Abstract
Background
Rift Valley fever (RVF) is endemic to the tropical regions of eastern and southern Africa. The seroprevalence of RVF was investigated among the human population in Borno State, Nigeria to determine the occurrence of the disease in the study area in comparison with that of Lassa fever and Crimean-Congo Hemorrhagic fever.
Methods
Recombinant nucleoprotein (rNP)-based IgG-ELISAs for the detection of serum antibodies against RVF virus (RVFV), Lassa fever virus (LASV), and Crimean-Congo hemorrhagic fever virus (CCHFV) were used to test human sera in Borno State, Nigeria. The presence of neutralizing antibody against the RVFV-glycoprotein-bearing vesicular stomatitis virus pseudotype (RVFVpv) was also determined in the human sera.
Results
Of the 297 serum samples tested, 42 (14.1%) were positive for the presence of RVFV-IgG and 22 (7.4%) and 7 (2.4%) of the serum samples were positive for antibodies against LASV and CCHFV, respectively. There was a positive correlation between the titers of neutralizing antibodies obtained using RVFVpv and those obtained using the conventional neutralization assay with the attenuated RVFV-MP12 strain.
Conclusions
The seroprevalence of RVF was significantly higher than that of LASV and CCHF in Borno State, Nigeria. The RVFVpv-based neutralization assay developed in this study has the potential to replace the traditional assays based on live viruses for the diagnosis and seroepidemiological studies of RVF.
Keywords: Nigeria, Rift Valley fever, Seroprevalence
Introduction
Rift Valley fever virus (RVFV) is a zoonotic mosquito-borne virus belonging to the genus Phlebovirus in the Family Bunyaviridae. It causes severe diseases in humans and livestock throughout Africa1 and the Arabian Peninsula2. RVFV is also considered to be a potential bioterrorism agent. In the last few decades, Rift Valley fever (RVF) outbreaks have been reported in eastern and southern Africa (e.g. Kenya, Somalia, United Republic of Tanzania, Madagascar and South Africa).3–7 In contrast, there have been very few reports on the recent occurrence of RVF in western and central Africa. Significant high- and low-prevalence clusters of RVF in sub-national areas on the African continent have been reported.8 Since the spread of RVFV largely depends on the mosquito vectors and the translocation of animal hosts, an endemic situation usually occurs in the restricted geographical areas inhabited by their hosts and vectors. In Nigeria, RVFV antibodies have been found in sheep, goats, cattle, horses and camels in the northern states of Kaduna and Sokoto9 and in the plateau area10 suggesting that the virus may be enzootic in Nigeria. In addition, serological studies conducted on human sera have confirmed the existence of the disease in Nigeria.11 The specific geographical location of Borno State in northeastern Nigeria, which shares international borders with three other African countries (Cameroun, Chad and Niger), makes it vulnerable to the transboundary spread of various diseases, including viral hemorrhagic fevers (VHFs). In addition, Borno State has been reported as the niche for Lassa fever virus (LASV) and possibly other VHFs. However, the epidemiology of RVF and other VHFs has not been extensively investigated in Borno State. A detailed and accurate investigation of the seroprevalence is necessary to ascertain the occurrence and spread of RVF in this area.
RVFV possesses a single-stranded tripartite RNA genome composed of three segments: S, M and L. The S segment encodes the nucleocapsid protein (NP) and non-structural (NS) protein, using an ambisense strategy. The M segment encodes the precursor for the glycoproteins Gn and Gc and two non-structural proteins of 78 kDa and 14 kDa. The L segment encodes the L protein.12 The nucleotide sequence of the NP gene is highly conserved among various RVFV strains.13 Serum antibodies against NP are readily detected early after infection and in convalescent individuals, providing a basis for the diagnosis of RVF.14,15
The traditional diagnostic assays for VHFs are based on immunoassays that use live viruses as the source of capture antigens. The use of highly attenuated RVFV (RVFV-MP12) does not require stringent biosafety measures and could readily be adopted in laboratories in developing countries where infrastructures for biosafety level 3 or 4 containments are lacking. The usefulness of recombinant viral nucleoprotein (rNP)-based serological assays, such as IgG-ELISAs and immunofluoresence assays (IFAs) for the detection of antibodies against VHFs such as Crimean-Congo hemorrhagic fever virus (CCHFV) and LASV have been reported.16–18 Recombinant protein technology does not require high containment biosafety facilities and could readily meet the demand for a simple and reliable system not only for diagnosis of VHFs but also for comparative seroepidemiology of various VHFs in a cohort study.
