Abstract
The present study was designed to evaluate the effects of tea saponins on oxidative stress induced by cyclophosphamide in chickens. One hundred twenty chickens were randomly divided into 5 groups. Groups 3 to 4 received intramuscular injection of cyclophosphamide to induce oxidative stress and immunosuppression. After that, groups 2 and 4 were orally administered tea saponins in drinking water for 7 d. Then, groups 1 to 4 were immunized with a live, bivalent vaccine of Newcastle disease virus and infectious bronchitis virus. Blood samples were collected for analysis of oxidative parameters and specific antibody titers, and splenocytes were prepared for lymphocyte proliferative assay. The results showed that administration of tea saponins significantly increased total antioxidant capacity, total superoxide dismutase, catalase, glutathione peroxidase, glutathione, ascorbic acid, and α-tocopherol, and decreased malondialdehyde and protein carbonyl. Enhanced immune responses, such as lymphocyte proliferation induced by concanavalin A and lipopolysaccharides, and serum Newcastle disease virus- and infectious bronchitis virus-specific antibodies were also observed in chickens injected with or without cyclophosphamide. In addition, no side effects were found in chickens throughout the study. Therefore, tea saponins may be a potential agent to improve imunosuppression induced by oxidative stress in chickens.
Keywords: Tea saponin, oxidative stress, cyclophosphamide, immunosuppression
INTRODUCTION
Oxidative stress is caused by the imbalance between pro-oxidants and antioxidants at either cellular or individual level (Voljč et al., 2011). During the normal respiration, oxygen is progressively reduced to yield water (Panda and Cherian 2013). However, the incomplete reduction of oxygen during this process leads to formation of chemical entities that have powerful oxidizing properties and is known as reactive oxygen species (ROS). ROS are constantly produced in vivo during the course of physiological metabolism in living tissues. Over production or insufficient elimination of ROS will lead to oxidative stress (Yu et al., 2015b). Oxidative stress constitutes an important factor of biological damage and is regarded as the cause of several pathological conditions that affect growth and development (Avanzo et al., 2001; Iqbal et al., 2001), and it may predispose to the development of cancer (Kim et al., 2014), diabetes (Gomes 2014), and cardiovascular diseases (Chan and Chan 2013).
Various stresses are responsible for decreasing productive and reproductive performance of growing chickens in the commercial poultry industry. When oxidative stress is more severe, the pro-oxidant systems outbalance the antioxidant systems, potentially producing oxidative damage to lipids, proteins, carbohydrates, and nucleic acids, ultimately leading to cell death (Surai 2000). Such uncontrolled oxidative reactions also can result in severe oxidative stress and metabolic diseases (Tavárez et al., 2011). Therefore, decreasing oxidative stress is very important to maintain feed and food quality and enhance bird health and welfare. Less effective immune responses were found after vaccination in some cases, such as individuals influenced by oxidative stress and numerous other factors (Peyre et al., 2009). Therefore, it is necessary to improve the immunization with currently available vaccines so as to effectively protect the host from infections.
The medicinal use of tea has a long history in Asian countries such as China, Japan, India and Thailand. Tea is the most widely consumed beverage, second only to water, with a per capita worldwide consumption of approximately 0.12 liter per d (Pastore and Fratellone 2006). Tea seeds and leaves contain variety of biological active compounds like polyphenols, saponins, vitamins, oil minerals, and trace elements (Cabrera et al., 2003). The main antioxidants in tea are saponins, catechins, flavonols, tannins, and phenolic compounds. The antioxidant properties of green tea polyphenols and the pro-oxidant effects of these compounds have been recommended as potential candidates for cancer prevention (Yashin et al., 2011). The beneficial effects of tea have been attributed to its strong antioxidant activity (Jang et al., 2007). Recently, saponins isolated from ginseng stem-leaf (GSLS) have been found to be an immune-stimulating agent in chickens (Zhai et al., 2011a,b; Zhai et al., 2014). GSLS not only affected oxidative stress but also improved immune responses in chickens (Yu et al., 2015a,b). Therefore, we hypothesized that tea saponins (TS) have both antioxidative and immunomodulatory properties. At the present study, we evaluated the effect of TS on the antioxidative activities as well as the immune responses to a live bivalent vaccine of Newcastle disease virus (NDV) and infectious bronchitis virus (IBV) vaccine in chickens in oxidative stress induced by cyclophosphamide (Cy).
MATERIALS AND METHODS
Tea Saponins
Standardized TS were purchased from Zhejiang Qingtian Zhongye Natural Plant Co. Ltd. (Hangzhou, China). TS was water-soluble powder with light-yellow color and 82.8% of the saponins are triterpenoid, having a foamility score of 188 mm and pH of 6.0.
Chickens
Day-old specific-pathogen-free (SPF) chickens (male) were purchased from Zhejiang Shennong Stock Breeding Inc. (Ningbo, China), and housed in separated units. The house was set at 35°C for the first 3 d and then adjusted to 26°C. Feed and water were supplied ad libitum. All the birds were treated according to the Zhejiang University Committee on Animal Care and Use.
Vaccines
Live bivalent vaccine of NDV (Strain La Sota) and infectious bronchitis virus (IBV, Strain H120) were purchased from Zhejiang EBVAC Bioengineering Co. Ltd.
Reagents
The antigen and positive control serum used for NDV-specific hemagglutination inhibition (HI) test were purchased from Qingdao Yebio Bioengineering Co. Ltd. (Qingdao, China). The infectious bronchitis virus antibody test kit was purchased from IDEXX Laboratories Inc. (Westbrook, Maine). Cyclophosphamide (Cy) was purchased from Pude Pharmaceutical Co. Ltd. (Shanxi, China). Concanavalin A (ConA), lipopolysaccharides (LPS) and thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma-Aldrich Inc. (St. Louis, MO). Detection kits for test of total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), catalase (CAT), glutathione peroxidase (GSH-PX), reduced glutathione (GSH), ascorbic acid (VC), α-tocopherol (VE), malondialdehyde (MDA), protein carbonyl were purchased from the Institute of Nanjing Jiancheng Bioengineering (Nanjing, China). All other chemicals were analytic grade.
