ABSTRACT
Vaccination programs against infectious bronchitis virus (IBV) in Egypt depend on both classical and/or imported variant IBV strain vaccines. However, many IBV outbreaks associated with respiratory distress, nephropathy, and high mortalities were attributed to the circulation of both classical and new nephropathogenic IBV variant 2 strains. In the present study, we report the development of attenuated IBV candidate vaccines using the classic IBV strains (IBM41 and IB2) and a nephropathogenic strain (IBvar2). The wild-type (WT) viruses were attenuated through serial passages in embryonated specific pathogen free (SPF) chicken eggs. Virulence of the attenuated viruses was then tested via the ocular route inoculation and the in vivo back passage in day-old SPF chickens. Efficacy against homologous challenge was investigated also in day-old SPF chickens. Results showed that the viruses were successfully adapted to the embryo by the 100th (IBM41 and IB2) and 110th passages (IBvar2). The attenuated viruses were safe and showed no change of virulence in day-old SPF chickens up to the 10th back passages. The efficacy experiment showed that the attenuated vaccines showed 90 to 100% protection against the homologous challenge based on ciliostasis score and protection percent. The att-IBM41 and att-IB2 vaccines were able to reduce the shedding of the challenge at 3 days post-infection (DPI) and no virus shedding was detected in both vaccinated groups by 5 DPI. In the att-IBvar2 vaccinated birds, only 20% of vaccinated birds shed the challenge virus with low titers (102.10±0.3 EID50/mL) at 3 DPI. In conclusion, the attenuated strains IBM41, IB2, and IBvar2 are efficient vaccine candidates against currently circulating classic and variant IB viruses, respectively. Further studies to evaluate the field efficacy and combining these attenuated IBV strains to induce a wider protection against heterologous IBV challenge are suggested.
Keywords: attenuated vaccine, classic, infectious bronchitis, variant 2, Middle East
INTRODUCTION
Avian infectious bronchitis (IB) is a highly contagious disease of chickens affecting the respiratory, renal, and reproductive systems. The IB was described as early as 1931 in North Dakota, USA. Since then, the IB virus (IBV) is circulating in different parts of the world with several IBV serotypes that led the IB control and prevention problem to persist (Fabricant, 1998). Though the disease affects chickens of all ages, the clinical disease is more severe in young birds with high mortalities (Cavanagh, 2005). The secondary bacterial infections and/or other respiratory pathogens have been reported to exaggerate both clinical outcome and mortality during IBV infections (Haghighat-Jahromi et al., 2008; Hassan et al., 2016, 2017).
The IBV is a single-stranded enveloped RNA virus and is a member of the genus Gammacoronavirus belonging to the family Coronaviridae (Cavanagh, 2001). The clinical signs of the IBV infection vary depending on the tissue tropism and the pathogenicity. Clinical signs include coughing, sneezing, tracheal rales, and watery eyes. Lesions in infected birds include degeneration of renal and ciliated respiratory epithelia (Cavanagh, 2007). The IBV epidemiology in Egypt reported the circulation of the virus in poultry flocks with continuous evolution despite massive vaccination programs. Different strains similar to Massachusetts, D3128, D274, D08880, and 793B are frequently isolated from Egyptian poultry (Abdel-Moneim et al., 2006; Abdel-Moneim et al., 2012; Zanaty et al., 2016).
Studies classified the Egyptian IBV strains on the basis of their HVR3 sequences into Egyptian Variant 1 (Egy/Var-I), represented by the strain Egypt/Beni-Suef/01 (Abdel-Moneim et al., 2006) and Egyptian Variant 2 (Egy/Var-II), including recent Egyptian IBV strains that resemble the Israeli IS/885/00 strain (Abdel-Moneim et al., 2012). The continuous mutation and recombination events and the emergence of novel IBV variants urge researchers to establish a simple phylogeny-based classification system. Thus, a recent study indicated that both Egy/Var-I and Egy/Var-II Egyptian IBV strains belong to a unique wild-type (WT) cluster confined to the Middle East region (GI-23 lineage) (Valastro et al., 2016).
Vaccination remains the main control approach of IBV infection. However, the continuous genetic, antigenic, and tissue tropism changes of the circulating IBV caused continual vaccine failure events (Cook et al., 2012). IBV vaccines are based on live attenuated or killed vaccines derived from classical or variant serotypes. In Egypt, Mass-type and variant (e.g., 4/91 and CR88) vaccine strains were employed to provide broader protection in poultry. However, the uniqueness of the WT IBV lineage in the Middle East (Valastro et al., 2016) and the lack of cross protection between imported vaccine strains and field strains may explain the failure to establish an effective vaccination program against IBV (Kim et al., 2013; Toro et al., 2015).
