Abstract
Objectives. To investigate whether cyclophilin A (CypA) can up-regulate the expression of MMP-2 and MMP-9 in monocytes/macrophages and whether CD147 facilitates this regulation in RA.
Methods. Peripheral blood monocytes were isolated from RA patients and differentiated into macrophages by M-CSF (15 ng/ml). Under CypA stimulation (200 ng/ml), the protein release and activation of MMPs were detected by gelatin zymography and invasion assay. Human monocyte cell line THP-1 cells were selected for the advanced searching for potential interaction between CypA and CD147 in production of MMPs and cell adhesion to extracellular matrix (ECM).
Results. CypA significantly increased production and activation of MMP-9, not MMP-2, in the monocytes/macrophages derived from RA SF. CSA and HAb18G/CD147 antagonistic peptide AP-9 against CD147, respectively, dramatically decreased MMP-2 and MMP-9 expression, both in the absence or presence of CypA. Similar effects of CypA on MMP-9 production and cell invasion were observed in THP-1 cells. CypA-induced nuclear factor κB (NF-κB) activity for MMP-9 transcription were strongly blocked by extracellular signal-regulated kinase (ERK), c-Jun amino terminal kinase (JNK) inhibitors (U0126 and SP600125, respectively), but not by p38 mitogen-activated protein kinase (MAPK) inhibitors (SB203580). CypA also induced calcium mobilization and increased the adhesion of THP-1 cells to ECM.
Conclusions. These findings suggest that in RA, the abundant CypA, by its direct binding to CD147, up-regulates MMP-9 expression and adhesion of monocytes/macrophages to ECM, and the cyclophilin-CD147 interactions might contribute to the destruction of cartilage and bone.
Keywords: Rheumatoid arthritis, Monocytes/macrophages, CD147, Cyclophilin A, Matrix metalloproteinase
Introduction
Rheumatoid arthritis (RA) is a chronic destructive autoimmune disease characterized by the inflammation and progressive destruction of distal joints [1]. In RA, the number of macrophages, which secrete MMP-2 and MMP-9, significantly increases both in the lining and sublining areas of RA synovium [2] and the increase correlates with the severity of cartilage destruction [3]. MMPs, such as MMP-2, -3 and -9, higher in SF of RA patients than those in OA or controls, are involved in the degradation of joint collagen [4, 5].
Cyclophilin A (CypA), a ubiquitously distributed intracellular protein belonging to the immunophilin family [6], is the major target of immunosuppressive drug CSA [7]. It is secreted by cells in response to inflammatory stimulation and is a potent neutrophil, eosinophil and monocyte chemoattractant in vitro or in vivo [8, 9]. It is also abundant in RA SF and its specific peptidyl-prolyl-isomerase activity (PPIase) for protein folding and transduction has been detected in SF from RA patients, but not in SF from OA patients [10]. CD147, also named ECM metalloproteinase inducer (EMMPRIN), is a regulator of MMP production by MAPK pathway [11, 12] and serves as a signalling receptor for extracellular cyclophilins [13, 14]. In RA, our previous studies and some other researches have shown that CD147 overexpression on synoviocytes in RA synovium enhances the production of MMP-2 and MMP-9 and the invasiveness of synoviocytes, and membrane CD147 expression on monocytes/macrophages in RA synovium is much higher than that on monocytes/macrophages derived from RA patient's peripheral blood [11, 15–18].
Recently, the potential role of CypA and CD147, ligand and receptor, in some immunological diseases has been identified, such as AIDS and severe acute respiratory syndrome (SARS), in which CypA is found to integrate with the structural protein on virus and enhance viral infection to the host cells by binding to CD147 directly [19–21]. Constant and others [22, 23] also showed that the cyclophilin–CD147 interactions might also play a potential role for activated leucocytes with up-regulated expression of CD147 in tissue inflammation. However, the cyclophilin–CD147 interactions for monocytes/macrophages in RA remains unclear.
Since CypA is abundant in RA SF and CD147 expresses high on monocytes/macrophages in RA synovium, we wanted to observe whether CypA could increase MMP expression, invasiveness and adhesion of monocytes/macrophages and whether it needed the CypA–CD147 interactions. These results of the study we believe may help us to gain a better understanding of the potential role of the CypA–CD147 interactions in RA pathogenesis.
Patients and methods
RA patients
Samples of SF in knee joints and peripheral blood were obtained from eight patients with active RA. All the patients met the 1987 revised diagnostic criteria of the ACR [24]. The mean age of these patients was 45 yrs (range 28–66 yrs) and the mean disease duration was 5 yrs. Samples of normal control peripheral blood were taken from eight normal human donor volunteers, who had no significant age or sex differences compared with those of the RA patients, these donor volunteers did not show immunological diseases. The Ethics Committee at Xijing Hospital granted ethical approval for this study and all the subjects provided their informed consent.
Cells isolation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque (1.077 g/ml; Invitrogen, Carlsbad, CA, USA) density gradient centrifugation. Monocytes were purified from PBMCs by using the Monocyte Negative Isolation Kits (Dynal, Biotech ASA, Oslo, Norway), and 1 × 106 cells/ml were cultured in 2 ml RPMI 1640 with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) with recombinant human M-CSF (rM-CSF, 15 ng/ml; R&D Systems, Inc., Minneapolis, MN, USA) in 6-well plates at 37°C. Media were changed every 3 days. Macrophages were used after 7 days of culture. CD14, CD68 and membrane CD147 expression on monocytes and macrophages were detected by flow cytometry. For SF, hyaluronidase (5 U/ml) was added to digest hyaluronic acid. monocytes/macrophages in RA SF, then monocytes/macrophages were isolated by Dynabeads® M-450 CD14 (Dynal Biotech ASA).
THP-1 cells (ATCC, Manassas, VA, USA) were cultured in RPMI 1640 with 10% FBS at 37°C in 5% CO2. For the induction of cell differentiation, THP-1 cells (5 × 105 to 106/ml) were stimulated with 100 nM phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St Louis, MO, USA) for 48 h. After incubation, the non-adherent cells were removed by aspiration and the adherent cells were differentiated to macrophages (dTHP-1). The THP-1 cells in RPMI 1640 without PMA stimulation were used as control cells (the undifferentiated THP-1, uTHP-1) [25].
Flow cytometry analysis
Monocytes/macrophages (3 × 105) were incubated with anti-CD14, CD68 and CD147 monoclonal antibodies (Serotec, Oxford, UK; Becton-Dickinson, Franklin Lakes, NJ, USA) according to manuscripts. Positive cell count and mean fluorescence intensity (MFI) were determined by flow cytometry (Becton-Dickinson). The data were processed using Cell Quest software (Becton-Dickinson).