Experimental Design
One hundred twenty chickens were randomly divided into 5 groups, each consisting of 24 birds. Chickens in groups 3 and 4 received intramuscular injection of Cy at 100 mg/kg body weight (BW) for 3 d to induce immunosuppression. Groups 1, 2, and 5 were injected with saline solution in the same way as groups 3 and 4. Then, groups 2 and 4 were orally administered TS of 5 mg/kg BW in drinking water for 7 d. Similar way to Yu et al (2015b) was used to administration of TS. To make the chickens drink the TS water sufficiently, the birds were kept thirsty for 2 h before TS medication. Groups 5 were not medicated and served as a control (Table 1). After that, group 1 to 4 were intranasally immunized twice with a live bivalent vaccine of NDV and IBV at 2-wk intervals. Blood samples were collected before Cy treatment and 7, 14, and 21 d after immunization for analysis of oxidative parameters, as well as measurement of NDV-specific HI titers and IBV-specific antibody titers. Splenocytes were prepared from 6 birds of each group before Cy treatment and 7, 14, and 21 d after immunization for lymphocyte proliferative assay. The dose of TS used in this study was based on our previous investigation where 5 mg/kg was found optimal to enhance the serum specific HI response to NDV vaccine when chickens were orally administered TS at the doses of 3, 5, 7, and 9 mg/kg.
Table 1.
Experimental design.
| Group | n | Cy injection | TS (5 mg/kg BW) | Vaccination |
|---|---|---|---|---|
| 1 | 24 | + | ||
| 2 | 24 | + | + | |
| 3 | 24 | + | + | |
| 4 | 24 | + | + | + |
| 5 | 24 |
Biochemical Test
T-AOC activity was measured by using the ferric-reducing antioxidant ability assay (Benzie and Szeto 1999). The assay for T-SOD activity involved inhibition of nitroblue tetrazolium reduction with xanthine oxidase used as a superoxide generator (Sun et al., 1988). CAT was measured by evaluation of hydrogen peroxide based on the formation of stable complex with ammonium molybdate (Aebi 1984). GSH-PX was measured by monitoring the reduction of t-butyl hydroperoxide (Wheeler et al., 1990). GSH was assayed by 5,5΄-dithiobis-(2-nitrobenzoic acid) reagent (Abegg et al., 2012). VC was measured by turning ferric iron to ferrous iron (Zannoni et al., 1974). VE was estimated by the reducing properties of tocopherol (Emmerie and Engel 1938). MDA was performed by the spectrophotometric method based on the reaction 2-thiobarbituric acid (Mihara and Uchiyama 1978). Protein carbonyl was measured by oxidative damage to proteins (Pirinccioglu et al., 2010).
Hemagglutination Inhibition Test
Serum NDV-specific HI titers were determined as described by Zhai et al. (2011a). Briefly, 2-fold serial dilution of serum samples (1:2 to 1:2048) was made in a V-shaped bottom 96-well microtiter plate. Afterwards, 4 hemagglutination units of NDV antigen was added to each well. After incubation at 37°C for 30 min, 25 μL of 1% rooster erythrocyte suspension was added and incubated at 37°C for 20 min. All samples were tested in duplicate, and the positive and negative serum controls were included on each plate. The HI titers were defined as the reciprocal of the last dilution of serum causing complete inhibition of hemagglutination. The data were expressed as log 2 of the highest dilution that exhibited HI.
Test of IBV-Specific Antibody Titers
Serum IBV-specific antibody titers were determined using detection kits according to the manufacturers’ protocols. Test samples were diluted 500-fold (1:500) with sample diluent prior to being assayed and the samples were thoroughly mixed prior to dispensing into the coated plate. All reagents were allowed to come to 18 to 26°C before use. Next, 100 μL of diluted sample was added into appropriate wells and 100 μL of undiluted negative control and positive control were added into duplicate wells. After incubation at 26°C for 30 min, the solution was removed and washed each well 5 times with approximately 350 μL of distilled water. After that, 100 μL of conjugate was dispensed into each well, then incubated at 26°C for 30 min. Each well was washed as described above and 100 μL of TMB substrate was dispensed. After incubation at 26°C for 15 min, 100 μL of stop solution was added. The mean optical density (OD) was read at 650 nm. The relative level of antibody titer in the unknown was determined by calculating the sample to positive (S/P) ratio as [(mean of sample optimal density) – (mean of negative control optimal density)] / [(mean of positive control optimal density) – (mean of negative control optimal density). Endpoint titers were calculated with the equation: log 10 titer = 1.09 (log 10 S/P) + 3.36 (Flock Check program, IDEXX).
Lymphocyte Proliferation Test
The test was performed as previously (Li et al., 2009). Briefly, spleens collected into Hank's balance salt solution (HBSS) were minced and passed through a fine steel mesh to obtain the cell suspension under aseptic conditions. The cells were washed in HBSS 3 times by centrifugation (500 × g at 4°C for 10 min), and suspended in cold RPMI 1640 supplemented with 0.05 mM 2-mercaptoethanol, 100 IU/mL penicillin, 100 μg/mL streptomycin and 10% heat inactivated fetal calf serum (FCS). Cell viability was assessed with trypan blue exclusion stain, and the cell suspension was adjusted to 5.0 × 106/mL. Mitogen-induced proliferation was performed by incubating 100 μL of lymphocyte suspension with 100 μL of either ConA (40 μg/mL) or LPS (35 μg/mL) in each well for the MTT assay. RPMI 1640 medium (100 μL) was added to the cell suspension in control wells. Cells were added in triplicate, and incubated in a humidified atmosphere at 5% CO2 at 40°C for 44 h. After that, 50 μL MTT (2 mg/mL) was added to each well and incubated for an additional 4 h. The plates were centrifuged (1,000 × g for 10 min), and the supernatant was carefully removed. Added 150 μL of acidic dimethyl sulfoxide (DMSO, 0.04 N HCl) to each well and mixed thoroughly by slightly shaking to solubilize the MTT formazan. The mean optical density (OD) was read at 570 nm. The stimulation index (SI) was calculated based on the formula: +SI = OD value of mitogen-stimulated cells divided by OD value of non-stimulated cells.
Statistical Analysis
Analysis of data was performed using SPSS software (version 20.0, SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) with Duncan post hoc test was used for multiple comparisons between groups. Values were expressed as the mean ± standard deviation (SD). P-values of less than 0.05 were considered statistically significant.
