Abstract
Background and Aims:
5-aminosalicylates (5-ASA) are frequently continued in patients with moderate-severe ulcerative colitis (UC), even after escalation to biologic agents, without evaluation of the benefit of this approach. We conducted an individual participant data (IPD) pooled analysis of trials of infliximab and golimumab in UC, to evaluate whether concomitant use of 5-ASA modifies clinical outcomes among anti-tumor necrosis factor (TNF)-α-treated patients.
Methods:
We included IPD from 5 trials of infliximab and golimumab in patients with moderate-severe UC (ACT-1 and −2, PURSUIT-SC, PURSUIT-M, NCT00336492). Patients treated with infliximab or golimumab were categorized as receiving concomitant 5-ASA or not at time of trial entry. Primary outcome was clinical remission (Mayo Clinic Score <3) at last follow-up for each trial; secondary outcomes were clinical response and mucosal healing. Using multivariable logistic regression analysis, we evaluated association between concomitant 5-ASA and clinical remission, after adjusting for sex, smoking, baseline disease activity, disease extent, biochemical variables (C-reactive protein, albumin, hemoglobin) and concomitant prednisone and immunomodulators.
Results:
We included 2183 infliximab- or golimumab-treated patients (1715 [78.6%] on 5-ASA). Concomitant use of 5-ASA was not associated with odds of achieving clinical remission (adjusted OR, 0.67 [95% CI, 0.45–1.01], p=0.06), clinical response (aOR, 0.89 [0.60–1.33], p=0.58) or mucosal healing (aOR, 1.12 [0.82–1.51], p=0.48). These results were consistent in trials of induction and maintenance therapy, and in trials of infliximab and golimumab.
Conclusions:
Based on IPD pooled analysis, in patients with moderate-severe UC who are escalated to anti-TNF therapy, continuing 5-ASA does not improve clinical outcomes.
Keywords: YODA project, Open science, posthoc analysis, value-based care, complications
INTRODUCTION
5-aminosalicytes (5-ASA) are the first-line therapy for patients with mild-moderate ulcerative colitis (UC), with >90% patients receiving 5-ASA within 1 year of diagnosis and 60–87% patients continuing 5-ASA on long-term follow-up.(1, 2) While many patients have a good prognosis, a smaller but important proportion of patients experience an aggressive course requiring immunosuppressive therapy including biologics. In patients who escalate to biologic therapy after failure of 5-ASA, there is a paucity of evidence to inform whether or not 5-ASA should be continued or discontinued. Specifically, no randomized trials have addressed this question. In clinical trials of biologics and small molecules in patients with moderate-severe UC, approximately 60–80% were concomitantly receiving 5-ASA at time of trial enrolment indicating that the majority of clinicians choose to continue 5-ASAs, despite treatment failure.(3–5) Similarly, in a community cohort of UC patients in Denmark, 80% of azathioprine-treated patients with UC were concomitantly receiving 5-ASA.(6) In a survey of gastroenterologists, 65% reported they would continue 5-ASA therapy in a patient hospitalized for intravenous corticosteroids for acute severe colitis, and 62% reported they would continue 5-ASA in an outpatient being started on an oral corticosteroid, despite lack of evidence of benefit.(7)
As a low-prevalence, high-cost condition, there is considerable interest in decreasing healthcare costs associated with inflammatory bowel diseases (IBD) through initiatives, such as the ‘Choosing wisely’ campaign that are focused on identifying and eliminating sources of low-value care.(8, 9) Although 5-ASAs may be relatively inexpensive compared to biologic therapies, their cumulative use is likely to have considerable financial burden to patients, payers and healthcare agencies. Annual wholesale cost of mesalamine 2.4g/d is estimated to be approximately $1,476 (range, $738-$2,951) per patient.(10) Furthermore, whilst they are perceived to be a safe drug class, rare but serious adverse events such a renal toxicity can occur and lead to substantial morbidity.(11) Thus, continuing 5-ASA in patients with moderate-severe UC who have failed therapy and escalated to biologic agents may represent a source of low-value care, if there is no evidence of benefit.