CypA stimulation of monocytes/macrophages
Each (3 × 105) of the THP-1 cells and human monocytes/macrophages was plated in 24-well plates and cultured with 500 µl of serum-free RPMI 1640. Cells were pre-treated, respectively, with CSA (200 ng/ml), HAb18G/CD147 antagonist peptide AP-9 (200 μg/ml, amino acid sequence was YKLPGHHHHYRP, designed and produced by our lab) [20] and siRNA against CD147 for 24 h, and then were stimulated with CypA (200 ng/ml in PBS, no endotoxin) (Alexis Biochemicals, San Diego, CA, USA). After 24 h of CypA stimulation, the supernatants were collected for gelatin zymography. In the same way, cells with various treatments were collected for the experiments of quantitative RT-PCR (qRT–PCR) of MMP-2, -9, CD147 mRNA and invasion assay.
siRNA targeted against CD147
Two different sequences of small interfering RNA (siRNA) fragments against CD147 were designed and synthesized by Ambion (Austin, TX, USA). The first siRNA fragment's (siRNA I) sequence was: sense 5′-GGUUCUUCGUGAGUUCCUCtt-3′ and anti-sense 5′-GAGGAACUCACGAAG AACCtg-3′; the second siRNA fragment's (siRNA II) sequence was: sense 5′-GCCAAUGCUGUCUGGUUGCtt-3′ and anti-sense 5′-GCAACCAGACAGCAUU GGCtt-3′. The negative control siRNA (sequence not shown, Ambion) was used as the negative control for RNAi. Cells (3 × 105) of the uTHP-1 and dTHP-1 cells were plated in a 24-well plate with 500 μl serum-free RPMI 1640. According to the LipofectamineTM 2000 (Invitrogen) manual, 50 pmol of siRNA I or siRNA II was transfected with 2 μl LipofectamineTM 2000 (optimized) for one well, and growth culture was added in wells after 5 h of transfection. After 48 h, the cells were collected for the flow cytometry and qRT–PCR analysis.
Gelatin zymography
For analysis of MMP expression and their proteolytic capacity, conditioned media were collected and centrifuged to remove cellular debris and the supernatants were collected. Each sample was followed by detection of gelatinolytic activity and quantity in the conditioned media by gelatin zymography [25]. In brief, the conditioned medium was resolved by SDS–PAGE under non-reducing conditions. The gels were then washed twice in 2.5% (v/v) Triton X-100 for 30 min at room temperature (RT) and incubated in reaction buffer for 16 h at 37°C. Then, the gels were subsequently stained with 0.5% Coomassie blue (R-250) and destained to visualize these zones of digestion as light areas against the darkly stained protein background. The zymography gels were scanned and analysed using US National Institutes of Health Image 1.6 software.
Besides the supernatants were collected for gelatin zymography, the cells were collected for invasion assay at the same time.
Invasion assay
The invasion assay was performed using 24-well transwell units (Costar, Cambridge, NY, USA). Each well was coated with Matrigel Matrix (5 µg/ml, BD Pharmingen, San Diego, CA, USA) to form a continuous thin layer. After pretreatment with CSA, siRNA or AP-9, the uTHP-1 and dTHP-1 cells (3 × 105) were plated on the surface of the Matrigel with serum-free medium and cultured for 24 h under CypA stimulation at 37°C in a 5% CO2 incubator. The cells remaining in the upper compartment were completely removed by gently swabbing. The number of cells invading through the filter into the lower compartment was determined using colorimetric haematoxylin and eosin stain assay. The cells that invaded through the filter into the lower surface of the filter in five microscopic fields of 200 × magnification were counted in each filter. Triplicate samples were conducted and the data were expressed as the average cell number of 15 fields.
qRT–PCR
After THP-1 cells were treated with various agents for 48 h, mRNA was extracted and reverse-transcribed. qRT–PCR was performed using a CFD3120 MiniOpticon Detector (Bio-Rad, Hercules, CA, USA) with Sybr Green I (Molecular Probes, Leiden, The Netherlands). PCR reactions comprised 1 µl cDNA, 3 µl 25 mM MgCl2, 2 µl dNTPs (10 mM), 2.5 µl 10 × Sybr Green I PCR buffer, 1 µl of 5 mM forward and reverse primer mix, 0.5 µl Taq DNA polymerase. Cycling parameters were 95°C for 30 s, 40 s ramp, 55°C for 30 s, 20 s ramp and 72°C for 1 min, 17 s ramp. After qRT–PCR, the data were processed with Opticon Monitor™ Version 3.1 (Hercules, CA, USA), the quantity of CD147 and MMPs mRNA was first normalized by GAPDH and then results were compared with those of the normal negative control of uTHP-1 cells. The CD147's primers: sense 5′-GAGTGAAGGCTGTGAAGTCG-3′ and anti-sense 5′-AACTCACGAAGAACCTGCT-3′. The GAPDH's primers: sense 5′-TCATCAGCAATGCCTCCT-3′ and anti-sense 5′-CATCACGCCACAGTTTCC-3′. The MMP-2 primers: sense 5′-TTGACGGTAAGGACGGACTC-3′ and anti-sense 5′-ACTTGCAGTACTCCCCATCG-3′. The MMP-9's primers: sense 5′-TTGACAGCGACAAGAAGTGG-3′ and anti-sense 5′-CCCTCAGTGAAGCGGTACAT-3′.
IF assay
For IF, the uTHP-1 and dTHP-1 cells were pre-incubated with AP-9 for 1 h, then activated with either lipopolysaccharide (LPS) (1 μg/ml) or CypA (200 ng/ml) for 2 h in RPMI 1640 medium with 0.1% FBS. For detection of nuclear factor-κB (NF-κB), the THP-1 cells (1 × 105) were fixed with 4% formaldehyde and put onto the slide glass. The fixed cells were dried for 1 h at RT and washed with PBS for 5 min. The cells were then treated with anti-p50 monoclonal antibody (SC-8414, Santa Cruz, CA, USA), followed by incubation, respectively, with FITC-conjugated rabbit anti-mouse IgG antibody (Serotec) or Cy3-conjugated sheep anti-mouse IgG antibody (C2181, Sigma-Aldrich; FITC for the uTHP-1 and Cy3 for the dTHP-1) according to manufacturer's instructions. After incubation, the cells were stained with 4′-6-diamidino-2-phenylindole (DAPI, Invitrogen). The slides were observed under IF microscopy.
Effect of MAPK inhibitors on MMP-9 secretion and NF-κB activity
For the MAPK inhibitors experiment, the THP-1 cells were cultured for 24 h in a serum-free medium before treatment with/without MAPK inhibitors (10 μM, SB203580 for P38, SP600125 for JNK, UO126 for ERK1/2, Alexis Biochemicals). CypA (200 ng/ml) was added after 2 h of inhibitor treatment and the cells were incubated for 48 h. Culture supernatants were collected for gelatin zymography and cells were used for detection of NF-κB activity by IF.
Calcium measurements
For detection of calcium (Ca2+) mobilization, the THP-1 cells were loaded with 3 μM Fluo-3/AM for 30 min at 37°C. After washing off extracellular Fluo-3/AM, the final cell concentration was adjusted to 1 × 105/ml in Dulbecco's phosphate-buffered saline (DPBS), pH 7.4. Stimulation was induced by adding various agonists at 37°C. Variations in cytosolic fluorescence intensity were detected by laser scanning confocal fluorescence microscope (MRC 1024, Bio-Rad) and the levels of cytosolic Ca2+ were calculated by fluorescence intensity value. The curves of each sample showed Ca2+ mobilization under the different stimulators.
Adhesion assay
For adhesion assay, freshly cultured THP-1 cells (100 μl, 10 × 104/well) were pre-incubated in DPBS/0.5% BSA with increasing concentrations of the pro-adhesive factors for 10 min at 37°C and were then distributed into Matrigel Matrix-coated (1 µg/ml) 96-well microtitre plates for an additional 30 min incubation at 37°C. Thereafter, the plates were washed extensively to remove the non-adherent cells and the remaining firmly attached cells were fixed with 4% formaldehyde, pH 7.8, for 20 min at 4°C. Adherent cells were stained with 0.2% Crystal Violet (100 μl/well) in PBS for 10 min at RT. The plates were washed by distilled water to remove the unfixed Crystal Violet and were dried in air. The adherent cells in wells were lysed by 10% SDS (100 μl/well) for 10 min at RT. The staining absorbance of each well was measured by Universal Microplate Reader (Bio-Tek ELx800, Hercules, CA, USA) at 490 nm. Cell adhesion was estimated by using standard curves where absorbance was related to THP-1 cell numbers and results were expressed as a percentage of the initially added cells remaining fixed to the substrates.