Several studies indicated the inability of the currently available vaccines to provide adequate protection against the Egy/Var-I and Var-II IBV strains (Hassan et al., 2016; Zanaty et al., 2016). Therefore, this study aimed to develop and evaluate live attenuated vaccines from IBV strains currently circulating in the Middle East including an Egy/Var-II strain belonging to the unique Middle East lineage (GI-23). The candidate vaccine's safety was evaluated in both day-old and layer specific pathogen free (SPF) chickens. Finally, the protective efficacy of the attenuated strains was investigated against WT virus challenge.
MATERIALS AND METHODS
Viruses
The IBV viruses used were obtained from the Reference Laboratory for Veterinary Quality Control on Poultry Production (NLQP), Animal Health Research Institute, Giza, Egypt. The IBV strain IBV-EG/11539F/2011 (accession no.: JQ839289) and IBV-EG/ M41-ME01/2011 (accession no.: MG334195), classic Mass-like strains belonging to the GI-1 lineage Both viruses were isolated from 21- to 23-d-old commercial broilers suffering from respiratory problem. These chickens had a history of vaccination against IBV using H120 vaccine. The IBV strain Eg/1212B/2012 (accession no.: JQ839287) was isolated from 14-d-old vaccinated chick suffering from respiratory symptoms and renal disease (Zanaty et al., 2016) and identified as Egy/Var-II. The viruses were designated IB2, IBM41, and IBvar2 for IBV-EG/11539F-2011, IBV-EG/M41-ME01/2011, and Eg/1212B/2012, respectively. The WT IBM41, IB2, and IBvar2 viruses were used for the homologous challenge at titers of 106.2, 106.0, and 105.0 EID50/0.2 mL, respectively.
Viruses Attenuation
For attenuation purposes, the viruses were confirmed to be pure from extraneous viruses and bacteria at the Reference Laboratory for Veterinary Quality Control on Poultry Production (NLQP), Animal Health Research Institute, Giza, Egypt in accordance with OIE guidelines (Eterradossi and Britton, 2013) (Data not shown). The viruses passaged for 100 times for IBM41 and IB2 viruses and for 110 times for IBvar2 via allantoic sac inoculation in 10-d-old SPF eggs. Inoculated eggs were incubated for 40 h at 37°C. Eggs that died within 24 h of inoculation were discarded. The allantoic fluid was harvested for subsequent passages. Every 10 passages, viruses were confirmed by RT-PCR (Huo et al., 2016). The final embryo infective dose 50% (EID50) of the attenuated viruses were determined in SPF embryonated chicken eggs (Reed and Munech, 1938). The attenuated viruses were designated att-IB2, att-IBM41, and att-IBvar2.
Chicken Experiments
In all experiments, white leghorn SPF chickens were used to determine the safety and efficacy of attenuated viruses. The SPF chickens were purchased from Nile SPF company (Kom-Osheim, Fayoum, Egypt). Birds were kept in biosafety level III chicken isolators and all experimental procedures were reviewed and approved by the Ethical and Animal Welfare Committee at ME VAC Company.
Safety of Attenuated IBV Viruses in Day-old SPF Chickens
A total of 140-d-old SPF white leghorn chicks were randomly divided into 7 groups of 20 chickens each. The chickens were maintained in biosafety level III chicken isolators. The first group was kept as a PBS-inoculated negative control. For each virus strain, 2 chicken groups were inoculated via the ocular route with 104 EID50/bird of either the WT or the attenuated virus separately. All groups were observed for 21 days post-inoculation (DPI), and the IBV clinical signs were scored according to Zhao et al. (2015) as follows: 0 = normal, 1 = mild depression, 2 = severe depressed, 3 = paralysis/prostration, and 4 = death. Three chicks from each group were sacrificed at 3, 5, and 7 DPI for ciliostasis evaluation as well as kidney and tracheal gross lesions scoring (Huang and Wang, 2006).
Safety of Attenuated IBV Viruses in 25-wk-old Layer Chickens
Forty-nine 25-wk-old white leghorn chicks were randomly divided into 7 groups (7 chickens/group). The chickens were maintained in isolators for adaptation and until they reached their normal egg production levels. The first group was kept as a PBS-inoculated negative control. Two groups for each virus strain were inoculated via the ocular route with 104 EID50/bird of either the WT or the attenuated virus separately. All groups were observed for 14 DPI for clinical signs, daily egg production, and egg quality.
