Learning Point for Clinicians.
Simultaneous coinfection with Cryptococcus neoformans and Mycobacterium tuberculosis was seldom reported. We present a case of tuberculous lymphadenopathy concomitant with pulmonary cryptococcus infection. Prompt investigation for other occult infection should be conducted if the clinical response is insufficient during administration of the appropriate antimicrobial treatment.
Case report
A previously healthy 58-year-old woman experienced fever and productive cough with yellowish sputum 2 weeks prior to admission. Empiric antibiotic therapy with flomoxef was prescribed, but intermittent fever was still noted.
On admission, vital signs were as follows: blood pressure, 126/68 mm Hg; pulse rate, 92 beats/min; respiration rate, 18 breaths/min, and body temperature, 38.6°C. Complete blood counts showed leukocytosis with predominant neutrophils. No gross hematuria or pyuria was indicated by the urinary analysis results. Biochemical analysis results were within the normal range, but the C-reactive protein level was elevated.
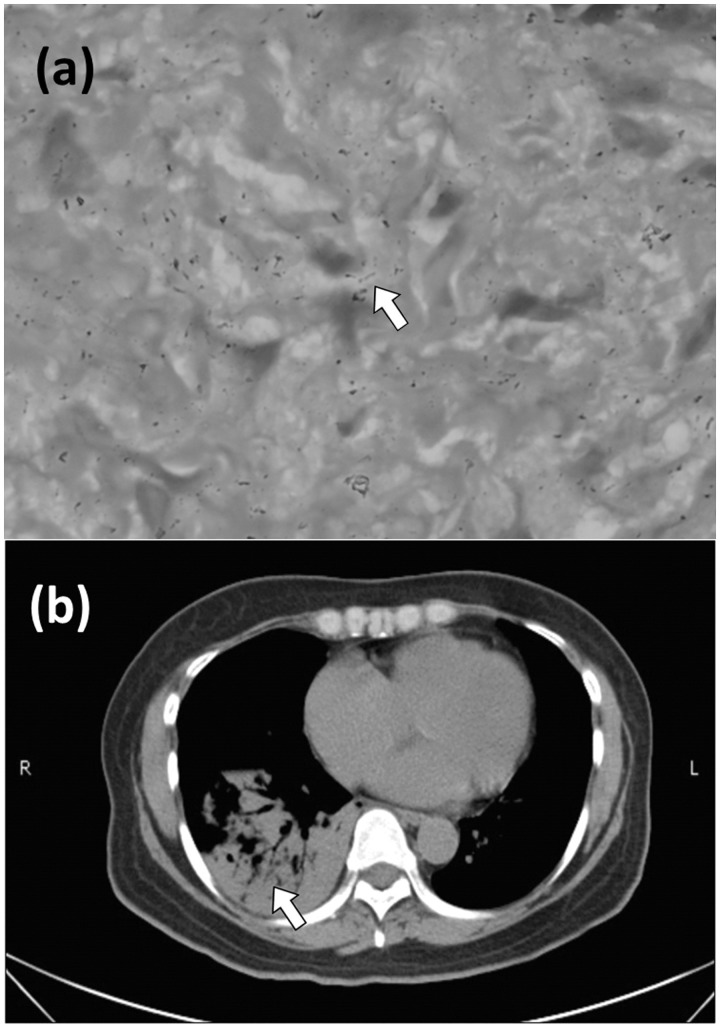
Physical examination revealed an enlarged level III neck lymph node on left side, which was excised by biopsy. The pathological report presented tuberculosis and granulomatous formation in lymph nodes (Figure 1a). Sputum culture and acid-fast staining yielded negative results. Based on these findings, treatment with anti-tuberculous agents was initiated. Chest radiographies revealed ill-defined patchy air-space consolidations in right lower lobe and left upper lobe of lung. Computed tomography of chest revealed multiple ill-defined foci, patchy opacities in left upper lobe of lung and ill-defined patchy consolidated opacities (>9 cm) in the right lower lobe of lung (Figure 1b). Several tests were conducted to identify the pneumonia-causing pathogen, including blood culture, sputum culture, Pneumococcus antigen test, Legionella antigen test, Mycoplasma test, Chlamydia antigen test, the test of human immunodeficiency virus (HIV), acid-fast stain and the polymerase chain reaction of tuberculosis in sputum, but the results were negative.