Statistical analysis
All values were expressed as the mean ± s.d. Statistical analyses were performed with Student's t-test using SPSS software (Chicago, IL, USA) and P-values<0.05 were considered significant.
Results
CD147 expression on monocytes/macrophages and THP-1 cells
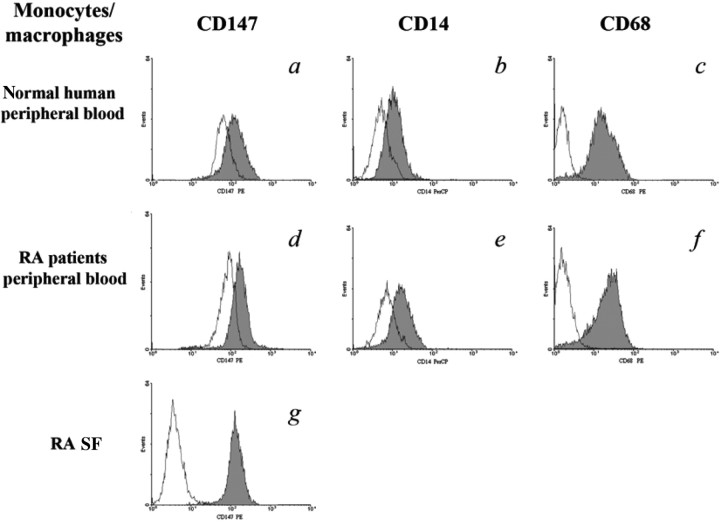
As in our previous studies, the values of MFI (Fig. 1) showed CD147 expression on monocytes/macrophages in RA SF (140.5 ± 27.51) was higher than that in normal human peripheral blood (75.13 ± 15.32) or from RA patients’ peripheral blood (96.29 ± 17.65; P < 0.05), but the percentage of positive staining of CD147 on these cells was not significantly different (P > 0.05). After differentiation, CD147 expression on macrophages (shaded peaks) from monocytes of normal human peripheral blood (135.76 ± 25.33) or RA patients’ peripheral blood (176.44 ± 34.21) were both increased (P < 0.05). MFIs of CD14 and CD68 were also increased after differentiation of monocytes, which showed the maturation of macrophages (shaded peaks). Because THP-1 cells were selected for advanced functional tests, CD147 expression on THP-1 also needed detection. It was the same with monocytes/macrophages of RA SF. CD147 expression on the dTHP-1 cells (dTHP-1) was higher than that on the uTHP-1 cells (uTHP-1) (P < 0.05).
Fig. 1.
Detection of CD147, CD14 and CD68 expressions in monocytes/macrophages by flow cytometry. Flow cytometry showed that CD147, CD14 and CD68 expressions were all increased both in mature macrophages (shaded peaks) differentiated from monocytes of normal human and RA peripheral blood (unshaded peaks). CD147 expression on monocytes/macrophages was highest among these cells. Data are expressed as means ± s.d. (n = 8).
Here, we used RNAi against CD147 expression for blocking CypA binding. The results of flow cytometry and qRT–PCR showed that both of siRNA I and siRNA II significantly blocked CD147 expression (P < 0.05), while the suppression efficiencies of siRNA I (70% for the uTHP-1 cells, 85% for the dTHP-1 cells) were better than those of siRNA II (60% for the uTHP-1 cells, 68% for the dTHP-1 cells).The siRNA I was selected for the next experiments.
MMP expression and invasive potential of monocytes/macrophages and THP-1 with CypA stimulation
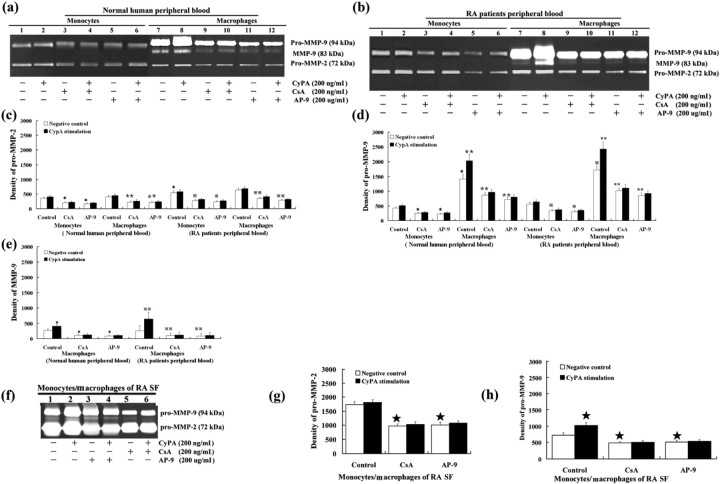
To determine whether the abundant CypA in RA SF could enhance MMP expression and invasive potential of monocytes/macrophages. We used CypA (200 ng/ml) to stimulate monocytes/macrophages. Without CypA stimulation, the secretion and activation of pro-MMP-2 and pro-MMP-9 significantly increased in monocytes/macrophages derived from RA SF compared with those in monocytes from normal human or RA patients’ peripheral blood (P < 0.05) (Fig. 2). After differentiation, pro-MMP-9 and MMP-9 production was significantly increased in macrophages (P < 0.05), while pro-MMP-2 production was not increased (P > 0.05). Under CypA stimulation, the secretion and activation of pro-MMP-9 and MMP-9 were increased in macrophages (P < 0.05), while pro-MMP-9 was a little increased in monocytes (P > 0.05). No significant changes of MMP production with CypA stimulation were observed when cells were, respectively, treated with CSA or AP-9 (P > 0.05). For pro-MMP-2, its secretion and activation had no significant changes in monocytes or macrophages after CypA stimulation (P > 0.05).
Fig. 2.
Gelatin zymography of culture medium conditioned by monocytes/macrophages with CypA stimulation. The protein expression of pro-MMP-9 (d) and MMP-9 (e) were significantly increased when monocytes were differentiated into marophages. CypA increased the secretion and activation of pro-MMP-9, but not that of pro-MMP-2, in macrophages and monocytes/macrophages from RA SF. Both CSA and AP-9 could inhibit CypA's effect on MMP-9 production. Data are expressed as means ± s.d. (n = 8). *P < 0.05, **P < 0.05 vs negative control of monocytes and macrophages derived from normal human peripheral blood, respectively.  P < 0.05,
P < 0.05,  P < 0.05 vs negative control of monocytes and macrophages derived from RA patients’ peripheral blood; ★P < 0.05 vs negative control of monocytes/macrophages RA patients’ SF, respectively.
P < 0.05 vs negative control of monocytes and macrophages derived from RA patients’ peripheral blood; ★P < 0.05 vs negative control of monocytes/macrophages RA patients’ SF, respectively.