In this study, the seroprevalence of RVFV infection in humans in Borno State, Nigeria, was determined using rNP-based IgG ELISAs, and the prevalence of RVFV antibody was compared with those of other hemorrhagic fever virus infections including LASV and CCHFV. In addition, we developed virus neutralization assays using vesicular stomatitis virus (VSV) pseudotype virus-bearing glycoproteins of RVFV, and the usefulness of the VSV pseudotype system was determined for a high throughput screening of neutralizing antibodies against RVFV.
Materials and methods
Serum samples
Two hundred and ninety-seven serum samples were collected between September 2011 and February 2012 from patients attending health facilities (government hospitals, private hospitals or clinics) in 10 out of the 27 local government areas (LGAs) in Borno State in northern Nigeria. A simple random sampling technique was used to obtain human sera from the selected LGAs, which consisted of at least three LGAs from each of the three senatorial districts (North, Central and South), and also from the town of Lassa.
Expression and purification of rNPs
Insect Tn5 cells19 were infected with recombinant baculoviruses expressing rNPs of RVFV, LASV or CCHFV to produce recombinant His-tagged RVFV-rNP, LASV-rNP or CCHFV-rNP, respectively.16,17,19 The rNPs were purified by Ni2+ column chromatography (QIAGEN GmbH, Hilden, Germany), as described previously.17 The negative control antigen (ΔP) was prepared from a baculovirus (Ac-ΔP) that lacks polyhedrin expression using the same protocols as for the rNPs. Expression of the His-tagged rNPs and ΔP was analyzed by SDS-PAGE gels (12% polyacrylamide) stained with Coomassie blue (Bio-Rad Laboratories, Hercules, CA, USA) (Supplementary Figure 1).
IgG-ELISA
IgG-ELISA was performed as described previously.16 Briefly, 96- well ELISA plates were coated with the predetermined optimal quantity of purified RVFV-rNP, LASV-rNP or CCHFV-rNP (approximately 100 ng/well) at 4°C overnight. Each well of the plates was then covered with 200 µl of PBS containing 5% skim milk and 0.05% Tween 20 (Sigma, St. Louis, MO, USA) (PBST-M), followed by incubation for 1 h at 37°C for blocking. The plates were washed three times with PBS containing 0.05% Tween 20 (PBST) and then inoculated with test serum (100 μl/well), which was diluted 1:400 and 1:1600 with PBST-M. After a 1 h incubation period, the plates were washed three times with PBST and then were inoculated with goat anti-human IgG antibody labeled with HRP (1:1000 dilution; Zymed Laboratories, Inc., South San Francisco, CA, USA). After a further 1 h incubation period, the plates were washed and 100 µl of ABTS solution (Roche Diagnostics, Mannheim, Germany) was added to each well. The plates were incubated for 30 min at room temperature, and the optical density at 405 nm (OD405) was measured against a reference of 490 nm. The adjusted OD405 value was calculated by subtracting the OD405 value of the negative Ag-coated wells from that of the corresponding wells. The mean plus three standard deviations (mean+3SD) of the ELISA indices for the IgG-ELISAs was calculated using human serum samples that were confirmed to be negative for infection with the respective viruses by IFA (data not shown) and was used as the cut-off value for the IgG-ELISAs. In order to minimize false-positive results that could occur with single serum dilution, IgG response was considered to be positive if the sample showed adjusted OD405 values above the cut-off at both 1:400 and 1:1600 dilutions.
Conventional neutralization assay
The conventional neutralization assay using infectious RVFV (RVFV-MP12 strain) was performed as described previously.20 Briefly, heat-inactivated serum samples were diluted three-fold (from 1:40 to 1:1080) with Eagle's minimum essential medium (MEM, Sigma, St. Louis, MO, USA) containing 2% FBS (Invitrogen, Carlsbad, CA, USA). Each test sample (50 µl) was then mixed with the same volume of RVFV-MP12 at an infectious dose of 100 plaque forming units. The mixture was then incubated for 1 h at 37°C for neutralization. After incubation, the mixtures were tested for neutralization by the cytopathic effect inhibition assay using Vero E6 cells.20 The neutralization antibody (NAb) titer was defined as the reciprocal of the highest dilution at which no cytopathic effect was observed.