To address this question, we evaluated the short- and medium-term impact of concomitant 5-ASA use on clinical outcomes in anti-tumor necrosis factor (TNF)-α-treated patients with moderate-severe UC. The study was performed through a pooled analysis of individual participant level data (IPD) from clinical trials of infliximab and golimumab, available through Yale Open Data Access (YODA) Project.(12) This was not an interventional study. Patients receiving concomitant 5-ASA in these trials had active disease while on stable doses of 5-ASA for at least 2 weeks before the screening period, and continued their medications at a stable dose throughout the trial period; patients not receiving these medications at trial entry were not permitted to start them during the trial. In the absence of prospective comparative effectiveness studies, this post-hoc analysis in a controlled clinical trial environment allows an assessment of potential benefits or harms of continuing 5-ASA in biologic-treated patients with UC.
METHODS
Data Source and Trials
Clinical trials of infliximab and golimumab in patients with moderate-severe UC were accessed through the YODA project. This pioneering data-sharing model, started in 2011, housed at Yale University, provides access to de-identified IPD data, shared by data holders, Johnson&Johnson, Medtronic, Inc. and SI-BONE, Inc.(12) A detailed research proposal for this project was approved by the YODA scientific committee (Protocol #2017–2306) on November 2, 2017. Through this project, we were able to access trials of infliximab and golimumab in UC including: ACT-1 (NCT00036439, C0168T37), ACT-2 (NCT00096655, C0168T46), an open-label trial of infliximab in UC (NCT00336492, C0168T72), PURSUIT-SC (NCT00487539, C0524T17) and PURSUIT-M (NCT00488631, C0524T18).
From these trials, we created a cohort of active biologic-treated patients (infliximab or golimumab) to study the impact of concomitant 5-ASA use on short- and intermediate-term outcomes in patients with UC. All trials included patients with moderate-severe disease activity, defined as a Mayo score of 6–12, with an endoscopic subscore ≥2 (despite baseline IBD-related medications), and had an inadequate response to, or intolerance to one or more of the following conventional therapies: oral 5-ASA, oral corticosteroids, immunomodulators (azathioprine, and/or 6-mercaptopurine), or were corticosteroid-dependent. Post-hoc analysis of the corresponding cohort of placebo-treated patients from these trials was performed.
Based on suggestions during the peer review period, a systematic literature search of trials of biologic therapy (adalimumab, golimumab, infliximab, vedolizumab, etrolizumab) and small molecules (tofacitinib, ozanimod) in patients with moderate-severe UC was performed, as outlined here.(13) The aim of this literature search was to identify study-level comparison of outcomes in biologic (or small molecule)-treated patients with moderate-severe UC who were vs. were not concomitantly on 5-ASA at time of trial entry.
Exposure
Patients who were concomitantly on 5-ASA (mesalamine, diazo-bonded 5-ASA or sulfasalazine) at time of trial enrolment were classified as ‘exposed’ and those who were not on 5-ASA were classified as ‘unexposed’. In all of these trials, patients received stable doses of 5-ASA and/or immunomodulators for at least 2 weeks before the screening period and had active disease despite continuation of these therapies. Patients were required to continue their concomitant medications at a stable dose throughout the trial period, and patients not receiving these medications were not permitted to start them during the trial. All patients on concomitant 5-ASA in these trials, regardless of whether they were randomized to active anti-TNF intervention or placebo, had failed 5-ASA therapy.
Outcome
Primary outcome of interest was achieving clinical remission, defined as Mayo Clinic Score [MCS] <3. Secondary outcomes of interest included: (a) clinical response (decrease in MCS by ≥3 points and at least 30% from baseline), (b) mucosal healing (MCS endoscopy sub-score of 0 or 1) and (c) biochemical remission (normalization of C-reactive protein in patients with elevated levels at baseline).
For the primary analysis, outcome was measured at last time point of trials (week 54 for ACT-1 and PURSUIT-M, week 30 for ACT-2, week 6 for PURSUIT-SC, week 8 for NCT00336492). Trials that reported outcomes for both induction and maintenance therapy were included in the corresponding analyses.
Confounding Variables
We abstracted data on relevant confounding variables including: age, sex, smoking status (classified as never smokers, prior smokers classified as those who quit >1 year prior to trial entry and <1 year prior to trial entry, and current smokers), disease duration, disease extent (limited to splenic flexure, extensive), baseline disease activity (UC: severe MCS>9, moderate 6–9, mild <6), disease duration, concomitant use of immunomodulators and/or corticosteroids, and baseline biochemical parameters including albumin, hemoglobin, C-reactive protein and fecal calprotectin, where available.