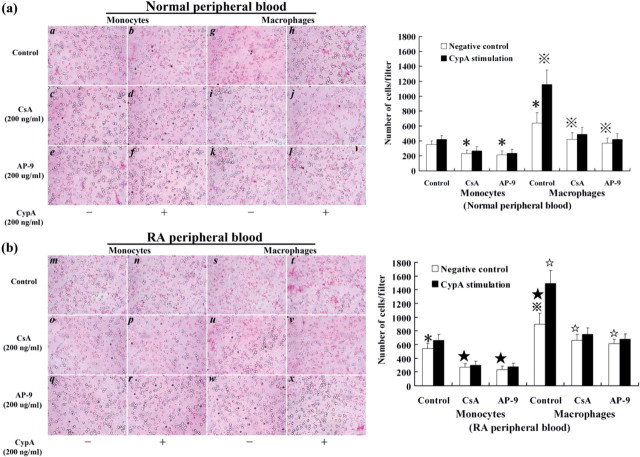
Similarly, for the invasive potential of monocytes/macrophages, higher numbers of macrophages (cells/filter) were found to have invaded through transwell chambers than those of monocytes (P < 0.05). CypA significantly increased the number of the invading macrophages (P < 0.05), while it had little effect on monocytes (P > 0.05). With or without CypA stimulation, the number of invading cells was both decreased when the monocytes or macrophages were pre-treated with CSA or AP-9, respectively (P < 0.05; Fig. 3).
Fig. 3.
Invasion assay of monocytes/macrophages with CypA stimulation. Monocytes and macrophages from normal human (a) and RA patients’ (b) peripheral blood were pre-treated, respectively with CSA, or AP-9, and then were stimulated by CypA for invasion assay. A higher number of macrophages (cells/filter) were found to have invaded through transwell chambers than monocytes. CypA significantly increased the number of the invading macrophages, while it had little effect on monocytes. CSA or AP-9 could inhibit CypA's function. Data are expressed as means ± s.d. (n = 8). *P < 0.05 and  P < 0.05 vs negative control of monocytes and macrophages from normal human peripheral blood, respectively. *P < 0.05 and
P < 0.05 vs negative control of monocytes and macrophages from normal human peripheral blood, respectively. *P < 0.05 and  P < 0.05 vs negative control of monocytes and macrophages from RA patients’ peripheral blood, respectively.
P < 0.05 vs negative control of monocytes and macrophages from RA patients’ peripheral blood, respectively.
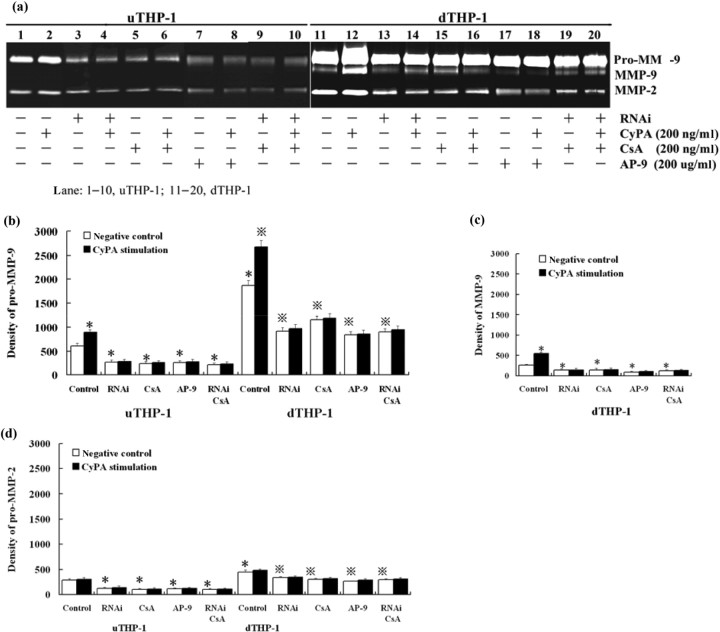
Since the THP-1 cells were selected for functional tests, we also need to observe MMP expression and invasive potential of THP-1 cells under CypA stimulation. The results showed that CypA increased pro-MMP-9 and MMP-9 (P < 0.05), not pro-MMP-2 (P > 0.05), in the uTHP-1 and dTHP-1 cells (Fig. 4), and RNAi, CSA or AP-9, respectively blocked the effect of CypA on MMPs. The production of increasing MMP-9 increased by CypA stimulation in dTHP-1 cells was higher than that in uTHP-1 cells (P < 0.05). No additional decrease or increase of MMP-9 production was observed when cells were treated with the combination of RNAi and CSA (P > 0.05) under CypA stimulation. CypA significantly increased the invading number of dTHP-1 cells (P < 0.05), while it had little effect on uTHP-1 cells (P > 0.05). With CypA or without CypA stimulation, the numbers of invading cells were both decreased when the uTHP-1 or dTHP-1 cells were pre-treated with RNAi, CSA or AP-9, respectively (P < 0.05).
Fig. 4.
Gelatin zymography of culture medium conditioned by THP-1 cells with CypA stimulation. (a) uTHP-1 and dTHP-1 cells were pre-treated, respectively, with siRNA, CSA or AP-9, and then were stimulated with CypA for 24 h. The supernatants were collected for gel zymography. The statistical data showed relative enzyme activity of pro-MMP-9 (b), MMP-9 (c) and pro-MMP-2 (d) with different treatments. CypA increased total MMP-9, but not MMP-2, secretion in the uTHP-1 and dTHP-1 cells. RNAi, CSA or AP-9 decreased the secretion of pro-MMP-2 and pro-MMP-9 in uTHP-1 or dTHP-1 cells, respectively, both in the absence or presence of CypA. Data are expressed as means ± s.d. (n = 3). *P < 0.05 vs the normal negative uTHP-1 cells.  P < 0.05 vs the normal negative dTHP-1 cells.
P < 0.05 vs the normal negative dTHP-1 cells.
These results confirmed that CypA could significantly increase MMP-9's expression and invasive potential of macrophages from RA, and needed binding to membrane CD147 on macrophages.
MMP-2, -9 and CD147 mRNA expression in THP-1 cells with CypA stimulation
Since CypA could increase MMP-9 protein level, we also wanted to observe its effect on mRNA level. We found that MMP-2 and MMP-9 mRNA expression in dTHP-1 cells was higher than that in uTHP-1 cells (P < 0.05). CypA increased MMP-9 mRNA expression, not MMP-2, in both uTHP-1 and dTHP-1 cells (P < 0.05), and the quantity of increasing MMP-9 mRNA by CypA stimulation in dTHP-1 cells was higher than that in uTHP-1 cells (P < 0.05). RNAi, CSA or AP-9 decreased MMP-2 and MMP-9 expression in uTHP-1 or dTHP-1 cells, respectively (P < 0.05), both in the absence or presence of CypA. No additional decrease of MMP-9 mRNA was observed when cells were treated with the combination of RNAi and CSA (P > 0.05) (Fig. 5).
Fig. 5.