RESULTS
Effect of Tea Saponins on Serum T-AOC, T-SOD, CAT, and GSH-PX
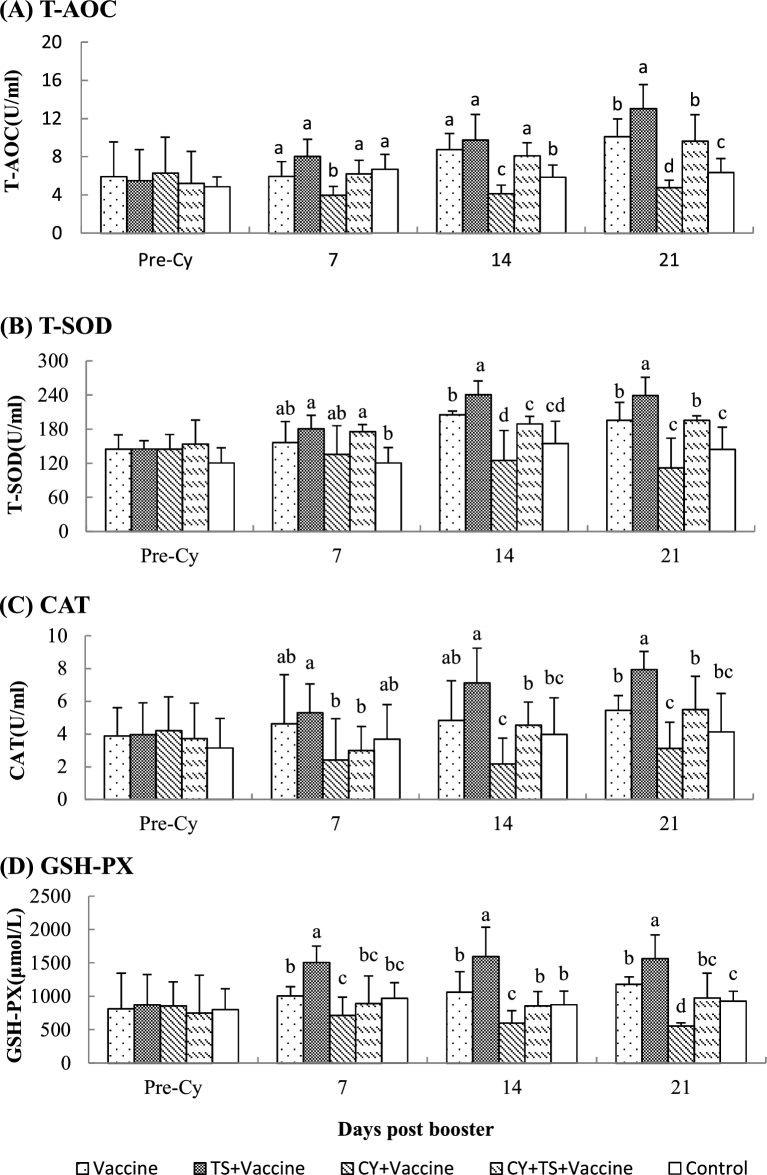
To investigate the effect of TS on oxidative stress, blood samples were collected to measure the serum T-AOC, T-SOD, CAT, and GSH-PX. Figure 1 showed that there were no significant difference for serum T-AOC, T-SOD, CAT, and GSH-PX between groups before medication. It was found that activities of T-AOC, T-SOD, CAT, and GSH-PX in birds treated with TS (TS + vaccine) were significantly higher than those without TS treatment (vaccine), and the activities in birds treated with Cy (Cy + vaccine) were significantly lower than those without Cy injection (vaccine). However, the antioxidant enzymes in birds treated with Cy significantly increased after administered TS (Cy + TS + vaccine) when compared to the birds treated without TS (Cy + vaccine).
Figure 1.
Serum T-AOC, T-SOD, CAT and GSH-PX. Chickens were i.m. injected with Cy at 100 mg/kg BW for 3 days to induce immunosuppression and oxidative stress. After that, the birds were orally administered TS (5 mg/kg BW) in drinking water for 7 days. Blood samples were collected from 6 birds of each group before Cy treatment and 7, 14, 21 days after booster for analysis of T-AOC, T-SOD, CAT and GSH-PX.Data were expressed as mean ± S.D. (n = 6). Bars with different letters are statistically different (P < 0.05).
Effect of Tea Saponins on Serum GSH, VC, VE, MDA, and Carbonyl
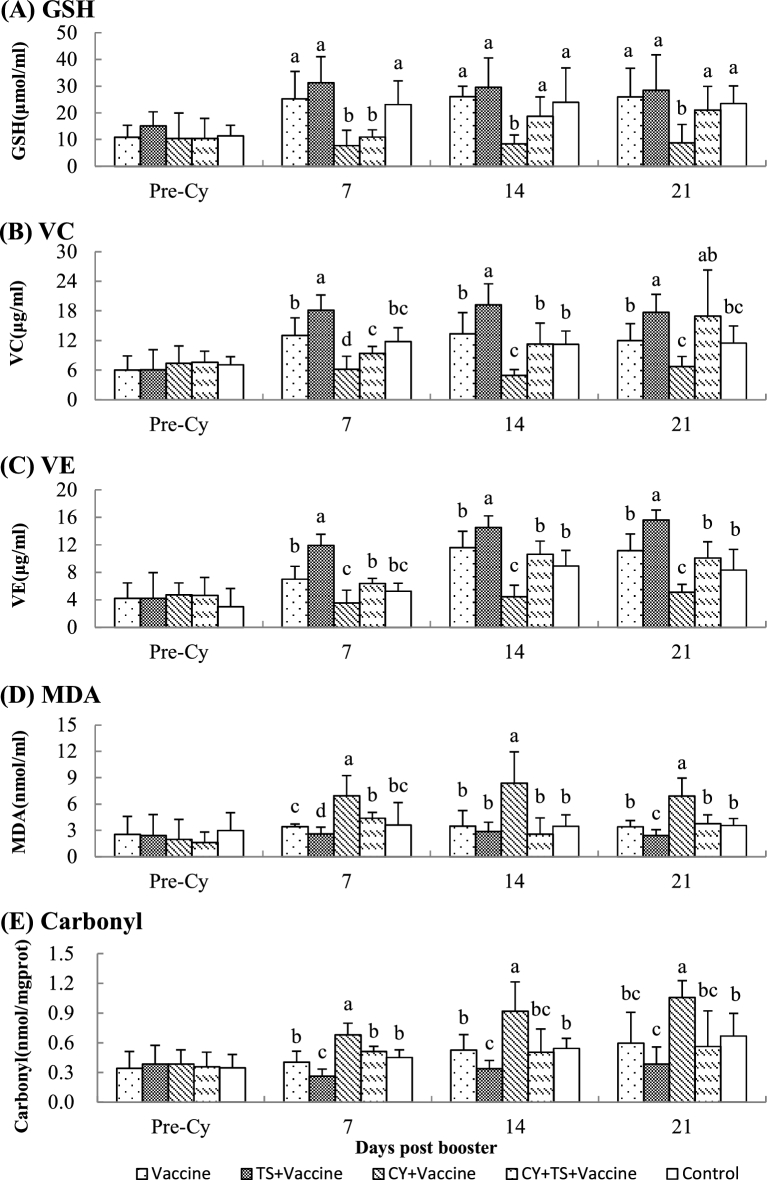
To investigate the effect of TS on serum GSH, VC, VE, MDA, and carbonyl, blood samples were collected after booster immunization. Figure 2 showed that there were no significant difference for serum GSH, VC, VE, MDA, and carbonyl between groups before medication; serum GSH, VC, and VE were significantly increased, and MDA and carbonyl were significantly decreased after birds were treated with TS (TS + vaccine); serum GSH, VC, and VE were significantly decreased, and MDA and carbonyl were significantly increased after birds were treated with Cy (Cy + vaccine). However, GSH, VC, and VE were significantly increased and MDA and carbonyl were significantly decreased in birds treated with Cy (Cy + vaccine) after they had been orally administered TS (Cy + TS + vaccine).