Figure 1.
(a) The neck lymph node specimen showing the presence of tuberculosis (white arrow). (b) The computed tomography scan of the chest revealed multiple ill-defined foci patchy opacities on the left upper lobe and ill-defined patchy consolidated opacities on the right lower lobe (white arrow).
Computed-tomography-guided lung biopsy was performed, and the pathological findings confirmed Cryptococcus neoformans. A subsequent lumbar puncture excluded central nervous system involvement. Antifungal treatment with fluconazaole was prescribed, and the patient recovered after 6-month courses of antifungal and anti-tuberculosis treatments.
Discussion
Cryptococcus neoformans is a heterobasidiomycetous and encapsulated fungus and is distributed worldwide. It is found in the excreta of certain birds, including pigeons, cockatoos and canaries, and in several species of eucalyptus trees. Inhalation of environmental propagules from the soil and bird droppings is the major portal of entry of C. neoformans. Clinical manifestations of C. neoformans infections are variable and range from asymptomatic colonization to life-threatening disease.1 The central nervous system and lung are the most common clinical infection sites.
Tuberculosis is one of the infections that require a cellular immune response for their control.2 Mycobacterial antigens present a unique ability to promote expression of inhibitory cytokines, in which the suppression of CD4 cell responses contributes to immunosuppression, thus, enhance susceptibility to other pathogens which requires cell-mediated immunity for infection control. Coinfection with Mycobacterium tuberculosis, ‘cytomegalovirus’, ‘severe acute respiratory syndrome-associated coronavirus’, Chlamydia trachomatis, Bartonella quintana, and cutaneous Leishmaniasis has been reported in the literature. Simultaneous coinfection with Cryptococcus neoformans and Mycobacterium tuberculosis was seldom reported.
Both cryptococcosis and tuberculosis infection could occur in apparently immunocompetent hosts. Previous studies have demonstrated that tuberculosis infection causes alteration in cellular immunity and is recognized as a predisposing factor for developing cryptococcosis.3 On the other hand, cryptococcosis inhibits the production of tumor necrosis factor alpha and predisposes patients to tuberculosis reactivation or infection.4 In the present case, it is difficult to know whether cryptococcosis infection preceded tuberculosis infection or vice versa. Coinfection with both pathogens was considered on the basis of the diagnosis of these two diseases at the same time.
Coinfection with tuberculosis and C. neoformans was seldom reported in the English literature over the previous 20 years. Most of reported coinfection cases with C. neoformans and tuberculosis involved central nervous system and lung, respectively. In this case, tuberculosis infection involved lymph node and C. neoformans infection involved lung, which was unique compared with previous reports.
In conclusion, simultaneously coinfection with cryptococcosis and tuberculosis is a rare but existing clinical entity that may occur not only in immunocompromised hosts but also in healthy individuals. The case represents a reminder of the importance of testing for occult infection if the clinical response is insufficient during administration of the appropriate antimicrobial treatment for definite infection.
Conflict of interest: None declared.
References
- 1.Perfect JR, Casadevall A. Cryptococcosis. Infect Dis Clin North Am. 2002;16:837–42. doi: 10.1016/s0891-5520(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 2.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottasso O, Bay ML, Besedovsky H, del Rey A. Immunoendocrine alterations during human tuberculosis as an integrated view of disease pathology. Neuroimmunomodulation. 2009;16:68–77. doi: 10.1159/000180261. [DOI] [PubMed] [Google Scholar]
- 4.Huffnagle GB, Chen GH, Curtis JL, McDonald RA, Strieter RM, Toews GB. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–16. [PubMed] [Google Scholar]