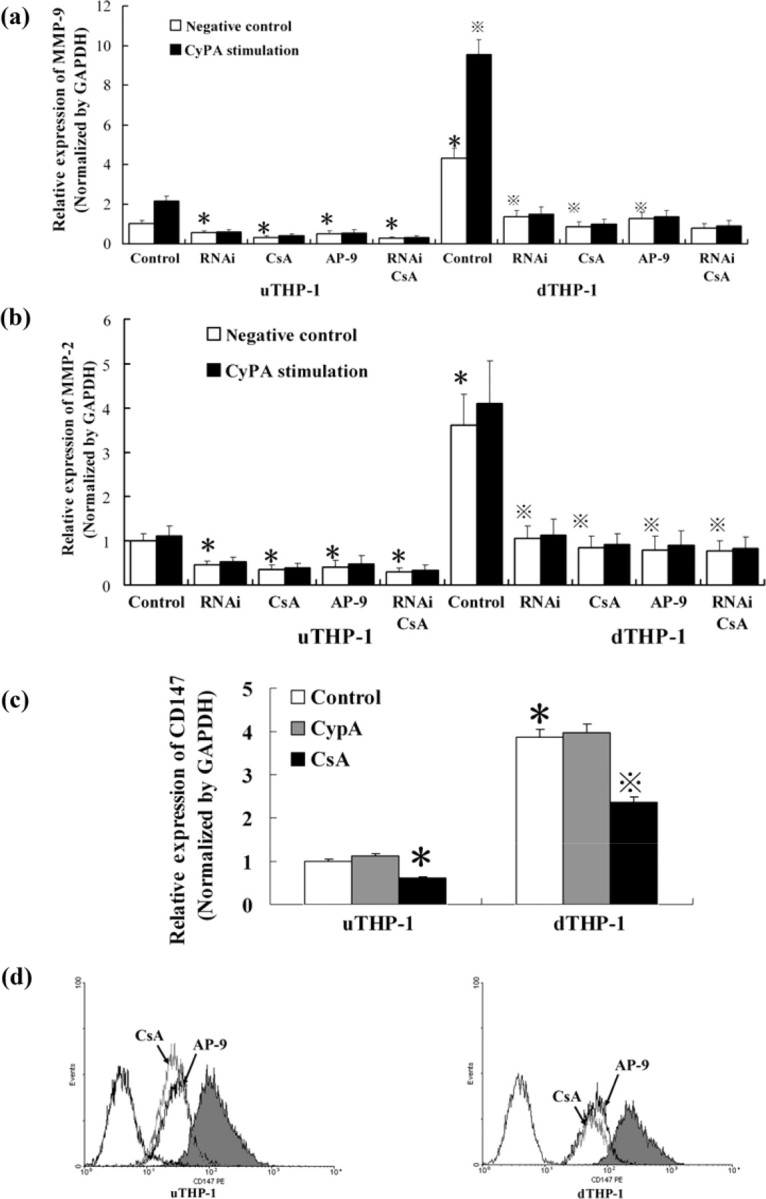
qRT–PCR of MMP-2, -9 and CD147 mRNA in THP-1 cells with CypA stimulation. The uTHP-1 and dTHP-1 cells were pre-treated, respectively, with CSA, RNAi or AP-9 against CD147 and then were stimulated by CypA. qRT–PCR was run for MMP-9 (a), MMP-2 (b) and CD147 (c) mRNA. CypA increased MMP-9, not MMP-2, mRNA expression, in both uTHP-1 and dTHP-1 cells (P < 0.05). RNAi, CSA or AP-9 decreased MMP-2 and MMP-9 expression in the uTHP-1 or dTHP-1 cells, respectively (P < 0.05), both in the absence or presence of CypA. (c) CypA had no significant effect on CD147 mRNA expression, while CSA dramatically decreased it. (d) Flow cytometry showed that CSA dramatically suppressed membrane CD147 expression ∼52% and AP-9 blocked membrane CD147 ∼ 63% in THP-1 cells. Data are expressed as means ± s.d. (n = 3). *P < 0.05 vs the normal negative uTHP-1 cells.  P < 0.05 vs the normal negative dTHP-1 cells.
P < 0.05 vs the normal negative dTHP-1 cells.
We confirmed that CypA up-regulated MMP-9 expression both on mRNA and molecular level via binding to CD147 on monocytes/macrophages. On the other hand, we also found that CypA had no significant effect on CD147 mRNA or protein expression (P > 0.05), while CSA dramatically decreased it by ∼52% (Fig. 5c) (P < 0.05). Flow cytometry (Fig. 5d) showed that AP-9 blocked membrane CD147 ∼63% in the THP-1 cells.
NF-κB activation in THP-1 cells with CypA stimulation
To confirm that whether CypA up-regulated MMP-9 expression via enhancing NF-κB activity, IF was used detecting NF-κB activity. IF analyses (Fig. 6) revealed that both in the uTHP-1 (FITC, green) and dTHP-1 cells (Cy3, red), CypA induced nuclear translocation of NF-κB after 1 h of its stimulation. When cells were pre-treated with AP-9 for 1 h or CypA was pre-incubated with CSA, the nuclear translocation of NF-κB induced by CypA was blocked. After CypA stimulating THP-1, the red color for NF-κB overlayed with the blue color for nucleus in cell nucleus under immunofluorescence microscopy, which showed plenty of p50 transducted into nucleus.
Fig. 6.
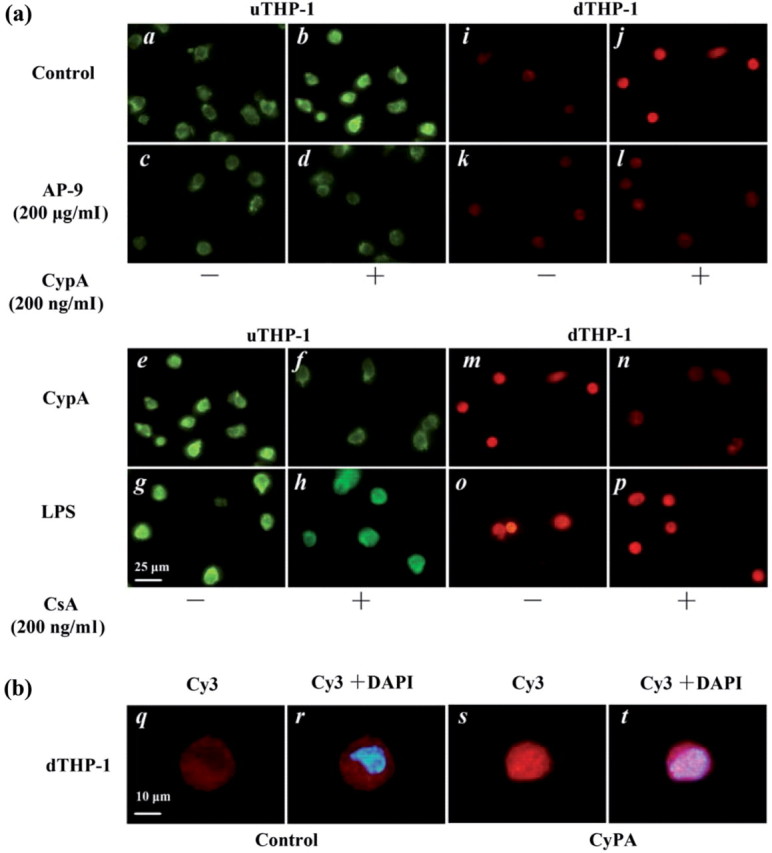
IF of NF-κB activity induced by CypA in the THP-1 cell. (a) The u and d THP-1 cells were precubated with control (a, b, i, j) and AP-9 (c, d, k, l), then stimulated with CypA (200 ng/mL, b, d, j, l), CypA/CSA (f, n), LPS (1 µg/mL, g, o) or LPS/CSA (h, p) respectively for 1h. The p50 protein of NF-κB activity was analysed by IF with FITC-conjugated anti-p50 antibody (green fluorescence) for the uTHP-1 and Cy3-conjugated anti-p50 antibody (red fluorescence) for the dTHP-1, respectively. CypA-induced nuclear translocation of NF-κB 2 h after treatment (b and h). Nuclear translocation of NF-κB induced by CypA was blockaded by AP-9 (d and l) or CSA (f and n) respectively. (b) Merged images of Cy3 and DAPI in the dTHP-1 were obtained using computer software. After CypA stimulating THP-1, the red color for NF-κB overlayed with the blue color for nucleus in cell nucleus under immunofluorescence microscopy, which showed plenty of p50 transducted into nucleus. The scale bars indicate 25 μm (a) and 10 μm (b). The data shown are representative of similar results from three independent experiments.