Figure 2.
Serum GSH, VC, VE, MDA and Carbonyl. Chickens were i.m. injected with Cy at 100 mg/kg BW for 3 days to induce immunosuppression and oxidative stress. After that, the birds were orally administered TS (5 mg/kg BW) in drinking water for 7 days. Blood samples were collected from 6 birds of each group before Cy treatment and 7, 14, 21 days after booster for analysis of serum GSH, VC, VE, MDA and Carbonyl. Data were expressed as mean ± S.D. (n = 6). Bars with different letters are statistically different (P < 0.05).
Effect of Tea Saponins on Lymphocyte Proliferative Responses to ConA and LPS
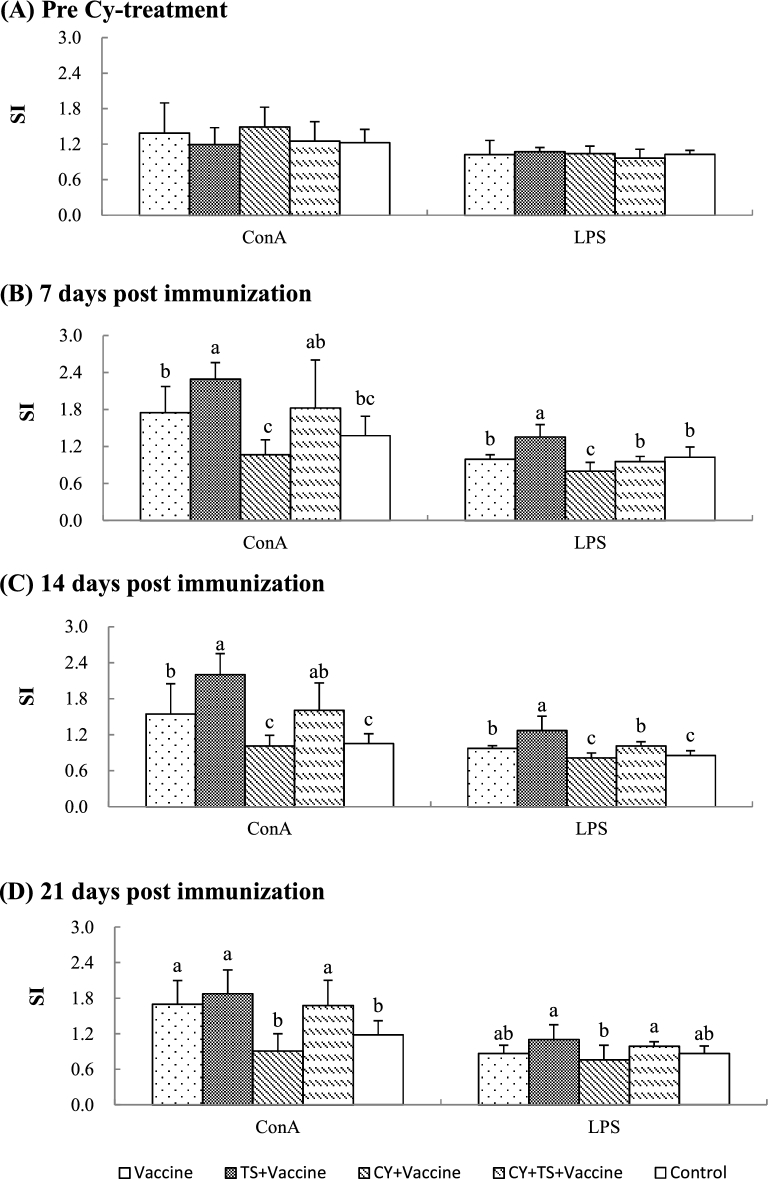
To investigate the effect of TS on the lymphocyte proliferative responses to mitogens in vitro, chickens were injected with Cy to induce immunosuppression and oxidative stress. After that, the birds were orally administered TS and then immunized with a bivalent vaccine of NDV and IBV. Splenocytes were prepared from the birds before and after immunization for lymphocyte proliferative assay. Figure 3 showed that no significant difference was found for stimulation indexes (SI) between groups before Cy treatment (Figure 3A). SI was significantly higher in birds treated with TS (TS + vaccine) (Figure 3B and C), and significantly lower in birds treated with Cy (Cy + vaccine) than the birds without TS treatment (vaccine) (Figure 3B, C and D). However, SI in birds treated with Cy significantly increased after administered TS (Cy + TS + vaccine) when compared to the birds treated with Cy (Cy + vaccine) (Figure 3B, C and D).
Figure 3.
Lymphocyte proliferative responses to ConA and LPS. Chickens were i.m. injected with Cy at 100 mg/kg BW for 3 days to induce immunosuppression and oxidative stress. After that, the birds were orally administered TS (5 mg/kg BW) in drinking water for 7 days, and then, intranasally immunized twice with a commercial live bivalent vaccine of NDV and IBV at 2 weeks intervals. Splenocytes were prepared from 6 birds of each group before Cy treatment and 7, 14, 21 days after booster for lymphocyte proliferative assay measured by the MTT method as described in the text. Data were expressed as mean ± S.D. (n = 6). Bars with different letters are statistically different (P < 0.05).