In Vivo Reversion of Virulence of the Attenuated IBV Viruses
To examine the in vivo reversion of virulence of the attenuated-IBM41, IB2, and IBvar2 viruses; 1-d-old SPF chicks were divided into 4 groups of 3 chicks. The 3 inoculated groups received 104 EID50/bird of the attenuated viruses separately via the ocular route. The fourth group was kept as a PBS-inoculated negative control. Birds were observed daily for clinical signs for 5 d. At 5 DPI, the virus detection was confirmed by RT-PCR in kidneys and tracheal homogenates using a specific primer set for amplification of the HVR-3 of the IBV-S1 gene as previously described (Selim et al., 2013). The tissue homogenates were then used to inoculate a second set of birds. The back passage of the attenuated viruses was repeated for 10 times. At the 1st, 5th, and 10th passages of each virus, tracheal and kidney lesions were scored as described in the safety study.
Efficacy of Attenuated IBV Viruses Against Homologous Virus Challenges
One hundred and twenty 1-d-old SPF white leghorn chicks were randomly divided into 6 groups of 20 chickens each. The first 3 groups were vaccinated via the ocular route with 103.5 EID50/bird of att-IBM41 virus, att-IB2, and att-IBvar2 viruses, respectively. Two groups served as unvaccinated challenge controls for WT IBM41, and IBvar2, respectively. The sixth group was kept as unvaccinated unchallenged control. At 21 days post-vaccination, birds in each group were challenged with 105 EID50/bird of the homologous WT virus as shown in Table 1. All birds were observed daily for IB infection clinical signs for 15 DPI. At 3 and 5 DPI, 3 birds from each group were euthanized for gross lesions observation and ciliary activity evaluation.
Table 1.
Experimental vaccination and virus challenge groups.
| No. of | Vaccine | Challenge virus | |||
|---|---|---|---|---|---|
| Group | chicks | dose | Day | Virus | Dose |
| att-IBM41 | 20 | 103.5 | 21 | WT IBM41 | 105 |
| att-IB2 | 20 | 103.5 | 21 | WT IBM41 | 105 |
| att-IBvar2 | 20 | 103.5 | 21 | WT IBvar2 | 105 |
| Challenge control-I | 20 | – | 21 | WT IBM41 | 105 |
| Challenge control-II | 20 | – | 21 | WT IBvar2 | 105 |
| Negative control | 20 | – | – | – | – |
WT, wild-type
Virus shedding detection in oropharyngeal swabs
Oropharyngeal swabs were collected from each group at 3, 5, and 7 DPI in 1 mL PBS to monitor virus shedding titers. The collected swab samples were processed on the same day of collection. Swabs were vortexed and then centrifuged at 2000 rpm for 10 minutes at 4°C to pellet the debris. The supernatants were then used for virus titration in 10-d-old embryonated SPF chicken eggs and the EID50/mL was calculated (Reed and Munech, 1938).
Ciliostasis Evaluation
In safety testing and post-challenge experiments, the tracheal ciliary activity was assessed as previously described (Cook et al., 1999). Briefly, 3 sections of the upper, middle, and lower parts of the trachea were analyzed. The rings were placed in a Petri dish containing Minimum Essential Medium with 10% fetal bovine serum. The integrity and the ciliary movement of the tracheal epithelial cells were evaluated by inverted light microscope. Ciliostasis scoring was 0 for the tracheal section showing complete movement, 1 for tracheal sections with 75 to 100% movement, 2 if the cilia in 50 to 75% of the trachea showed movement, 3 if the cilia in 25 to 50% of the trachea section showed movement, and 4 if the cilia in less than 25% of the trachea section showed movement or no movement at all. The average ciliostasis score for each group was calculated.
Statistical Analysis
The differences in ciliostasis scores and virus shedding titers at 3 DPI were estimated using one-way ANOVA with Tukey's post-test through GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com).
RESULTS
Viruses Attenuation
The 3 viruses (IBV-EG/11539F-2011, IBV-EG/M41-ME01/2011, and Eg/1212B/2012) were successfully adapted to embryonated chicken eggs and showed the typical embryonic changes including dwarfing, stunting, and curling of embryos by the 10th or 14th passages for the classic (IB2 and IBM41) and the variant virus (IBvar2) (data not shown). By the 100th passage, the classic IB2 and IBM41 were efficiently growing to titers of 107.2 and 106.9 EID50/mL, respectively. However, the IBvar2 virus consistently reached the titer of 106.8 EID50/mL by the 110th passage.