Effect of MAPK inhibitors on MMP-9 secretion and NF-κB activity in THP-1
To observe whether MAPK pathway was involved in CypA up-regulation of MMP-9, THP-1 cells were treated with MAPK inhibitors. Gelatin zymography showed that P38 MAPK inhibitor, SB203580 had no effect on the MMP-9 secretion in the uTHP-1 or dTHP-1 cells, while ERK1/2 and JNK inhibitors (U0126 and SP600125) significantly decreased MMP-9 secretion, both in the absence or presence of CypA (Fig. 7b). Similar results were observed in NF-κB activity by IF (Fig. 7a).
Fig. 7.
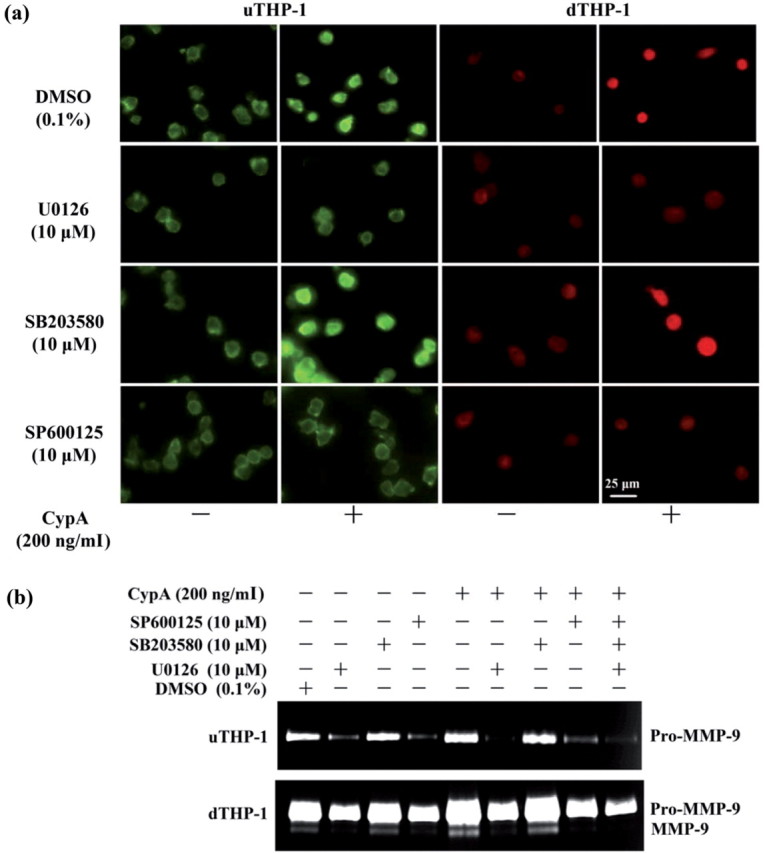
Effect of MAPK inhibitors on MMP-9 secretion and NF-κB sactivity in the THP-1. (a) The uTHP-1 and dTHP-1 cells were pre-treated with 10 μM U0126, SB203580 and SP600125 for 2 h, respectively and then were stimulated with CypA for 2 h. The cells were collected for detection of NF-κB activity by IF. The scale bars indicate 25 μm. (b) The uTHP-1 and dTHP-1 cells were pre-treated with U0126, SB203580 and SP600125 for 2 h, respectively and then were stimulated with CypA for 48 h in serum-free culture. The supernatants of each sample were collected for gelatin zymography. SB203580 had no effect on the MMP-9 secretion in the uTHP-1 or dTHP-1 cells, while U0126 and SP600125 significantly decreased MMP-9 secretion, both in the absence or presence of CypA. Similar results were observed in NF-κB activity by IF. The data shown are representative of similar results from three independent experiments.
Ca2+ mobilization and cell adhesion in the THP-1 cells with CypA stimulation
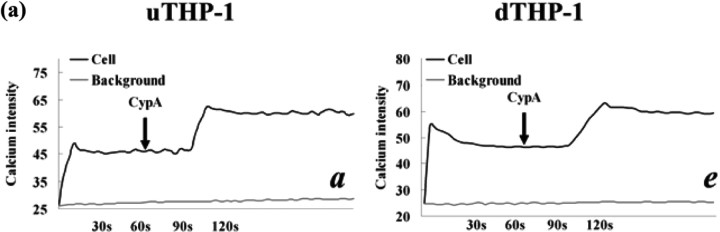
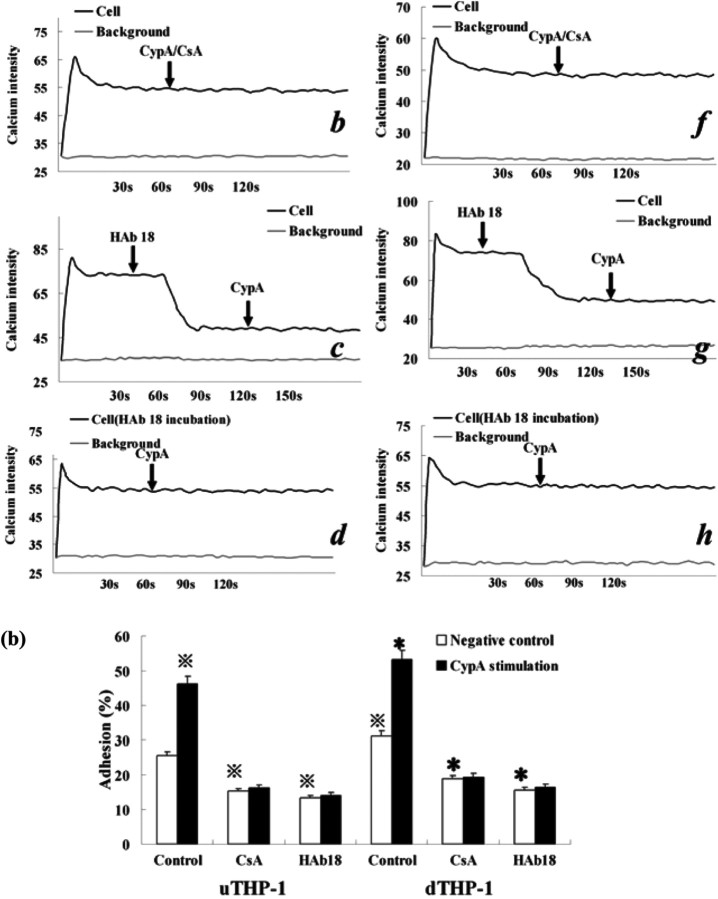
To observe CypA's influence on Ca2+ mobilization and cell adhesion in THP-1 cells, we used laser scanning confocal fluorescence microscope and adhesion assay. After the addition of CypA, Ca2+ mobilization in the uTHP-1 and dTHP-1 cells was increased (Fig. 8a). Anti-CD147 antibody HAb18 strongly decreased cytosolic Ca2+ concentration and CypA had no effect on Ca2+ concentration after HAb18 treatment. When CypA was pre-incubated with CSA or cells were pre-incubated with HAb18, no cytosolic Ca2+ mobilization response was observed. In the absence of exogeneous cyclophilins, the adhesion of uTHP-1 and dTHP-1 to ECM-coated plates was ∼25.5 and 31.2% of initially added cells, respectively (Fig. 8b). A significant increase in both the uTHP-1 and dTHP-1 cell adhesion was observed with CypA treatment (P < 0.05). In contrast, CSA or anti-CD147 antibody HAb18 dramatically reduced THP-1 cell adhesion, both in the absence or presence of CypA. These results confirmed that CypA enhanced Ca2+ mobilization and cell adhesion via direct binding to CD147 on THP-1 cells.