Effect of Tea Saponins on Serum Antibodies to NDV and IBV
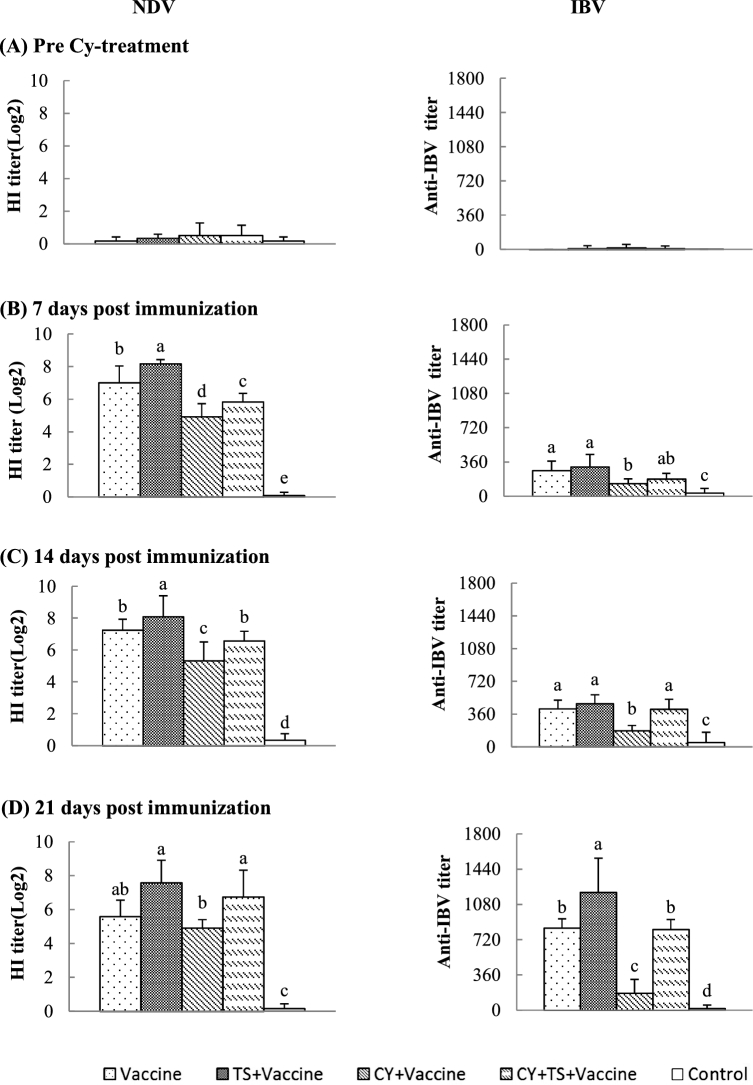
To investigate the effect of TS on the serum antibody responses to vaccination, blood samples were collected to measure the serum antibodies to NDV and IBV after immunization. Figure 4 showed that almost no specific NDV and IBV titers were detected in birds (Figure 4A) before immunization. However, antibody titers progressively increased after immunization. Significantly increased antibody titers to NDV (Figure 4B, C and D) and IBV (Figure 4D) in birds treated with TS (TS + vaccine) and decreased titers in birds treated with Cy (Cy + vaccine) (Figure 4B, C and D) were detected when compared to the birds without TS treatment (vaccine). However, the antibody titers in birds treated with Cy significantly increased after administered TS (Cy + TS + vaccine) when compared to the birds treated with Cy (Cy + vaccine).
Figure 4.
Antibody responses to a bivalent live vaccine of NDV and IBV. Chickens were i.m. injected with Cy at 100 mg/kg BW for 3 days to induce immunosuppression and oxidative stress. After that, the birds were orally administered TS (5 mg/kg BW) in drinking water for 7 days, and then, intranasally immunized twice with a commercial live vaccine of NDV and IBV at 2 weeks intervals. Blood samples were collected from 6 birds of each group before Cy treatment and 7, 14, 21 days after booster for analysis of NDV and IBV specific titers. Data were expressed as mean ± S.D. (n = 6). Bars with different letters are statistically different (P < 0.05).
Effect of Tea Saponins on Body Weight
No significant difference for body weight was found at each time point between the birds orally administered TS at 5 mg/kg BW and those without TS administration. And no abnormal behaviors and side effects were observed in chickens throughout the experiment.
DISCUSSION
The present study demonstrated that TS has antioxidant activities in chickens. Oral administration of TS enhanced enzymatic and non-enzymatic antioxidant defense mechanisms such as T-AOC, T-SOD, CAT, GSH-PX (Figure 1), GSH, VC, and VE (Figure 2A, B and C), and decreased the protein carbonyl content and MDA in oxidative stress induced by Cy (Figure 2D and E). No abnormal behaviors and side effects caused by TS were observed in chickens throughout the study.
In chickens, a balance between pro-oxidative and antioxidative activities is important. Overproduction or insufficient elimination of ROS will lead to oxidative stress (Yu et al., 2015b). There are enzymatic and non-enzymatic antioxidant defense mechanisms in the body. Enzymatic antioxidant defenses include total antioxidative capacity (T-AOC), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-PX), and catalase (CAT). Both T-SOD and CAT can degrade O2 and decompose H2O2, and result in a decrease in oxidative stress, which is an effective way of cell protection from damage (Nagi and Almakki 2009). Glutathione peroxidase (GSH-PX) is an enzyme responsible for scavenging ROS and its oxidation products to protect the body from lipid peroxidation (LPO) (Cui et al., 2010). The major function of GSH-PX, which uses glutathione as a substrate, is to reduce soluble hydrogen peroxide and alkyl peroxides (Bebe and Panemangalore 2003). All these enzymes work together to eliminate active oxygen species, and small deviations in physiological concentrations may have a dramatic effect on the resistance of cellular lipids, proteins, and DNA to oxidative damage (Ince et al., 2012).
Non-enzymatic antioxidants are represented by GSH, VC, and VE. GSH may protect cells against oxidative injury. The depletion of GSH due to Cy treatment will reduce its cellular level and lead to induction of oxidative stress (Yuan et al., 1991; Yousefipour et al., 2005). VC is a water-soluble antioxidant that helps to reduce the effect of oxidative stress (Eroglu et al., 2013). VC can easily react with free radicals in extracellular body fluids, as it is water soluble (Bendich 1990). VC exerts its antioxidant effects in both direct and indirect ways. VC directly scavenges the free radicals formed as a byproduct of metabolic reactions (Dawson et al., 1990). In an indirect way, VC helps recycling of oxidized VE, thus supplying active VE to fight against LPO (Netke et al., 1997). In addition, there are many studies demonstrating that VE protects cell membranes by preventing LPO. α-Tocopherol reacts with peroxyl radicals depending on its methylation state of the chromanol ring and the saturation grade of the side chain, forming tocopheroxyl radicals (Brigeliusflohé 2009). Tocopheroxyl radicals are converted to tocopherols by reacting with ascorbate (May et al., 1998). VE has been shown to play a role in various enzymes activities by enabling their translocation to the membrane (Kempná et al., 2004) or affecting their transcriptional activation process (Khor et al., 2000). In many studies, VE neutralizes LPO and unsaturated membrane lipids because of its oxygen scavenging effect (Aldana et al., 2001; John et al., 2001).