Generation of VSV pseudotyped with RVFV-glycoprotein
The glycoprotein (GP) cDNA of RVFV-MP12 was cloned into the pKS336 vector17 to construct an RVFV-GP expression plasmid, designated as pKS336-RVFV-GP. To generate the RVFV-GP-bearing VSV pseudotype (RVFVpv), a *G-VSVΔG/luc encoding luciferase gene (kindly provided by Dr. M. A. Whitt), instead of the VSVG gene, was inoculated into human kidney 293 T cells21 transfected with pKS336-RVFV-GP. After 24 h the culture supernatants were collected and used as a working seed for the RVFVpv.
Neutralization test using RVFVpv
The dilution of RVFVpv used was calculated to produce approximately 105 relative light units in control wells. Serum samples were mixed with RVFVpv at a dilution of 1:50 in MEM (Sigma) supplemented with 2% FBS (Invitrogen). Then, the mixture was incubated at 37°C for 1 h for neutralization. The serum-RVFVpv mixture was transferred to 96-well plates containing monolayers of Vero E6 cells.20 After 24 h the cells were lysed, and the luciferase activities were measured using the Bright-Glo Luciferase Assay System (Promega, Madison, WI, USA) according to the protocol recommended by the manufacturer.
Statistical methods
The sensitivity, specificity and predictive values for positive and negative tests were calculated by standard methods. Spearman's rank correlation coefficient test, ROC curves and two-graph-ROC (TG-ROC) curves22,23 were analyzed using the Stat Flex ver. 5 software program (Artech Co. Ltd., Osaka, Japan).
Results
rNP-based IgG-ELISA
In order to determine the seroprevalence of RVFV in humans in Borno State, Nigeria, sera were first subjected to an His-RVFV-rNP-based IgG-ELISA. An IgG response was considered to be positive if the sample had adjusted OD405 values higher than cut-off values at both 1:400 and 1:1600 dilutions. Of the 297 serum samples analyzed, 42 (14.1%) were positive for RVFV IgG (Table 1). Simultaneously, the serum samples were also tested for the presence of antibodies against LASV and CCHFV using the rNP-based IgG ELISAs, and a total of 22 (7.4%) and 7 (2.4%) of the samples were positive for antibodies against LASV and CCHFV, respectively (Table 1). The result indicated a high prevalence rate of RVF in the study area.
Table 1.
Summary of the results of the IgG ELISA (n=297), serum neutralization antibody (NAb) assay using VSV-RVFV-pV (n=278) and NAb assay using RVFV-MP12 (n=271) to determine seroprevalence of RVFV in humans in Borno State, Nigeria
| IgG-ELISAa |
NAb assay |
||||
|---|---|---|---|---|---|
| RVFV-rNP ELISA | LASV-rNP ELISA | CCHFV-rNP ELISA | RVFVpv NAbb | RVFV-MP12 NAb | |
| No. positive (%) | 42 (14.1) | 22 (7.4) | 7 (2.4) | 43 (15.5) | 34 (12.6) |
| No. negative (%) | 255 (85.9) | 275 (92.6) | 290 (97.6) | 235 (84.5) | 237 (87.4) |
| Total | 297 (100) | 297 (100) | 297 (100) | 278 (100) | 271 (100) |
CCHFV-rNP: Crimean-Congo hemorrhagic fever virus recombinant nucleoprotein; LASV-rNP: Lassa fever virus recombinant nucleoprotein; NAb: serum neutralization antibody; RVFV: Rift Valley fever virus; RVFV-MP12: highly attenuated MP12 strain of RVFV; RVFV-rNP: RVFV recombinant nucleoprotein; RVFVpv: RVFV-glycoprotein-bearing vesicular stomatitis virus pseudotype.
a IgG response considered positive if the sample had a positive titer at both 1:400 and 1:1600 dilutions.
b >75% inhibition considered positive.