Fig. 8.
CypA-mediated Ca2+ increase and adhesion of the THP-1 cells. (a) Ca2+ mobilization in the Fluo-3-loaded uTHP-1 (a ∼ d) and dTHP-1 (e ∼ h) cells was measured in the presence of CypA (200 ng/ml) (a, e), CypA/CSA (b, f), HAb18 pre-incubation (d, h) or pre-stimulation (c, g). The arrows indicate the addition of the agonist. The calcium intensity that was detected by intensity of green fluorescence correlated well with cytosolic Ca2+ concentration and showed the changing of Ca2+ levels in THP-1 cells. CypA-induced Ca2+ mobilization in both uTHP-1 (a) and dTHP-1 (e) cells while HAb 18 and CSA inhibited CypA-induced Ca2+ mobilization. (b) The uTHP-1 and dTHP-1 cells were pre-treated with HAb18 antibody or CSA and then were stimulated with CypA in Matrigel Matrix-coated 96-well microtitre plates. A significant increase in both uTHP-1 and dTHP-1 cell adhesion was observed with CypA. In contrast, CSA or HAb18 dramatically reduced THP-1 cell adhesion respectively, both in the absence or presence of CypA. Data are expressed as means ± s.d. (n = 3). 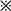 P < 0.05 vs the normal negative uTHP-1 cells. *P < 0.05 vs the normal negative dTHP-1 cells.
P < 0.05 vs the normal negative uTHP-1 cells. *P < 0.05 vs the normal negative dTHP-1 cells.
Discussion
CypA is a multifunctional cytokine involved in many biological processes, such as protein folding, cellular signalling, immunosuppression and apoptosis in many types of cells [26]. It is a secreted growth factor that augments the proliferation of human embryonic brain cells and vascular smooth muscle cells [27]. In RA synovial tissues, various cells, including macrophages and endothelial cells, are the sources of CypA [28]. CypA treatment has been reported to have induced macrophage chemoattractant protein-1 (MCP-1), TNF-α, IL-8 and IL-1β expression in a dose-dependent manner in THP-1 cells and RA synovium [29, 30], and anti-CD147 antibody or AP-9 has been reported to have inhibited CypA's chemotaxis to mononuclear cells in RA [18]. These data might show the role of CypA as one of the inflammatory mediators involved in the pathogenesis of RA.
Here in this study, we used M-CSF to differentiate monocytes into macrophages, which represented model of mature macrophages in RA SF. We found that CypA significantly up-regulated MMP-9 expression in monocytes/macrophages derived from RA SFs and M-CSF-differentiated macrophages, while it had less effect on monocytes. This difference suggested that, as one of inflammatory factors, the marked effect of CypA on the MMP-9 production in monocytes/macrophages might need monocytes’ differentiation and maturation. The highest productions of MMP-2 and MMP-9 in monocytes/macrophages from RA SF showed high activities of inflammation. Another phenomenon, that MMP-9 production and invasive ability of monocytes/macrophages from RA patients were higher than those from normal humans, suggested that the inflammation activity of monocytes/macrophages in RA patients might be higher than those cells in normal humans. As monocytes/macrophages, CypA also increased MMP-9 production in the THP-1 cells.
Another important molecule under study was CD147. CD147 is a transmembrane signalling receptor involved in neuronal function, inflammation, tumour invasion and metastasis. CD147 expression correlates well with MMP expression levels and cell invasive ability in some cancers, such as hepatoma and prostatic carcinoma [19], and the aggressive characteristics of RA synovium are similar to those of neoplastic tissues [31]. In our previous studies, CD147 expression on RA fibroblast (FLS) was found to be higher than that on OA FLS and its expression on monocytes/macrophages derived from RA SF was higher than that derived from normal human peripheral blood. High CD147 expression on monocytes/macrophages significantly up-regulated MMP-2 and MMP-9 production of RA FLS by interaction with CD147 itself [19], which formed the homologous or heterogenetic dimer to promote MMP expressions. Soluble CD147 was also found to have enhanced MMP-2 and MMP-9 production of RA FLS by its binding to membrane CD147 (data not shown). In this study, the results showed that CD147 expression increased when monocytes and uTHP-1 were differentiated into macrophages, and both of siRNA and AP-9 against CD147 decreased MMP-2 and MMP-9 expression in monocytes/macrophages. The reasons for this down-regulation might be that siRNA down-regulated the quantity of membrane CD147, which in turn reduced CD147 dimers, and that AP-9 inhibited the formation of CD147 dimers between these cells.
In this study, CSA, capable of binding and blocking CypA function was used as one way to interrupt the interaction between CypA and CD147. We found that CSA significantly decreased MMP-2 and MMP-9 expression on THP-1 by decreasing CD147 mRNA expression and membrane CD147 production, with or without CypA stimulation, while CypA had no effect on CD147 mRNA expression. These results suggested that CSA might down-regulate MMPs by decreasing CD147 expression, and CypA might up-regulate MMPs by direct binding to membrane CD147 on monocytes/macrophages and not by increasing CD147 expression. The results of no additional decrease or increase of MMP-9 production when cells were treated with the combination of RNAi and CSA under CypA stimulation suggested that RNAi and CSA targeted the same pathway in CypA-induced MMP-9 production. The increasing CD147 expression on macrophages might be one of the reasons for the difference of CypA's effect on MMP-9 between monocytes and macrophages, which means that the receptor of CypA on cells was increased for enhancing transduction signalling.
Kim et al. [29] revealed that CypA induced activation and nuclear translocation of NF-κB to enhance MMP-9 production in THP-1 cells, but the potential pathway was not reported. In this study, we found that CypA-mediated MMP-9 production needed CypA's direct binding to membrane CD147 to transduce signalling for NF-κB activity and that the inhibition of ERK1/2 or JNK MAPK pathway suppressed CypA-induced MMP-9 production by inhibiting the activity of NF-κB, while the inhibition of p38 failed. These results showed that CD147 might facilitate CypA-induced MMP-9 production by activating ERK1/2 and JNK MAPK pathway.
There are some reports about the pathway between CypA and CD147 in the development of such diseases as HIV-1 virus, SARS-CoV, pancreatic cancer, T-cell activation and adhesion to ECM, and the effective blocking of the pathway by pre-treatment with anti-CD147 antibody or CSA [19–21, 32, 33]. Constant and others [22, 23] revealed that the role for extracellular cyclophilins, via interaction with CD147, on the recruitment of circulating leucocytes to sites of inflammation, and cyclophilin–CD147 interactions might contribute directly to the pathogenesis of inflammatory diseases. In this study, we confirmed that in RA, the abundant CypA could increase MMP-9 expression and invasion ability of macrophages via direct binding to CD147, which showed the potential role of cyclophilin–CD147 interactions in RA pathogenesis. So, all these results showed the important role of cyclophilin–CD147 interactions during inflammatory responses, and targeting cyclophilin–CD147 interactions may provide a new approach for alleviating tissue inflammation.