Cyclophosphamide (Cy) is a cytotoxic alkylating agent with a wide of clinical uses and has been proved to be effective originally used in the treatment of some types of cancers and nonmalignant diseases. Cy is transformed in the liver into active metabolites (4-hydroxycyclophosphamide, aldophosphamide, phosphoramide mustard, and others), which interfere with cellular DNA synthesis in rapidly dividing cells, thus leading to cell death (Perini et al., 2007). In the early study, Cy has been found to increase superoxide anion and H2O2 formation in cultured rat hepatocytes (Fuhrman et al., 1997) and different kidney cells (Hussein et al., 2005) and induce oxidative stress and lipoperoxidation (Reiter et al., 1999). The deleterious effects of Cy was, at least in part, due to the increased production of free radicals and ROS, which is associated with enhanced ROS generation and oxidative stress (Maestroni and Conti 1989). Thus Cy is frequently used to induce oxidative stress models (Tripathi and Jena 2009; Oboh et al., 2011). In addition, Cy has been reported to suppress both cellular and humoral immune responses (Bear 1986; Hoover et al., 1990), such as inhibiting lymphocyte activity and specific antibodies to NDV and IBV (Reynolds and Maraqa 1999; Loa et al., 2002; Wang et al., 2011). In this study, injection of Cy generated oxidative stress and lowered immune responses by reducing antioxidant enzymes such as T-AOC, T-SOD, GSH, and CAT, as well as inhibiting lymphocyte proliferation and antibody responses to vaccination. Decreased antioxidant activities in animals due to Cy were reported in other studies (Popovic et al., 2007; Singh et al., 2010; Ognjanović et al., 2012; Yu et al., 2015b).
Oral administration of ginseng stem-leaf saponins has recently been reported to reduce oxidative stress induced by Cy in chickens (Yu et al., 2015b). Similarly, reduced oxidative stress in chickens by oral administration of TS was found in this study. A stressful condition is closely related to the immune system. During oxidative stress, the excessive production of free radicals results in an imbalance in the oxidant system, and suppresses the immune function (Kucinski 1990). The immune system is particularly sensitive to stress and specific effects of stress have been demonstrated by a number of studies (Mastorakos and Ilias 2000; Wu et al., 2000; Mcewen 2001). The immune responses can be improved by removing immunosuppression (Srikumar 2006). In the present study, the increased antioxidant activities by oral administration of TS paralleled the enhanced immune responses to vaccination. TS-treatment significantly increased serum antibodies to NDV and IBV as well as the lymphocyte proliferation induced by ConA and LPS. Newcastle disease and infectious bronchitis virus infection are fatal and could result in large amount of economic losses in poultry industry (Callison et al., 2007; Ge et al., 2007). Immunization using live, attenuated vaccines is a common practice for the control of NDV and IBV in birds (Seto et al., 1974; Gelb et al., 1998). Figure 4 showed that oral administration of TS improved antibody responses to NDV and IBV in the birds in healthy and immune-suppressed by Cy. The enhanced immune responses could be due to reduction in oxidative stress resulting from oral administration of TS.
In conclusion, antioxidative and immunopotentiating effects of tea saponins (TS) on oxidative stress induced by cyclophosphamide in chickens were demonstrated. Oral administration of TS at a dose of 5 mg/kg BW significantly improved the antioxidant activity, as indicated by increasing T-AOC, T-SOD, CAT, GSH-PX, GSH, VC, VE, as well as by decreasing MDA and the protein carbonyl. The increased antioxidant activity paralleled increased specific antibody responses to NDV and IBD vaccine, lymphocyte proliferative responses induced by ConA and LPS. Considering TS has its antioxidant activity and plentiful resources, TS may be a potential agent to relieve oxidative stress in immunosuppressed chickens in poultry industry.
Acknowledgements
This study was supported by the Special Fund for Agro-Scientific research in the Public Interest(2013203040). The authors wish to thank the students in the Laboratory of Traditional Chinese Veterinary Medicine (TCVM Lab) for their assistance in sample collection.
REFERENCES
- Abegg M. A., Alabarse P. V., Schüller A. K., Benfato M. S.. 2012. Glutathione levels in and total antioxidant capacity of Candida sp. cells exposed to oxidative stress caused by hydrogen peroxide. Rev. Soc. Bras. Med. Trop. 45:620–626. [DOI] [PubMed] [Google Scholar]
- Aebi H. 1984. Catalase in vitro. Method Enzymol. 105:121–126. [DOI] [PubMed] [Google Scholar]
- Aldana L., Tsutsumi V., Craigmill A., Silveira M. I., Gonzalez D. M. E.. 2001. alpha-Tocopherol modulates liver toxicity of the pyrethroid cypermethrin. Toxicol. Lett. 125:107–116. [DOI] [PubMed] [Google Scholar]
- Avanzo J. L., de Mendonça C. X. Jr., Pugine S. M., de Cerqueira Cesar M.. 2001. Effect of vitamin E and selenium on resistance to oxidative stress in chicken superficial pectoralis muscle. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 129:163–173. [DOI] [PubMed] [Google Scholar]
- Bear H. D. 1986. Tumor-specific suppressor T-cells which inhibit the in vitro generation of cytolytic T-cells from immune and early tumor-bearing host spleens. Cancer Res. 46:1805–1812. [PubMed] [Google Scholar]
- Bebe F. N., Panemangalore M.. 2003. Exposure to low doses of endosulfan and chlorpyrifos modifies endogenous antioxidants in tissues of rats. J. Envir. Sci. Health Pt. B. 38:349–363. [DOI] [PubMed] [Google Scholar]
- Bendich A. 1990. Antioxidant micronutrients and immune responses. Ann. N.Y. Acad. Sci. 587:168–180. [DOI] [PubMed] [Google Scholar]
- Benzie I. F., Szeto Y. T.. 1999. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 47:633–636. [DOI] [PubMed] [Google Scholar]
- Brigeliusflohé R. 2009. Vitamin E: the shrew waiting to be tamed. Free Radical Biol. Med. 46:543–554. [DOI] [PubMed] [Google Scholar]
- Cabrera C., Giménez R., López M. C.. 2003. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 51:4427–4435. [DOI] [PubMed] [Google Scholar]