Statistical Analysis
Baseline characteristics of trials participants were summarized as mean (standard deviation) or medians (range) for continuous variables, and as frequency (%) for categorical variables, Statistical differences in patient characteristics by concomitant exposure or non-exposure to 5-ASA were assessed using two sample t-tests for continuous variables and chi-squared test for categorical variables. We compared proportions of patients with the desired given outcomes (clinical remission, response or mucosal healing) in 5-ASA exposed and unexposed patients, across trials, logistic regression with a fixed covariate to control for heterogeneity across study. Subsequently, multivariable logistic regression was used to analyze the association between concomitant 5-ASA exposure and outcomes, with study as a fixed covariate to account for inter-study differences and adjusting for confounding variables (sex, smoking status, baseline disease activity, disease extent, concomitant corticosteroids, concomitant immunomodulators, baseline laboratory variables including C-reactive protein, albumin and hemoglobin); of note, age of participants was not reported in the YODA clinical trial platform for ACT trials, and hence, was not included in the multivariate analysis. Results were reported as odds ratios (OR) and 95% confidence intervals (CI), using non-exposure to 5-ASA as the reference category. A priori subgroup analyses to assess stability of the association were performed based on trial design (induction therapy [6–10 weeks] and maintenance trials [26–54 weeks]) and by medication (infliximab and golimumab). Findings from each trial were also reported separately. All analyses were performed using R (the R Project for Statistical Computing).
RESULTS
Patient Characteristics in Included Trials
In 5 trials (3 trials of infliximab, 2 trials of golimumab), we included 2183 patients receiving active biologic therapy, of whom 1715 (78.6%) who were concomitantly treated with 5-ASA at time of screening (range in individual trials, 45.5–83.6%). Table 1 lists the main characteristics of all patients, by concomitant 5-ASA exposure at trial entry. Approximately 36.8% biologic-treated patients were on corticosteroids at time of trial entry, and 26.7% were on immunomodulators. There was no difference in the age (p=0.74), sex distribution (p=0.58), distribution of disease severity (p=0.42) or concomitant immunomodulator use (p=0.44) at cohort entry in 5-ASA exposed and non-exposed patients; however, 5-ASA exposed patients were more likely to be on concomitant corticosteroids at enrolment (40.2% vs. 25.5%, p<0.01).
Table 1.
Baseline characteristics of patients with biologic-treated patients with ulcerative colitis, by exposure or non-exposure to concomitant 5-ASA
| Characteristics | All (n= 2183) | Concomitant 5-ASA: No (n=432) | Concomitant 5-ASA: Yes (n=1715) |
|---|---|---|---|
| Age, mean (SD)* | 40.20 (13.67) | 39.98 (14.15) | 40.28 (13.55) |
| Sex: Female | 894 (41.0 %) | 182 (42.1%) | 695 (40.5%) |
