pone.0119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Yang Y., Lin C., Wang Q.. 2010. Antioxidant attenuation of ROS-involved cytotoxicity induced by Paraquat on HL-60 cells. Health (Irvine. Calif). 2:253–261. Available at http://www.scirp.org/journal/PaperDownload.aspx?DOI=10.4236/health.2010.23036. [Google Scholar]
- Zhang L., Yue H. Y., Zhang H. J., Xu L., Wu S. G., Yan H. J., Gong Y. S., Qi G. H.. 2009. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult. Sci. 88:2033–2041. Available at http://www.ncbi.nlm.nih.gov/pubmed/19762854. [DOI] [PubMed] [Google Scholar]