- Callison S. A., Hilt D. A., Boynton T. O., Sample B. F., Robison R., Swayne D. E., Jackwood M. W.. 2007. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J. Virol. Methods 138:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. H., Chan J. Y.. 2013. Angiotensin-generated reactive oxygen species in brain and pathogenesis of cardiovascular diseases. Antioxid. Redox Sign. 19:1074–1084. [DOI] [PubMed] [Google Scholar]
- Cui J. J., Yuan J. F., Zhang Z. Q.. 2010. Anti-oxidation activity of the crude polysaccharides isolated from Polygonum cillinerve (Nakai) Ohwi in immunosuppressed mice. J. Ethnopharmacol. 132:512–517. [DOI] [PubMed] [Google Scholar]
- Dawson E. B., Harris W. A., Powell L. C.. 1990. Relationship between Ascorbic Acid and Male Fertility. World Rev. Nutr. Dietetics 62:1–26. [DOI] [PubMed] [Google Scholar]
- Emmerie A., Engel C.. 1938. Colorimetric determination of DL-alpha-tocopherol (vitamin E). Nature 142. [Google Scholar]
- Fuhrman B., Elis A., Aviram M.. 1997. Hypocholesterolemic effect of lycopene and beta-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem. Biophys. Res. Commun. 233:658–662. [DOI] [PubMed] [Google Scholar]
- Ge J., Deng G., Wen Z., Tian G., Wang Y., Shi J., Wang X., Li Y., Hu S., Jiang Y.. 2007. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J. Virol. 81:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M. B. 2014. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 6:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover S. K., Barrett S. K., Turk T. M. T.. 1990. Cyclophosphamide and abrogation of tumor-induced suppressor T cell activity. Cancer Immunol. Immunother. 31:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M. R., Abu-Dief E. E., El-Reheem M. H. A., Abd-Elrahman A.. 2005. Ultrastructural evaluation of the radioprotective effects of melatonin against X-ray-induced skin damage in Albino rats. Int. J. Exp. Pathol. 86:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince S., Keles H., Erdogan M., Hazman O., Kucukkurt I.. 2012. Protective effect of boric acid against carbon tetrachloride-induced hepatotoxicity in mice. Drug Chem. Toxicol. 35:285–292. [DOI] [PubMed] [Google Scholar]
- Iqbal M., Cawthon D., Wideman R. F., Bottje W. G.. 2001. Lung mitochondrial dysfunction in pulmonary hypertension syndrome. II. Oxidative stress and inability to improve function with repeated additions of adenosine diphosphate. Poult. Sci. 80:656–665. [DOI] [PubMed] [Google Scholar]
- Jang H. D., Chang K. S., Huang Y. S., Hsu C. L., Lee S. H., Su M. S.. 2007. Principal phenolic phytochemicals and antioxidant activities of three Chinese medicinal plants. Food Chem. 103:749–756. [Google Scholar]
- John S., Kale M., Rathore N., Bhatnagar D.. 2001. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J. Nutr. Biochem. 12:500–504. [DOI] [PubMed] [Google Scholar]
- Gelb J. Jr, Nix W. A., Gellman S. D.. 1998. Infectious bronchitis virus antibodies in tears and their relationship to immunity. Avian Dis. 42:364–374. [PubMed] [Google Scholar]
- Panda A. K., Cherian G.. 2013. Role of Vitamin E in Counteracting Oxidative Stress in Poultry. J. Poult. Sci. 51:109–117. [Google Scholar]
- Kempná P., Reiter E., Arock M., Azzi A., Zingg J. M.. 2004. Inhibition of HMC-1 mast cell proliferation by vitamin E: involvement of the protein kinase B pathway. J. Biol. Chem. 279:50700–50709. [DOI] [PubMed] [Google Scholar]
- Khor H. T., Ng T. T., Henry C. J. K.. 2000. Effects of administration of α-tocopherol and tocotrienols on serum lipids and liver HMG CoA reductase activity. Int. J. Food Sci. Nutr. 51:S3–S11. [PubMed] [Google Scholar]
- Kim S. H., Kwon C. H., Nakano I.. 2014. Detoxification of oxidative stress in glioma stem cells: mechanism, clinical relevance, and therapeutic development. J. Neurosci. Res. 92:1419–1424. [DOI] [PubMed] [Google Scholar]
- Kucinski B. J. 1990. Evidence that shock-induced immune suppression is mediated by adrenal hormones and peripheral beta-adrenergic receptors. Pharmacol. Biochem. Behav. 36:645–651. [DOI] [PubMed] [Google Scholar]
- Li R. L., Sakwiwatkul K., Li Y., Hu S. H.. 2009. Enhancement of the immune responses to vaccination against foot-and-mouth disease in mice by oral administration of an extract made from Rhizoma Atractylodis Macrocephalae (RAM). Vaccine 27:2094–2098. [DOI] [PubMed] [Google Scholar]
- Loa C. C., Lin T. L., Wu C. C., Bryan T., Hooper T., Schrader D.. 2002. The effect of immunosuppression on protective immunity of turkey poults against infection with turkey coronavirus. Comp. Immunol. Microbiol. Infect. Dis. 25:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A.. 1989. Beta-endorphin and dynorphin mimic the circadian immunoenhancing and anti-stress effects of melatonin. Int. J. Immunopharmacol. 11:333–340. [DOI] [PubMed] [Google Scholar]
- Mastorakos G., Ilias I.. 2000. Maternal hypothalamic-pituitary-adrenal axis in pregnancy and the postpartum period: postpartum-related disorders. Ann. N.Y. Acad. Sci. 900:95–106. [DOI] [PubMed] [Google Scholar]
- May J. M., Qu Z. C., Mendiratta S.. 1998. Protection and recycling of α-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch. Biochem. Biophys. 349:281–289. [DOI] [PubMed] [Google Scholar]
- Mcewen B. S. 2001. The neurobiology of stress: from serendipity to clinical relevance 1. Brain Res. 886:172–189. [DOI] [PubMed] [Google Scholar]
- Mihara M., Uchiyama M.. 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86:271–278. [DOI] [PubMed] [Google Scholar]
- Nagi M. N., Almakki H. A.. 2009. Thymoquinone supplementation induces quinone reductase and glutathione transferase in mice liver: possible role in protection against chemical carcinogenesis and toxicity. Phytother. Res. 23:1295–1298. [DOI] [PubMed] [Google Scholar]
- Netke S. P., Roomi M. W., Tsao C., Niedzwiecki A.. 1997. Ascorbic acid protects guinea pigs from acute aflatoxin toxicity. Toxicol. Appl. Pharmacol. 143:429–435. [DOI] [PubMed] [Google Scholar]
- Oboh G., Akomolafe T. L., Adefegha S. A., Adetuyi A. O.. 2011. Inhibition of cyclophosphamide-induced oxidative stress in rat brain by polar and non-polar extracts of annatto (Bixa orellana) seeds. Exp. Toxicol. Pathol. 63:257–262. [DOI] [PubMed] [Google Scholar]