Safety in Day-old SPF Chickens
The WT viruses induced depression, ruffled feathers, and respiratory rales in day-old SPF chicks; however, no mortality was recorded in any of the inoculated birds. Upon necropsy, tracheal congestion was observed in the trachea in all WT viruses. The WT IB-VAR2 induced kidneys swelling and urate deposition. The attenuated viruses did not show any clinical signs in inoculated birds. No gross lesions in both trachea and kidneys were observed as compared to birds inoculated with the WT viruses. Only the att-IBvar2 showed an average lesion score of 0.6 and 1.0 in the trachea and kidney, respectively. The WT viruses induced ciliostasis in the tracheal epithelium especially WT IBM41 and IBvar2 (3.6 ± 0. 14 and 3.9 ± 0.18, respectively) as compared to IB2 (3.1 ± 0.57). However, the attenuated strains had significantly (P < 0.001) lower tracheal ciliostasis (≤1.3) than those induced by WT viruses (Figure 1).
Figure 1.
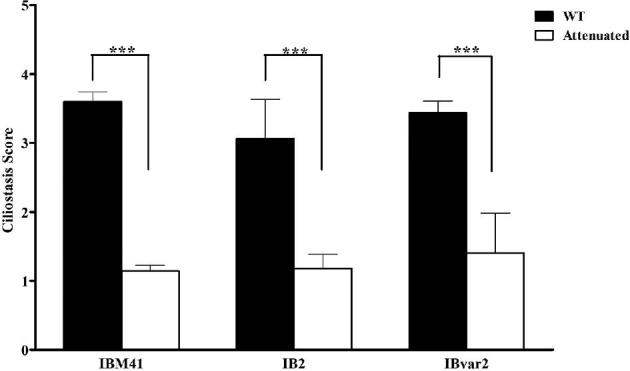
Trachea ciliostasis scores in day-old chickens inoculated with wild-type and attenuated IBM41, IB2, and IBvar2 strains. The significance of P < 0.001 is indicated (***).
Safety in 25-wk-old Layer Chickens
The egg production dropped in the 3 WT viruses-inoculated chicken groups. Egg quality deterioration including watery albumin, rough shell, and shelless eggs was also observed. Chickens also showed mild respiratory signs with no mortality in the inoculated birds. Neither clinical signs nor drop in egg production were observed in the attenuated viruses inoculated group with the exception of day 1 post inoculation where a slight drop was observed in all groups including the negative control group (Figure 2). The egg production declined to 28.6 to 42.8% between 3 and 7 DPI in WT viruses inoculated groups. Meanwhile, in all attenuated viruses inoculated groups, the egg production was comparable to the negative control group (71.4 to 85.7%).
Figure 2.
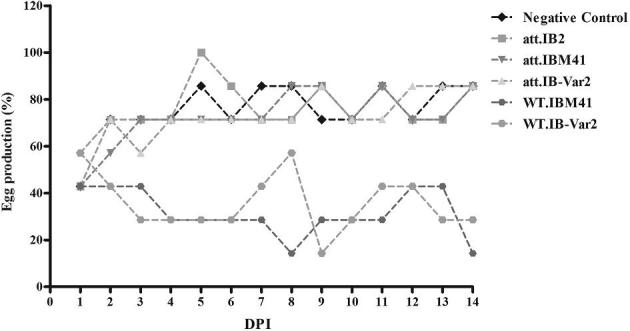
Daily egg production in 25-wk-old layer chickens inoculated with wild-type and attenuated IBM41, IB2, and IBvar2 strains.
In Vivo Reversion of Virulence of the Attenuated IBV Viruses
The 3 attenuated viruses were consistently detected in tissue homogenates of inoculated birds at all passages. No clinical signs were observed in any of the inoculated birds after 5 passages. No significant differences were found between the tracheal and renal lesion scores of the 1st, 5th, and 10th passages of the attenuated IBM41, IB2, and IBvar2 viruses (Table 2).
Table 2.