In order to confirm the efficacy of RVFV-rNP as an antigen and to determine the sensitivity and specificity of the RVFV-rNP based ELISA, the NAb assay was performed using RVFV-MP12. Of the 271 human serum samples examined, 34 (12.6%) were positive for NAb, with antibody titers ranging from 40 to 1080 (Table 1). Thirty-one out of 34 (sensitivity: 91.2%) NAb-positive samples were also positive in the IgG-ELISA, and 229 out of 237 (specificity: 96.6%) NAb-negative samples were also negative in the IgG-ELISA.
Neutralization assay with the VSV-based RVFV pseudotype
Virus neutralization assays using VSV pseudotype-bearing glycoproteins of viruses causing VHFs have been developed with high sensitivity and specificity.24 In order to determine whether a VSV-based RVFV pseudotype can be applied for the screening of NAbs against RVFV infection, we produced RVFVpv and then determined whether the human sera collected from the study area could neutralize its infectivity. Single serum dilution (1:50) assay rather than titration by endpoint dilutions was performed in order to establish a high-throughput screening of NAbs. Since the infectivity of the RVFVpv, harboring the luciferase gene, could be ascertained by determining its luciferase activity, the neutralization activities of the sera were represented as the percent neutralization calculated from the reduction in the luciferase expression. Of the 278 serum samples tested, 43 (15.5%) showed more than 75% luciferase activity neutralization, and the remaining 235 (84.5%) showed less than 75% neutralization, compared with the non-serum control (Table 1). The sensitivity of the RVFVpv-based neutralization assay was determined by comparing the results with those obtained from the conventional neutralization assay using RVFV-MP12. All 34 serum samples that tested positive in the neutralization assay with RVFV-MP12 showed more than 50% neutralization of RVFVpv (Table 2). Furthermore, of the 211 serum samples that had less than 50% neutralization, none (0%) were positive for the neutralization assay with RVFV-MP12 (Table 2).
Table 2.
The relationship between the results of the authentic virus (RVFV-MP12 strain)-based and RVFVpv-based neutralization assays (n=270)
| NAb for RVFVpv |
|||
|---|---|---|---|
| NAb for RVFV-MP12 | % Neutralization |
||
| >75 | 50–75 | <50 | |
| Positive | 32 (11.9%) | 2 (0.7%) | 0 (0%) |
| Negative | 11 (4.1%) | 14 (5.2%) | 211 (78.1%) |
| Total | 43 (15.9%) | 16 (5.9%) | 211 (78.1%) |
NAb: serum neutralization antibody; RVFV-MP12: highly attenuated MP12 strain of Rift Valley fever virus; RVFVpv: RVFV-glycoprotein-bearing vesicular stomatitis virus pseudotype.
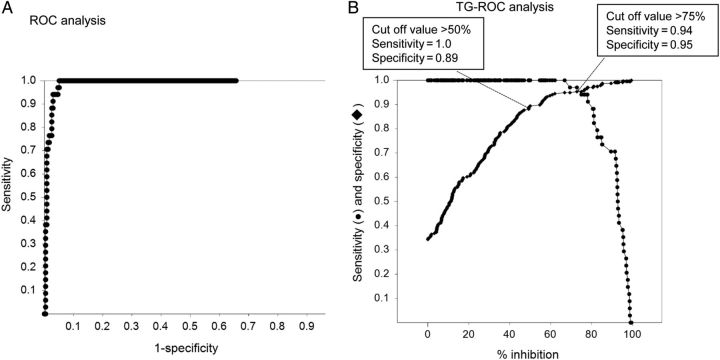
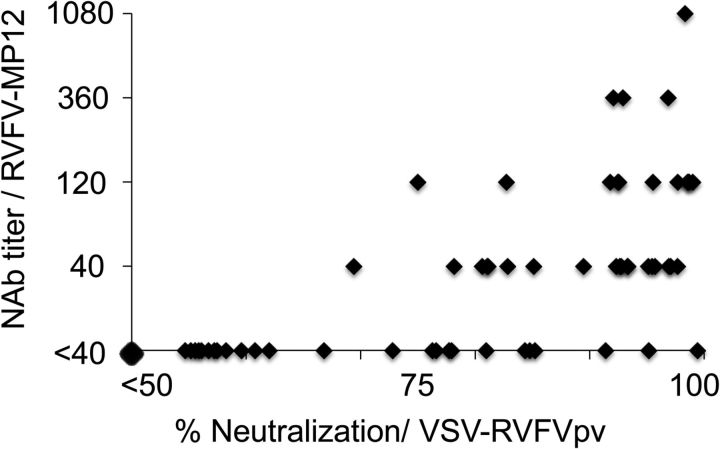
The ROC and TG-ROC analyses were performed in order to select cut-off points for the percent neutralization using RVFVpv (Figure 1). If the cut-off was defined as the intersection point of the sensitivity and specificity curves, the cut-off value was 75% neutralization, and the sensitivity and specificity were 94% and 95%, respectively. If the cut-off was defined as 50% neutralization, the sensitivity would increase to 100%, but specificity would decrease to 89% (Figure 1). There was a positive correlation between the titers of neutralizing antibodies obtained using RVFVpv and those obtained using the conventional neutralization assay with RVFV-MP12. Spearman's rank correlation coefficient (rs) was calculated to be 0.77 (Figure 2). Thus, NAbs against RVFV can be screened by the RVFVpv-based neutralization assay using the single serum dilution format.