The mobilization of cytosolic Ca2+ correlates well with the ability of cell–cell or cell–ECM adhesion, and CD147 facilities adhesion by interacting with integrins in T cells. In this study, the ability of adhesion of THP-1 to ECM was increased when cells differentiated, and CypA increased mobilization of cytosolic Ca2+ both in uTHP-1 cells and dTHP-1 cells. We found that anti-CD147 antibody decreased the ability of cell adhesion, which showed that CD147 might have the function of maintaining the normal level of cytosolic Ca2+ concentration in THP-1 cells, and also found that anti-CD147 antibody blocked CypA-induced cytosolic Ca2+ concentration and adhesion of THP-1 cells to ECM, which also showed that CD147 might be a co-stimulatory molecule in cyclophilin-mediated signalling events for the adhesion of monocytes/macrophages to ECM. CSA in this study blocked CypA-induced adhesion by interrupting the CypA-binding to CD147. These results suggested that CypA promotes adhesion of monocytes/macrophages to ECM by direct binding to CD147, and also suggested that during the progress of RA, CypA might enhance the adhesive and invasive abilities of monocytes/macrophages to synovium or cartilage and contribute to the joints’ impairment.
In conclusion, our findings indicate that CypA enhances the adhesion of monocytes/macrophages to ECM in RA, and up-regulates MMP-9's expression and potential invasion in monocytes/macrophages to degrade cartilage by direct binding to CD147 which enhances the calcium mobilization and NF-κB activity. These results suggest the potential role of cyclophilin–CD147 interactions in RA pathogenesis and may serve as a preliminary foundation for further researches on CypA–CD147 signalling pathway in RA and therapeutic intervention in RA.

Acknowledgements
Funding: This work was supported by grants from the Key Program projects, National Nature Science Foundation of China (30530720).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–24. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 4.Tchetverikov I, Ronday HK, Van El B, et al. MMP profile in paired serum and synovial fluid samples of patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:881–3. doi: 10.1136/ard.2003.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannelli G, Erriquez R, Iannone F, Marinosci F, Lapadula G, Antonaci S. MMP-2, MMP-9, TIMP-1 and TIMP-2 levels in patients with rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol. 2004;22:335–8. [PubMed] [Google Scholar]
- 6.Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Albers MW, Wandless TJ, et al. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992;31:3896–901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- 8.Sherry B, Yarlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci USA. 1992;89:3511–5. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Q, Leiva MC, Fischkoff SA, Handschumacher RE, Lyttle CR. Leukocyte chemotactic activity of cyclophilin. J Biol Chem. 1992;267:11968–71. [PubMed] [Google Scholar]
- 10.Billich A, Winkler G, Aschauer H, Rot A, Peichl P. Presence of cyclophilin a in synovial fluids of patients with rheumatoid arthritis. J Exp Med. 1997;185:975–80. doi: 10.1084/jem.185.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomita T, Nakase T, Kaneko M, et al. Expression of extracellular matrix metalloproteinase inducer and enhancement of the production of matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 2002;46:373–8. doi: 10.1002/art.10050. [DOI] [PubMed] [Google Scholar]
- 12.Lai WC, Zhou M, Shankavaram U, Peng G, Wahl LM. Differential regulation of lipopolysaccharide-induced monocyte matrix metalloproteinase (MMP)-1 and MMP-9 by p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Immunol. 2003;170:6244–9. doi: 10.4049/jimmunol.170.12.6244. [DOI] [PubMed] [Google Scholar]
- 13.Yurchenko V, Zybarth G, O’Connor M, et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002;277:22959–65. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 14.Pushkarsky T, Yurchenko V, Vanpouille C, et al. Cell surface expression of CD147/EMMPRIN is regulated by cyclophilin 60. J Biol Chem. 2005;280:27866–71. doi: 10.1074/jbc.M503770200. [DOI] [PubMed] [Google Scholar]
- 15.Konttinen YT, Li TF, Mandelin J, et al. Increased expression of extracellular matrix metalloproteinase inducer in rheumatoid synovium. Arthritis Rheum. 2000;43:275–80. doi: 10.1002/1529-0131(200002)43:2<275::AID-ANR6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Zhu P, Lu N, Shi ZG, et al. Cd147 overexpression on synoviocytes in rheumatoid arthritis enhances matrix metalloproteinase production and invasiveness of synoviocytes. Arthritis Res Ther. 2006;8:R44. doi: 10.1186/ar1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Zhu P, Jiang JL, et al. Involvement of CD147 in overexpression of MMP-2 and MMP-9 and enhancement of invasive potential of PMA-differentiated THP-1. BMC Cell Biol. 2005;6:25. doi: 10.1186/1471-2121-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu P, Ding J, Zhou J, Dong WJ, Fan CM, Chen ZN. Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther. 2005;7:R1023–33. doi: 10.1186/ar1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yurchenko V, Constant S, Bukrinsky M. Dealing with the family: CD147 interactions with cyclophilins. Immunology. 2006;117:301–9. doi: 10.1111/j.1365-2567.2005.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Mi L, Xu J, et al. Function of HAB18g/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J Infect Dis. 2005;191:755–60. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pushkarsky T, Zybarth G, Dubrovsky L, et al. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc Natl Acad Sci USA. 2001;98:6360–5. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arora K, Gwinn WM, Bower MA, et al. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol. 2005;175:517–22. doi: 10.4049/jimmunol.175.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J Leukoc Biol. 2007;82:613–8. doi: 10.1189/jlb.0506317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Stawowy P, Meyborg H, Stibenz D, et al. Furin-like proprotein convertases are central regulators of the membrane type matrix metalloproteinase-pro-matrix metalloproteinase-2 proteolytic cascade in atherosclerosis. Circulation. 2005;111:2820–7. doi: 10.1161/CIRCULATIONAHA.104.502617. [DOI] [PubMed] [Google Scholar]
- 26.Cande C, Vahsen N, Kouranti I, et al. AIF and cyclophilin A cooperate in apoptosis-associated chromatinolysis. Oncogene. 2004;23:1514–21. doi: 10.1038/sj.onc.1207279. [DOI] [PubMed] [Google Scholar]
- 27.Nahreini P, Hovland AR, Kumar B, Andreatta C, Edwards-Prasad J, Prasad KN. Effects of altered cyclophilin A expression on growth and differentiation of human and mouse neuronal cells. Cell Mol Neurobiol. 2001;21:65–79. doi: 10.1023/A:1007173329237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin ZG, Melaragno MG, Liao DF, et al. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–96. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 29.Kim H, Kim WJ, Jeon ST, et al. Cyclophilin A may contribute to the inflammatory processes in rheumatoid arthritis through induction of matrix degrading enzymes and inflammatory cytokines from macrophages. Clin Immunol. 2005;116:217–24. doi: 10.1016/j.clim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Shankar S, Handa R. Biological agents in rheumatoid arthritis. J Postgrad Med. 2004;50:293–9. [PubMed] [Google Scholar]
- 31.Koch AE. Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998;41:951–62. doi: 10.1002/1529-0131(199806)41:6<951::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.Allain F, Vanpouille C, Carpentier M, Slomianny MC, Durieux S, Spik G. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc Natl Acad Sci USA. 2002;99:2714–9. doi: 10.1073/pnas.052284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, Zhai Q, Bharadwaj U, et al. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006;106:2284–94. doi: 10.1002/cncr.21862. [DOI] [PubMed] [Google Scholar]