The results of in vivo reversion of virulence examination of the attenuated viruses in day-old specific pathogen free (SPF) chicks.
| Virusa | Passage no. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P5 | P10 | |||||||||||||
| C. signs | Lesions score | PCR detection | C. signs | Lesions score | PCR detection | C. signs | Lesions score | PCR detection | |||||||
| Trachea | kidneys | Trachea | kidneys | Trachea | kidneys | Trachea | kidneys | Trachea | kidneys | Trachea | kidneys | ||||
| att-IBM41 | None | 0 | 0.67 | + | + | None | 0 | 0 | + | + | None | 0 | 0 | + | + |
| att-IB2 | None | 0 | 0 | + | + | None | 0 | 0 | + | + | None | 0 | 0 | + | + |
| att-IBvar2 | None | 0.67 | 1.3 | + | + | None | 0.67 | 1.0 | + | + | None | 1 | 1.3 | + | + |
aDay-old SPF chicks received 104 EID50/bird of the attenuated viruses separately via the ocular route. Birds were observed daily for clinical signs for 5 d. At 5 days post-infection, the virus detection was confirmed by RT-PCR in kidneys and tracheal homogenates. The tissue homogenates were then used to inoculate a second set of birds. At the 1st, 5th, and 10th passages; tracheal and kidney lesions were scored.
WT, wild-type
Efficacy Against Homologous Challenge in SPF Chickens
Challenge experiments were conducted separately for birds vaccinated with the classic viruses, att-IBM41 and att-IB2 and those vaccinated with att-IBvar2 using the homologous virus in each group. The att-IBM41 and att-IB2 vaccine candidates provided complete protection of the respiratory tract against homologous strain WT IBM41 challenge. The ciliary activity of the trachea of vaccinated birds was significantly higher than unvaccinated challenge control birds (P < 0.001) at 3 and 5 DPI (Figure 3). Calculation of protection score and percentage showed that the vaccine conferred 90 to 100% protection against homologous challenge (Table 3).
Figure 3.
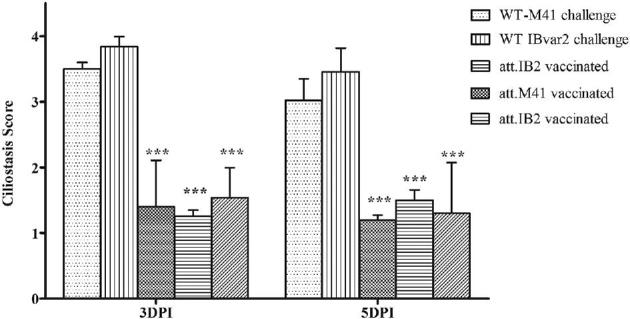
Tracheal ciliostasis scores in vaccinated chickens at 3 and 5 days post-infection after homologous wild-type virus challenge. The significance of P < 0.001 is indicated (***).
Table 3.
Protection scores and protection % in vaccinated specific pathogen free chickens attenuated IBM41, IB2, and IB-Var2 strains after homologous virus challenge.
| Group | Challenge virus | Protection Scorea | Protection %b |
|---|---|---|---|
| att-IBM41 vaccinated | WT-IBM41 | 90.1 | 100% |
| att-IB2 vaccinated | 88.2 | 100% | |
| att-IBvar2 vaccinated | WT-IBvar2 | 85.6 | 90% |
| Challenge control-IBM41 | WT-IBM41 | NAc | 0% |
| Challenge control-IBvar2 | WT-IBvar2 | NA | 0% |
| Negative control | None | NA | NA |
a,bProtection score [1 – mean ciliostasis score for vaccinated challenged/mean ciliostatsis score for challenge group] × 100 and % of protected chicks showed an average score ≤20 out of all tested chicks (Cook et al., 1999).
cNA; not applicable.
The att-IBM41 and att-IB2 vaccines were also able to reduce the shedding of the challenge at 3 DPI, since 40 and 50% of vaccinated birds, respectively, were active shedders with significantly low titers (P < 0.001). By 5 DPI, no virus shedding was detected in both vaccinated groups (Table 4). Similarly, the att-IBvar2 protected birds from homologous WT IB-VAR2 virus challenge. None of the birds showed clinical signs, and the average ciliostasis score in the att-IBvar2 vaccinated birds was 1.3±0.7 that was significantly lower (P < 0.001) than the WT virus non-vaccinated challenge group (Figure 3). Only 20% of vaccinated birds shed the challenge virus with low titers (102.10±0.3 EID50/mL) (Table 4).
Table 4.