Figure 1.
The results of the ROC and two-graph (TG)-ROC analyses of the vesicular stomatitis virus pseudotype virus-bearing glycoproteins of Rift Valley fever virus, (RVFVpv)-based neutralization assay. In (A) the ROC analysis graph, the specificity values are deducted from 1.0 in the x-axis, and the sensitivity vs 1-specificity data are plotted as dots. In (B) the TG-ROC analysis graph, sensitivity data are plotted as dots and specificity data are plotted as diamonds.
Figure 2.
A comparison of the percent neutralization obtained from virus pseudotype virus-bearing glycoproteins of Rift Valley fever virus (RVFVpv)-based neutralization assay with the serum neutralization antibody (NAb) titers obtained from authentic virus (RVFV-MP12)-based neutralization assays. The data are plotted as diamonds on the scatterplot. Spearman's rank correlation coefficient (rs) was calculated to be 0.77.
Discussion
In this study, the seroprevalence of RVF was investigated among human population in Borno State, Nigeria to determine the occurrence and spread of the disease in comparison with those of Lassa fever and CCHF. Since recombinant protein-based immunoassays with high sensitivity and specificity have been demonstrated to be useful for the diagnosis of VHFs in humans,15–17,25,26 we have used rNP-based IgG-ELISAs for the detection of serum antibodies against RVFV, LASV, and CCHFV. Of the 297 serum samples tested for RVFV-IgG, a total of 42 (14.1%) showed positive results (Table 1). The antibody prevalence observed in this study is in agreement with the results of a surveillance study carried out in the 1980s, when 14.8% of the sera collected in more than 30 locations throughout Nigeria were found to be positive for a hemagglutination-inhibiting antibody against RVFV.11 In this study a significant difference in the prevalence of antibodies was observed (RVFV rNP-ELISA [14.1%], LASV rNP-ELISA [7.4%] and CCHFV rNP-ELISA [2.4%]), and the highest prevalence was noted for RVFV rNP-ELISA antibody. In addition, more than 12% of the samples tested had NAb activity against RVFV. These results indicate that RVFV is more actively circulating in the study area compared with LASV and CCHFV. It is therefore important to undertake a risk assessment of RVFV infection in humans in Nigeria.
RVFV is transmitted through contact with body fluids from infected humans and animals or by mosquito bites and/or aerosols. The particular location of Borno State, with its shared geographical borders with three other African countries, indicates that a regional epidemiological study should be conducted not only in the LGAs in Nigeria, but also in the neighboring countries, to identify the possible risk factors for transboundary RVFV infection. The borders are porous and unrestricted human and animal trafficking across borders is common throughout the year. In spite of the active circulation of RVFV observed in this study, no outbreak of the disease in humans or animals has been reported in the study area. This could be as a result of poor surveillance for the outbreak of the disease or a lack of expertise in disease recognition.10,11 It is also possible that RVFV circulating in the region is a non-pathogenic strain. RVF in humans usually begins with a non-specific influenza-like acute fever, but can progress to serious hemorrhagic fever in some cases. VHFs, particularly Lassa fever, an acute viral hemorrhagic fever caused by LASV, has been reported to be endemic in Nigeria.27,28 The symptoms of the disease also vary from a non-specific febrile illness to fatal viral hemorrhagic fever. Therefore, there is the possibility that patients with non-specific febrile illness caused by RVFV infection could be misdiagnosed with other endemic infectious diseases, such as malaria, typhoid fever or Lassa fever.