- Ognjanović B. I., Djordjević N. Z., Matić M. M., Obradović J. M., Mladenović J. M.. 2012. Lipid peroxidative damage on cisplatin exposure and alterations in antioxidant defense system in rat kidneys: a possible protective effect of selenium. Int. J. Molec. Sci. 13:1790–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore R. L., Fratellone P.. 2006. Potential health benefits of green tea (Camellia sinensis): A narrative review. J. Sci. Healing 2:531–539. [DOI] [PubMed] [Google Scholar]
- Perini P., Calabrese M., Rinaldi L., Gallo P.. 2007. The safety profile of cyclophosphamide in multiple sclerosis therapy. Expert Opin. Drug Saf. 6:183–190. [DOI] [PubMed] [Google Scholar]
- Peyre M., Fusheng G., Desvaux S., Roger F.. 2009. Avian influenza vaccines: a practical review in relation to their application in the field with a focus on the Asian experience. Epidemiol. Infect. 137:1–21. [DOI] [PubMed] [Google Scholar]
- Pirinccioglu A. G., Gökalp D., Pirinccioglu M., Kizil G., Kizil M.. 2010. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin Biochem 43:1220–1224. [DOI] [PubMed] [Google Scholar]
- Popovic M., Kolarovic J., Mikov M., Trivic S., Kaurinovic B.. 2007. Anthracycline-based combined chemotherapy in the mouse model. Eur. J. Drug Metab. Pharm. 32:101–108. [DOI] [PubMed] [Google Scholar]
- Reiter R. J., Tan D. X., Cabrera J., Arpa D. D, Sainz R. M., Mayo J. C., Ramos S.. 1999. The oxidant/antioxidant network: role of melatonin. Biol. Sign. Recept. 8:56–63. [DOI] [PubMed] [Google Scholar]
- Reynolds D. L., Maraqa A. D.. 1999. A technique for inducing b-cell ablation in chickens by in ovo injection of cyclophosphamide. Avian Dis. 43:367–375. [PubMed] [Google Scholar]
- Eroglu S., Pandir D., FG. 2013. Protective role of vitamins C and E in diclorvos-induced oxidative stress in human erythrocytes in vitro. Biol. Res. 46:33–38. [DOI] [PubMed] [Google Scholar]
- Seto J. T., Becht H., Rott R.. 1974. Effect of specific antibodies on biological functions of the envelope components of Newcastle disease virus. Virology 61:354–360. [DOI] [PubMed] [Google Scholar]
- Singh G., Maulik S. K., Jaiswal A., Kumar P., Parshad R.. 2010. Effect on antioxidant levels in patients of breast carcinoma during neoadjuvant chemotherapy and mastectomy. Malaysian J. Med. Sci. 17:24–28. [PMC free article] [PubMed] [Google Scholar]
- Srikumar R. 2006. Effect of Triphala on oxidative stress and on cell-mediated immune response against noise stress in rats. Mol. Cell Biochem. 283:67–74. [DOI] [PubMed] [Google Scholar]
- Sun Y., Oberley L. W., Li Y.. 1988. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 34:497–500. [PubMed] [Google Scholar]
- Surai P. F. 2000. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 41:235–243. [DOI] [PubMed] [Google Scholar]
- Tavárez M. A., Boler D. D., Bess K. N., Zhao J., Yan F., Dilger A. C., Mckeith F. K., Killefer J.. 2011. Effect of antioxidant inclusion and oil quality on broiler performance, meat quality, and lipid oxidation. Poult. Sci. 90:922–930. [DOI] [PubMed] [Google Scholar]
- Tripathi D. N., Jena G. B.. 2009. Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: A study in mice. Chem.-Biol. Interact 180:398. [DOI] [PubMed] [Google Scholar]
- Voljč M., Frankič T., Levart A., Nemec M., Salobir J.. 2011. Evaluation of different vitamin E recommendations and bioactivity of α-tocopherol isomers in broiler nutrition by measuring oxidative stress in vivo and the oxidative stability of meat. Poult. Sci. 90:1478–1488. [DOI] [PubMed] [Google Scholar]
- Wang H., Wang M., Chen J., Tang Y., Dou J., Yu J., Xi T., Zhou C.. 2011. A polysaccharide from Strongylocentrotus nudus eggs protects against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int. Immunopharmacol. 11:1946–1953. [DOI] [PubMed] [Google Scholar]
- Wheeler C. R., Salzman J. A., Elsayed N. M., Omaye S. T., Jr K. D.. 1990. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 184:193–199. [DOI] [PubMed] [Google Scholar]
- Wu W., Yamaura T., Murakami K., Murata J., Matsumoto K., Watanabe H., Saiki I.. 2000. Social isolation stress enhanced liver metastasis of murine colon 26-L5 carcinoma cells by suppressing immune responses in mice. Life Sci. 66:1827–1838. [DOI] [PubMed] [Google Scholar]
- Yashin A., Yashin Y., Nemzer B.. 2011. Determination of antioxidant activity in tea extracts, and their total antioxidant content. Am. J. Biomed. Sci. 3:322–335. [Google Scholar]
- Yousefipour Z., Ranganna K., Newaz M. A., Milton S. G.. 2005. Mechanism of acrolein-induced vascular toxicity. J. Physiol. Pharmacol. 56:337. [PubMed] [Google Scholar]
- Yu J., Shi F. S., Hu S.. 2015a. Improved immune responses to a bivalent vaccine of Newcastle disease and avian influenza in chickens by ginseng stem-leaf saponins. Vet. Immunol. Immunopathol. 167:147–155. [DOI] [PubMed] [Google Scholar]
- Yu J., Chen Y., Zhai L., Zhang L., Xu Y., Wang S., Hu S.. 2015b. Antioxidative effect of ginseng stem-leaf saponins on oxidative stress induced by cyclophosphamide in chickens. Poult. Sci. 94:927–933. [DOI] [PubMed] [Google Scholar]
- Yuan Z. M., Smith P. B., Brundrett R. B., Colvin M., Fenselau C.. 1991. Glutathione conjugation with phosphoramide mustard and cyclophosphamide. A mechanistic study using tandem mass spectrometry. Drug Metab. Disp. 19:625–629. [PubMed] [Google Scholar]
- Zannoni V., Lynch M., Goldstein S., Sato P.. 1974. A rapid micromethod for the determination of ascorbic acid in plasma and tissues. Biochem. Med. 11:41–48. [DOI] [PubMed] [Google Scholar]
- Zhai L., Li Y., Wang W., Wang Y., Hu S.. 2011a. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine 29:5007–5014. [DOI] [PubMed] [Google Scholar]
- Zhai L., Li Y., Wang W., Hu S.. 2011b. Enhancement of humoral immune responses to inactivated Newcastle disease and avian influenza vaccines by oral administration of ginseng stem-and-leaf saponins in chickens. Poult. Sci. 90:1955–1959. [DOI] [PubMed] [Google Scholar]
- Zhai L., Wang Y., Yu J., Hu S.. 2014. Enhanced immune responses of chickens to oral vaccination against infectious bursal disease by ginseng stem-leaf saponins. Poult. Sci. 93:2473–2481. [DOI] [PubMed] [Google Scholar]