Viral shedding titers in oropharyngeal swab samples from the vaccinated birds after wild-type (WT) virus challenge.
| Classical IBV challenge | Variant IBV challenge | ||||
|---|---|---|---|---|---|
| DPIa | WT IBM41 challenge control | att-IBM41 vaccinated | att-IB2 vaccinated | WT IBvar2 challenge control | att-IBvar2 vaccinated |
| 3 | 4.26 ± 0.98b | 1.14 ± 1.38* | 2.14 ± 0.83* | 4.48 ± 0.59 | 2.10 ± 0.28 |
| (10/10) | (4/10) | (5/10) | (10/10) | (2/10) | |
| 5 | 3.33 ± 0.77 | NDc | ND | 3.05 ± 0.75 | ND |
| (7/10) | (10/10) | ||||
| 7 | 1.57 ± 0.52 | ND | ND | 2.17 ± 0.57 | ND |
| (6/10) | (7/10) | ||||
aDPI; days post-infection.
bMean EID50/ml (positive/total tested).
cND; not detected.
*Indicate significant difference compared to the corresponding challenge virus group (P < 0.01).
DISCUSSION
The widespread exposure to several serotypes and genotypes of IBV with limited cross-protection has led to the failure of currently available vaccines to protect chickens from the heterologous challenge (Gelb et al., 1991). Though the IBV vaccination programs in Egypt depend on both classical (Mass 41 and H120) and/or imported variant (D278, CR88 or 4/91 strains) strain vaccines, many IBV outbreaks associated with respiratory distress, nephropathy, and high mortalities (>30%) were observed during last 10 yr (Sid et al., 2015; Hassan et al., 2016). Most of the outbreaks were mainly attributed to the nephropathogenic IBV variant 2 strains (Hassan et al., 2016; Zanaty et al., 2016; Abozeid et al., 2017). Recent studies also indicated that the IBV strains circulating in Egypt represent a distinct WT cluster confined to the Middle East region (GI-23 lineage) (Valastro et al., 2016).
The recently described protection studies with the Chinese QX-like IBV (Feng et al., 2015; Huo et al., 2016; Lim et al., 2015; Zhao et al., 2015) and Korean nephropathogenic IBV strain (Lee et al., 2010) further confirmed the concept that the best protection against IBV challenge can be achieved by using homologous strain vaccines. In the present study, we report the development of an attenuated IBV candidate vaccines using the Egyptian classic IBV strains (IBM41 andIB2) and a nephropathogenicIB-VAR2 strain belonging to the widely spread GI-23 lineage in the Middle East.
The WT viruses were attenuated through serial passage onto SPF eggs. Though the attenuation of IBV via egg passage is laborious and time-consuming, it is the most common technique of IBV vaccine development (Jackwood et al., 2003). By the 100th (att-IBM41 and IB2) and 110th passage (IB-VAR2), the viruses were successfully adapted to the embryo with acceptable growth characteristics. Virulence of the attenuated virus was then tested via the ocular route in day-old SPF chickens in comparison with the corresponding WT viruses. The effect of attenuated viruses on egg production in layer chickens and the in vivo back passage of the attenuated viruses was evaluated. The results confirmed that the viruses were efficiently attenuated compared to the WT parent viruses. The absence of adverse effect on egg production or change of virulence in day-old SPF chickens up to the 10th passage further confirmed the safety of the attenuated viruses.
It is worthy to note that the att-IBvar2 kidney lesion scores reported were mainly due to virus tropism to the kidney tissues which was reported with other nephropathogenic strains even after attenuation (Hodgson et al., 2004; Lim et al., 2015). The mild ciliostasis observed in all attenuated strains seems to be consistent with other research studies that reported certain degrees of tracheal tissue damage which may be needed to induce IBV local immunity (Jackwood et al., 2003; Jackwood et al., 2015). The levels of local tracheal immunity associated with variable tracheal tissue damage degrees were found to be positively correlating with the protection levels of IBV vaccines (Awad et al., 2016).
Efficacy of attenuated viruses against homologous challenge was investigated in day-old SPF chickens. The heterologous challenge was not considered as both field data and broilers results confirmed that Mass-type vaccines are not sufficient to protect birds against heterologous variant IBV strains (Meir et al., 2004; Bru et al., 2017). Recent studies highlighted the importance of depending on both quantification of ciliostasis and detection of viral shedding for the evaluation of protection levels afforded by the IBV vaccine candidates (Bande et al., 2015).
In the current study, both evaluation criteria were taken into consideration. The attenuated IBM41, IB2, and IB-VAR2 were capable to significantly reduce the clinical signs, lesions, and ciliostasis induced by the homologous challenge. Moreover, a significant reduction of the virus shedding titers was observed in the vaccinated birds at 3 DPI and no virus was detected at 5 and 7 DPI. These findings indicate that the attenuated viruses are suitable vaccine candidates that can provide protection against the challenge with homologous strains.