A VSV-based pseudotype system has been designed to enable the high-throughput screening of NAbs against viral infections.29 We also developed a novel serum neutralization test using RVFVpv to detect serum antibody against RVFV. The reliability and usefulness of the assay were evaluated by comparing the results of the assay with those obtained from the widely used ‘gold-standard’ neutralization assay using infectious RVFV. Of the 43 serum samples that showed more than 75% neutralization by the RVFVpv-based assay, 11 were negative by the conventional RVFV-MP12 neutralization assay (Table 2). It is possible that the results of 11 negative samples were due to false-positive reactions in the VSV-RVFVpv-based assay. However, among these serum samples, one showed a higher OD405 value (0.443) than the cut-off OD405 value at 1:400 dilution, and another one was identified as a positive by the rNP-based ELISA, with OD405 values of 1.318 and 0.364 at the 1:400 and 1:1600 dilutions, respectively (Supplementary Table 1), indicating that the serum contained antibody to RVFV. Another possibility is that the VSV-RVFVpv-based assay was more sensitive than the standard neutralization assay using RVFV-MP12. This assumption is supported by the observation that the NAb titers measured using pseudotyped VSV bearing the GP of Nipah virus or SARS-coronavirus are higher than those obtained by using infectious viruses.21,29,30
The ROC and TG-ROC analyses indicated an appropriate cut-off value for the percent neutralization (75%) to demonstrate that the NAbs in the sera had high sensitivity and specificity. The conventional assay for measuring NAbs requires serial-dilutions of the test serum and takes several days for the virus to replicate to a level which results in plaque-forming or cytopathic effects in infected cells. However, the new assay based on RVFVpv uses a single serum dilution (1:50) and has a quantitative nature, where the luciferase activity can be determined one day after inoculation onto cells. Finally, pseudotyped VSVs do not produce infectious progeny viruses, thereby ensuring their safe use as diagnostic tools. Taken together, our results indicate that the RVFVpv-based assay for measuring NAb has safe, rapid and high-throughput diagnostic capabilities.
Our study has limitations, one of which is the relatively small sample size compared to the previous study, carried out in the 1980s, employing more than 3000 human sera from the different ecological zones in Nigeria.11 In addition, we could not access detailed information on the subjects (age, profession, history of illness etc.). Although our data were obtained using the most recent serological procedures, the lack of demographic information on the study subjects makes it difficult to provide an advanced epidemiological understanding of VHFs in Nigeria. However, this study has important strengths: it provides information on RVFV infection with a high prevalence in human population in Borno State and demonstrates the usefulness of the VSV-based neutralization assay for the epidemiological investigations.
Conclusions
The results of rNP-based ELISA have shown that approximately 14% of the study population in Borno State, Nigeria, have a history of RVFV infection, and the seroprevalence of RVF was higher than those of other viral hemorrhagic fevers such as Lassa fever and CCHF. In addition, the RVFVpv-based NAb assay developed in this study has the potential to replace traditional assays based on live viruses for the diagnosis and seroepidemiological analysis of RVF in endemic and non-endemic countries.
Supplementary Material
Acknowledgments
Authors' contributions: DNB, FK and SSB conceived the study; SF and MS designed the study protocol; DNB, HT, TY, ST, KI and AF carried out the serological assays, and analysis and interpretation of the data. DNB and SF drafted the manuscript; MS, SM, MS and SSB critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. SF and MS are guarantors of the paper.
Acknowledgements: The authors wish to thank Dr. M.A. Whitt for providing the *G-VSVΔG/luc construct. We also thank Ms. M. Ogata for her valuable assistance. The senior author (DNB) was a Visiting Scientist at the National Institute of Infectious Diseases, Tokyo, Japan, under the sponsorship of the Tertiary Education Trust Fund, Nigeria. He is also a postgraduate student in the Department of Veterinary Microbiology and Parasitology, University of Maiduguri, Nigeria.