| Male | 1289 (59.0 %) | 250 (57.9%) | 1020 (59.5%) |
| Smoking | |||
| • Current | 19 (3.8%) | 7 (5.8%) | 12 (3.3%) |
| • Past (Unknown) | 170 (33.6%) | 34 (28.1%) | 126 (34.8%) |
| • Remote past | 31 (6.1%) | 8 (6.6%) | 21 (5.8%) |
| • Recent past (<1y) | 5 (1.0%) | 0 (0.0%) | 5 (1.4%) |
| • Never | 281 (55.5%) | 72 (59.5%) | 198 (54.7%) |
| Disease extent | |||
| • Limited (up to splenic flexure) | 1253 (57.5%) | 251 (58.4%) | 984 (57.4%) |
| • Extensive (extends beyond splenic flexure) | 926 (42.5%) | 179 (41.6%) | 729 (42.6%) |
| Disease duration, Mean (SD), in y | 4.1 (6.0) | 3.8 (4.9) | 4.1 (6.0) |
| Disease activity | |||
| • Mayo Clinic Score, median (IQR) | 8 (3) | 8 (2) | 8 (3) |
| Severity | |||
| • Mild | 287 (14.9%) | 66 (16.4%) | 216 (14.6%) |
| • Moderate | 1178 (61.3%) | 236 (58.7%) | 923 (62.2%) |
| • Severe | 456 (23.7%) | 100 (24.9%) | 344 (23.2%) |
| Co-interventions | |||
| • Prednisone | 799 (36.8%) | 110 (25.5%) | 689 (40.2%) |
| • Immunomodulators | |||
| ∘ Azathioprine | 439 (20.2%) | 98 (22.7%) | 341 (19.9%) |
| ∘ 6-mercaptopurine | 54 (3.2%) | 21 (4.9%) | 86 (5.0%) |
| ∘ Methotrexate | 71 (3.3%) | 2 (0.6%) | 16 (1.2%) |
| • Biologic agents | |||
| ∘ Infliximab | 506 (23.2%) | 121 (28.0%) | 362 (21.1%) |
| ∘ Golibumab | 1677 (76.8%) | 311 (72.2%) | 1353 (78.9%) |
| Laboratory variables | |||
| • Albumin (g/dL) | |||
| ∘ Mean (SD) | 4.19 (0.44) | 4.14 (0.46) | 4.20 (0.43) |
| ∘ Low | 90 (4.9%) | 23 (6.5%) | 64 (4.5%) |
| • Hemoglobin (g/dL) | |||
| ∘ Mean (SD) | 12.9 (1.9) | 12.8 (2.0) | 12.9 (1.9) |
| ∘ Low | 577 (32.7%) | 124 (35.4%) | 438 (31.7%) |
| • C-reactive protein (mg/L) | |||
| ∘ Mean (SD) | 12.0 (19.3) | 13.0 (19.2) | 11.5 (18.6) |
| ∘ Normal | 794 (37.0%) | 154 (36.4%) | 633 (37.5%) |
| ∘ High | 1352 (63.0%) | 269 (63.6%) | 1055 (62.5%) |
| • Fecal Calprotectin (μg/g) | |||
| ∘ Mean (SD) | 1735.9 (3172.6) | 1973.9 (3379.0) | 1672.0 (3127.0) |
| ∘ Normal | 164 (10.3%) | 25 (8.4%) | 138 (10.8%) |
| ∘ High | 1423 (89.7%) | 271 (91.6%) | 1142 (89.2%) |
Age reported in only 3 trials
[Abbreviations: SD=Standard deviation; g=grams; mg=milligrams; dL=decilitre; 5-ASA=5-aminosalicylates]
Impact of Concomitant 5-ASA Use on Clinical Remission
In anti-TNF-treated patients with moderate-severe UC, concomitant 5-ASA use was not associated with increased odds of achieving clinical remission (5-ASA-exposed vs. 5-ASA-unexposed – 31.4% vs. 34.9%; OR, 0.84 [95% CI, 0.64–1.12], p=0.23). Results were unchanged after adjusting for key covariates (aOR, 0.67 [0.45–1.01], p=0.06) (Table 2, Figure 1). Concomitant 5-ASA was not associated with higher rates of induction of remission patients with active disease, or maintenance of remission in patients with quiescent disease (Table 3). On analyzing by type of biologic, results were comparable in trials of infliximab and golimumab (p-value for difference in groups, clinical remission=0.29) (Table 4). On evaluating individual trials, patients in ACT-2, concomitantly on 5-ASA were less likely to achieve remission as compared to patients not on 5-ASA (eTables 1–5).
Table 2.