These findings provide evidence of the efficacy of the attenuated IBV strains IBM41, IB2, and IB-VAR2 as vaccine candidates against Middle Eastern classic and variant GI-23 IBV strains, respectively with protection % of ≥90%. However, further studies are required to evaluate the field safety and effectiveness of attenuated viruses, compared to other IBV vaccines. Also, further research should be done to investigate the use of combinations of these attenuated IBV strains to induce a wider protection against heterologous IBV challenge considering the fact that several virulent IBV strains are co-circulating in the Middle East.
ACKNOWLEDGEMENTS
The authors would like to thank Middle East for Veterinary Vaccines (ME VAC) Co. research & development and laboratory animal team for their technical support during experimental safety and challenge studies.
Notes
This work is partially funded by Faculty of Veterinary Medicine, Beni-Suef University, and Middle East for Veterinary Vaccines (ME VAC) Co.
REFERENCES
- Abdel-Moneim A. S., Afifi M. A., El-Kady M. F.. 2012. Emergence of a novel genotype of avian infectious bronchitis virus in Egypt. Arch. Virol. 157:2453–2457. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A. S., El-Kady M. F., Ladman B. S., Gelb J. Jr.. 2006. S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol. J. 3:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abozeid H. H., Paldurai A., Khattar S. K., Afifi M. A., El-Kady M. F., El-Deeb A. H., Samal S. K.. 2017. Complete genome sequences of two avian infectious bronchitis viruses isolated in Egypt: evidence for genetic drift and genetic recombination in the circulating viruses. Infect. Genet. Evol. 53:7–14. [DOI] [PubMed] [Google Scholar]
- Awad F., Hutton S., Forrester A., Baylis M., Ganapathy K.. 2016. Heterologous live infectious bronchitis virus vaccination in day-old commercial broiler chicks: clinical signs, ciliary health, immune responses and protection against variant infectious bronchitis viruses. Avian Pathol. 45:169–177. [DOI] [PubMed] [Google Scholar]
- Bande F., Arshad S. S., Bejo M. H., Moeini H., Omar A. R.. 2015. Progress and challenges toward the development of vaccines against avian infectious bronchitis. J. Immunol. Res. 2015:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru T., Vila R., Cabana M., Geerligs H. J.. 2017. Protection of chickens vaccinated with combinations of commercial live infectious bronchitis vaccines containing Massachusetts, Dutch and QX-like serotypes against challenge with virulent infectious bronchitis viruses 793B and IS/1494/06 Israel variant 2. Avian Pathol. 46:52–58. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. 2001. A nomenclature for avian coronavirus isolates and the question of species status. Avian Pathol. 30:109–115. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. 2005. Coronaviruses in poultry and other birds. Avian Pathol. 34:439–448. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. 2007. Coronavirus avian infectious bronchitis virus. Vet. Res. 38:281–297. [DOI] [PubMed] [Google Scholar]
- Cook J. K., Jackwood M., Jones R. C.. 2012. The long view: 40 years of infectious bronchitis research. Avian Pathol. 41:239–250. [DOI] [PubMed] [Google Scholar]
- Cook J. K., Orbell S. J., Woods M. A., Huggins M. B.. 1999. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 28:477–485 [DOI] [PubMed] [Google Scholar]
- Eterradossi N., Britton P.. 2013. Avian infectious bronchitis. In the OIE Manual of Diagnostic Tests and Vac-cines for Terrestrial Animals. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.02_AIB.pdf. Accessed May 2017. [Google Scholar]
- Fabricant J. 1998. The early history of infectious bronchitis. Avian Dis. 42:648–650. [PubMed] [Google Scholar]
- Feng K., Xue Y., Wang J., Chen W., Chen F., Bi Y., Xie Q.. 2015. Development and efficacy of a novel live-attenuated QX-like nephropathogenic infectious bronchitis virus vaccine in China. Vaccine 33:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb J. Jr., Lunt R. L., Metz A. L., Fries P. A.. 1991. Attenuation of avian infectious bronchitis virus by cold-adaptation. Avian Dis. 35:847–853. [PubMed] [Google Scholar]