Funding: This work was supported in part by a grant-in-aid from the Ministry of Health Labor and Welfare of Japan and the Japan Society for the Promotion of Science [H22-Shinko-Ippan-009, H22-Shinko-Ippan-006, and H25 Shinko-Ippan-004].
Competing interests: None declared.
Ethical approval: This study protocol was approved by the Borno State Ministry of Health, Nigeria and the Ethics Committee of the National Institute of Infectious Diseases, Tokyo, Japan [No. 371].
References
- 1.Faye O, Diallo M, Diop D, et al. Rift Valley fever outbreak with East-Central African virus lineage in Mauritania, 2003. Emerg Infect Dis. 2007;13:1016–23. doi: 10.3201/eid1307.061487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoemaker T, Boulianne C, Vincent MJ, et al. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000–01. Emerg Infect Dis. 2002;8:1415–20. doi: 10.3201/eid0812.020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andriamandimby SF, Randrianarivo-Solofoniaina AE, Jeanmaire EM, et al. Rift Valley fever during rainy seasons, Madagascar, 2008 and 2009. Emerg Infect Dis. 2010;16:963–70. doi: 10.3201/eid1606.091266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archer BN, Weyer J, Paweska J, et al. Outbreak of Rift Valley fever affecting veterinarians and farmers in South Africa, 2008. S Afr Med J. 2011;101:263–6. doi: 10.7196/samj.4544. [DOI] [PubMed] [Google Scholar]
- 5.Gachohi JM, Bett B, Njogu, et al. The 2006–2007 Rift Valley fever outbreak in Kenya: sources of early warning messages and response measures implemented by the Department of Veterinary Services. Rev Sci Tech. 2012;31:877–87. doi: 10.20506/rst.31.3.2163. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich N, Saathoff E, Weller N, et al. High seroprevalence of Rift Valley fever and evidence for endemic circulation in Mbeya region, Tanzania, in a cross-sectional study. PLoS Negl Trop Dis. 2012;6:e1557. doi: 10.1371/journal.pntd.0001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nderitu L, Lee JS, Omolo J, et al. Sequential Rift Valley fever outbreaks in eastern Africa caused by multiple lineages of the virus. J Infect Dis. 2011;203:655–65. doi: 10.1093/infdis/jiq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements AC, Pfeiffer DU, Martin V, Otte MJ. A Rift Valley fever atlas for Africa. Prev Vet Med. 2007;82:72–82. doi: 10.1016/j.prevetmed.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Ezeifeka GO, Umoh JU, Belino ED, Ezeokoli CD. A serological survey for Rift Valley fever antibody in food animals in Kaduna and Sokoto States of Nigeria. Int J Zoonoses. 1982;9:147–51. [PubMed] [Google Scholar]
- 10.Olaleye OD, Tomori O, Schmitz H. Rift Valley fever in Nigeria: infections in domestic animals. Rev Sci Tech. 1996;15:937–46. doi: 10.20506/rst.15.3.966. [DOI] [PubMed] [Google Scholar]
- 11.Olaleye OD, Tomori O, Ladipo MA, Schmitz H. Rift Valley fever in Nigeria: infections in humans. Rev Sci Tech. 1996;15:923–35. doi: 10.20506/rst.15.3.967. [DOI] [PubMed] [Google Scholar]
- 12.Giorgi C, Accardi L, Nicoletti L, et al. Sequences and coding strategies of the S RNAs of Toscana and Rift Valley fever viruses compared to those of Punta Toro, Sicilian Sandfly fever, and Uukuniemi viruses. Virology. 1991;180:738–53. doi: 10.1016/0042-6822(91)90087-r. [DOI] [PubMed] [Google Scholar]
- 13.Bird BH, Khristova ML, Rollin PE, et al. Complete genome analysis of 33 ecologically and biologically diverse Rift Valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry. J Virol. 2007;81:2805–16. doi: 10.1128/JVI.02095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fafetine JM, Tijhaar E, Paweska JT, et al. Cloning and expression of Rift Valley fever virus nucleocapsid (N) protein and evaluation of a N-protein based indirect ELISA for the detection of specific IgG and IgM antibodies in domestic ruminants. Vet Microbiol. 2007;121:29–38. doi: 10.1016/j.vetmic.