Rates of clinical remission, response and mucosal healing in biologic-treated patients in trials of induction therapy and maintenance therapy, by exposure or non-exposure to concomitant 5-ASA [variables adjusted for: sex, smoking status, baseline disease activity, disease extent, concomitant corticosteroids, concomitant immunomodulators, baseline laboratory variables including C-reactive protein, albumin and hemoglobin]
| Outcome | Concomitant 5-ASA: No | Concomitant 5-ASA: Yes | p-value |
|---|---|---|---|
| Clinical Remission | |||
| • Proportion (n/N) | 106 / 304 (34.9%) | 402 / 1279 (31.4%) | |
| • Unadjusted OR (95% CI) | 1.0 | 0.84 (0.64, 1.12) | 0.23 |
| • Adjusted OR (95% CI) | 1.0 | 0.67 (0.45, 1.01) | 0.06 |
| Clinical Response | |||
| • Proportion (n/N) | 152 / 298 (51.0%) | 590 / 1270 (46.5%) | |
| • Unadjusted OR (95% CI) | 1.0 | 0.95 (0.73, 1.23) | 0.69 |
| • Adjusted OR (95% CI) | 1.0 | 0.89 (0.60, 1.33) | 0.58 |
| Mucosal Healing | |||
| • Proportion (n/N) | 169 / 393 (43.0%) | 637 / 1451 (43.9%) | |
| • Unadjusted OR (95% CI) | 1.0 | 1.11 (0.88, 1.40) | 0.39 |
| • Adjusted OR (95% CI) | 1.0 | 1.12 (0.82, 1.51) | 0.48 |
| Biochemical Remission* | |||
| • Proportion (n/N) | 93 / 269 (34.6%) | 402 / 1055 (38.1%) | |
| • Unadjusted OR (95% CI) | 1.0 | 1.18 (0.88, 1.58) | 0.27 |
| • Adjusted OR (95% CI) | 1.0 | 0.94 (0.61, 1.46) | 0.79 |
Biochemical remission was defined as normalization of C-reactive protein in a subset of patients with elevated C-reactive protein at baseline
Figure 1.
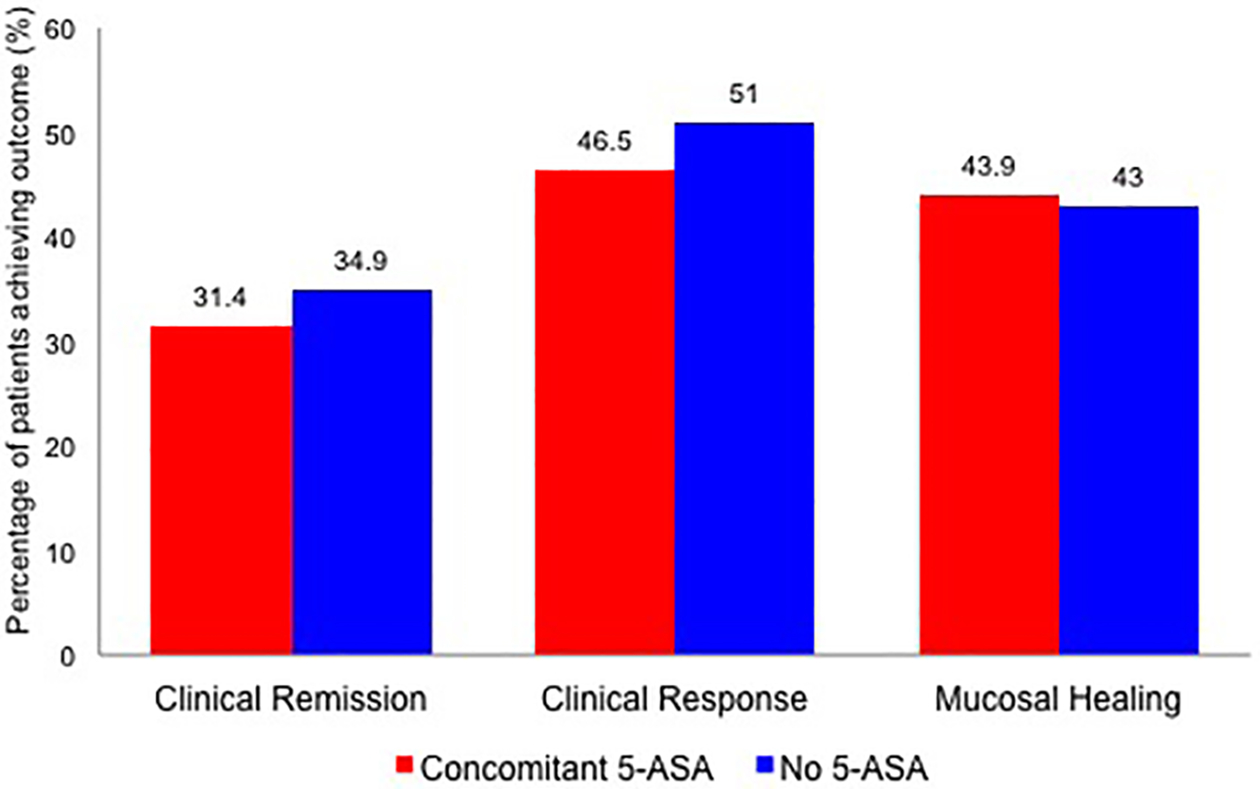
Clinical remission, clinical response, mucosal healing and biochemical remission in anti-TNF-treated patients with moderate-severe ulcerative colitis with or without concomitant 5-aminosalicylates
Table 3. Subgroup analysis, by trial design.