- Haghighat-Jahromi M., Asasi K., Nili H., Dadras H., Shooshtari A. H.. 2008. Coinfection of avian influenza virus (H9N2 subtype) with infectious bronchitis live vaccine. Arch. Virol. 153:651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan K. E., Ali A., Shany S. A. S., El-Kady M. F.. 2017. Experimental co-infection of infectious bronchitis and low pathogenic avian influenza H9N2 viruses in commercial broiler chickens. Res. Vet. Sci. 115:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan K. E., Shany S. A., Ali A., Dahshan A. H., El-Sawah A. A., El-Kady M. F.. 2016. Prevalence of avian respiratory viruses in broiler flocks in Egypt. Poult. Sci. 95:1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson T., Casais R., Dove B., Britton P., Cavanagh D.. 2004. Recombinant infectious bronchitis coronavirus Beaudette with the spike protein gene of the pathogenic M41 strain remains attenuated but induces protective immunity. J. Virol. 78:13804–13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. P., Wang C. H.. 2006. Development of attenuated vaccines from Taiwanese infectious bronchitis virus strains. Vaccine 24:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y. F., Huang Q. H., Lu M., Wu J. Q., Lin S. Q., Zhu F., Zhang X. M., Huang Y. Y., Yang S. H., Xu C. T.. 2016. Attenuation mechanism of virulent infectious bronchitis virus strain with QX genotype by continuous passage in chicken embryos. Vaccine 34:83–89. [DOI] [PubMed] [Google Scholar]
- Jackwood M. W., Hilt D. A., Brown T. P.. 2003. Attenuation, safety, and efficacy of an infectious bronchitis virus GA98 serotype vaccine. Avian Dis. 47:627–632. [DOI] [PubMed] [Google Scholar]
- Jackwood M. W., Jordan B. J., Roh H. J., Hilt D. A., Williams S. M.. 2015. Evaluating protection against infectious bronchitis virus by clinical signs, ciliostasis, challenge virus detection, and histopathology. Avian Dis. 59:368–374. [DOI] [PubMed] [Google Scholar]
- Kim B. Y., Lee D. H., Jang J. H., Lim T. H., Choi S. W., Youn H. N., Park J. K., Lee J. B., Park S. Y., Choi I. S., Song C. S.. 2013. Cross-protective immune responses elicited by a Korean variant of infectious bronchitis virus. Avian Dis. 57:667–670. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Youn H. N., Kwon J. S., Lee Y. J., Kim J. H., Lee J. B., Park S. Y., Choi I. S., Song C. S.. 2010. Characterization of a novel live attenuated infectious bronchitis virus vaccine candidate derived from a Korean nephropathogenic strain. Vaccine 28:2887–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. H., Youn H. N., Yuk S. S., Kwon J. H., Hong W. T., Gwon G. B., Lee J. A., Lee J. B., Lee S. W., Song C. S.. 2015. Successful cross-protective efficacy induced by heat-adapted live attenuated nephropathogenic infectious bronchitis virus derived from a natural recombinant strain. Vaccine 33:7370–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir R., Rosenblut E., Perl S., Kass N., Ayali G., Perk S., Hemsani E.. 2004. Identification of a novel nephropathogenic infectious bronchitis virus in Israel. Avian Dis. 48:635–641. [DOI] [PubMed] [Google Scholar]
- Reed L. J., Munech H.. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27:493–497. [Google Scholar]
- Selim K., Arafa A., Hussein A., El-Sanousi A. A.. 2013. Molecular characterization of infectious bronchitis viruses isolated from broiler and layer chicken farms in Egypt during 2012. Int. J. Vet. Sci. Med. 1:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sid H., Benachour K., Rautenschlein S.. 2015. Co-infection with multiple respiratory pathogens contributes to increased mortality rates in algerian poultry flocks. Avian Dis. 59:440–446. [DOI] [PubMed] [Google Scholar]
- Toro H., van Santen V. L., Ghetas A. M., Joiner K. S.. 2015. Cross-protection by infectious bronchitis viruses under controlled experimental conditions. Avian Dis. 59:532–536. [DOI] [PubMed] [Google Scholar]
- Valastro V., Holmes E. C., Britton P., Fusaro A., Jackwood M. W., Cattoli G., Monne I.. 2016. S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infect. Genet. Evol. 39:349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanaty A., Naguib M. M., El-Husseiny M. H., Mady W., Hagag N., Arafa A. S.. 2016. The sequence of the full spike S1 glycoprotein of infectious bronchitis virus circulating in Egypt reveals evidence of intra-genotypic recombination. Arch Virol 161:3583–3587. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Cheng J. L., Liu X. Y., Zhao J., Hu Y. X., Zhang G. Z.. 2015. Safety and efficacy of an attenuated Chinese QX-like infectious bronchitis virus strain as a candidate vaccine. Vet. Microbiol. 180:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