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Jansen van Vuren P, Potgieter AC, Paweska JT, van Dijk AA. Preparation and evaluation of a recombinant Rift Valley fever virus N protein for the detection of IgG and IgM antibodies in humans and animals by indirect ELISA. J Virol Methods. 2007;140:106–14. doi: 10.1016/j.jviromet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Saijo M, Georges-Courbot MC, Marianneau P, et al. Development of recombinant nucleoprotein-based diagnostic systems for Lassa fever. Clin Vaccine Immunol. 2007;14:1182–9. doi: 10.1128/CVI.00101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saijo M, Qing T, Niikura M, et al. Recombinant nucleoprotein-based enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Crimean-Congo hemorrhagic fever virus. J Clin Microbiol. 2002;40:1587–91. doi: 10.1128/JCM.40.5.1587-1591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saijo M, Qing T, Niikura M, et al. Immunofluorescence technique using HeLa cells expressing recombinant nucleoprotein for detection of immunoglobulin G antibodies to Crimean-Congo hemorrhagic fever virus. J Clin Microbiol. 2002;40:372–5. doi: 10.1128/JCM.40.2.372-375.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukushi S, Nakauchi M, Mizutani T, et al. Antigen-capture ELISA for the detection of Rift Valley fever virus nucleoprotein using new monoclonal antibodies. J Virol Methods. 2012;180:68–74. doi: 10.1016/j.jviromet.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Saijo M, Ogino T, Taguchi F, et al. Recombinant nucleocapsid protein-based IgG enzyme-linked immunosorbent assay for the serological diagnosis of SARS. J Virol Methods. 2005;125:181–6. doi: 10.1016/j.jviromet.2005.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukushi S, Mizutani T, Saijo M, et al. Evaluation of a novel vesicular stomatitis virus pseudotype-based assay for detection of neutralizing antibody responses to SARS-CoV. J Med Virol. 2006;78:1509–12. doi: 10.1002/jmv.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greiner M, Sohr D, Gobel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods. 1995;185:123–32. doi: 10.1016/0022-1759(95)00121-p. [DOI] [PubMed] [Google Scholar]
- 23.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]
- 24.Fukushi S, Tani H, Yoshikawa T, et al. Serological assays based on recombinant viral proteins for the diagnosis of arenavirus hemorrhagic fevers. Viruses. 2012;4:2097–114. doi: 10.3390/v4102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackel S, Eiden M, Balkema-Buschmann A, et al. A novel indirect ELISA based on glycoprotein Gn for the detection of IgG antibodies against Rift Valley fever virus in small ruminants. Res Vet Sci. 2013;95:725–30. doi: 10.1016/j.rvsc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Nakauchi M, Fukushi S, Saijo M, et al. Characterization of monoclonal antibodies to Junin virus nucleocapsid protein and application to the diagnosis of hemorrhagic fever caused by South American arenaviruses. Clin Vaccine Immunol. 2009;16:1132–8. doi: 10.1128/CVI.00163-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehichioya DU, Hass M, Becker-Ziaja B, et al. Current molecular epidemiology of Lassa virus in Nigeria. J Clin Microbiol. 2011;49:1157–61. doi: 10.1128/JCM.01891-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monath TP. Lassa fever: review of epidemiology and epizootiology. Bull World Health Organ. 1975;52:577–92. [PMC free article] [PubMed] [Google Scholar]
- 29.Kaku Y, Noguchi A, Marsh GA, et al. Second generation of pseudotype-based serum neutralization assay for Nipah virus antibodies: sensitive and high-throughput analysis utilizing secreted alkaline phosphatase. J Virol Methods. 2012;179:226–32. doi: 10.1016/j.jviromet.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Kaku Y, Noguchi A, Marsh GA, et al. A neutralization test for specific detection of Nipah virus antibodies using pseudotyped vesicular stomatitis virus expressing green fluorescent protein. J Virol Methods. 2009;160:7–13. doi: 10.1016/j.jviromet.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.