Rates of clinical remission, response and mucosal healing in biologic-treated patients in trials of induction therapy and maintenance therapy, by exposure or non-exposure to concomitant 5-ASA [variables adjusted for: sex, smoking status, baseline disease activity, disease extent, concomitant corticosteroids, concomitant immunomodulators, baseline laboratory variables including C-reactive protein, albumin and hemoglobin]
| Outcome | Concomitant 5-ASA: No | Concomitant 5-ASA: Yes | p-value |
|---|---|---|---|
| INDUCTION PHASE (Range 6–10 weeks) | |||
| Clinical remission | |||
| • Proportion (n/N) | 58 / 240 (24.2%) | 200 / 905 (22.1%) | |
| • Unadjusted OR (95% CI) | 1.0 | 0.97 (0.69, 1.37) | 0.86 |
| • Adjusted OR (95% CI) | 1.0 | 0.64 (0.38, 1.11) | 0.10 |
| Clinical Response | |||
| • Proportion (n/N) | 137 / 235 (58.3%) | 469 / 893 (52.5%) | |
| • Unadjusted OR (95% CI) | 1.0 | 0.91 (0.67, 1.26) | 0.52 |
| • Adjusted OR (95% CI) | 1.0 | 0.77 (0.47, 1.24) | 0.28 |
| Mucosal Healing | |||
| • Proportion (n/N) | 134 / 257 (52.1%) | 486 / 959 (50.7%) | |
| • Unadjusted OR (95% CI) | 1.0 | 1.05 (0.79, 1.39) | 0.75 |
| • Adjusted OR (95% CI) | 1.0 | 0.99 (0.70, 1.55) | 0.70 |
| MAINTENANCE PHASE (Range 26–54 weeks) | |||
| Clinical remission | |||
| • Proportion (n/N) | 72 / 159 (45.3%) | 281 / 685 (41.0%) | |
| • Unadjusted OR (95% CI) | 1.0 | 0.79 (0.55, 1.13) | 0.20 |
| • Adjusted OR (95% CI) | 1.0 | 0.62 (0.37, 1.06) | 0.08 |
| Clinical Response | |||
| • Proportion (n/N) | 83 / 158 (52.5%) | 319 / 687 (46.4%) | |
| • Unadjusted OR (95% CI) | 1.0 | 0.87 (0.61, 1.25) | 0.46 |
| • Adjusted OR (95% CI) | 1.0 | 1.01 (0.58, 1.76) | 0.97 |
| Mucosal Healing | |||
| • Proportion (n/N) | 98 / 245 (40.0%) | 361 / 844 (42.8%) | |
| • Unadjusted OR (95% CI) | 1.0 | 1.17 (0.87, 1.58) | 0.31 |
| • Adjusted OR (95% CI) | 1.0 | 1.18 (0.75, 1.84) | 0.48 |
Table 4. Subgroup analysis, by biologic medication.
Rates of clinical remission, response and mucosal healing in biologic-treated patients in trials of infliximab and golimumab, by exposure or non-exposure to concomitant 5-ASA [variables adjusted for: sex, smoking status, baseline disease activity, disease extent, concomitant corticosteroids, concomitant immunomodulators, baseline laboratory variables including C-reactive protein, albumin and hemoglobin]
| Outcome | Concomitant 5-ASA: No | Concomitant 5-ASA: Yes | p-value |
|---|---|---|---|
| Infliximab | |||
| Clinical remission | |||
| • Proportion (n/N) | 60 / 84 (43.4%) | 66 / 247 (26.7%) | |
| • Unadjusted OR (95% CI) | 1.0 | 0.48 (0.28, 0.80) | 0.01 |
| • Adjusted OR (95% CI) | 1.0 | 0.45 (0.19, 1.04) | 0.07 |
| Clinical Response | |||
| • Proportion (n/N) | 60 / 84 (71.4%) | 162 / 255 (63.5%) | |
| • Unadjusted OR (95% CI) | 1.0 | 0.70 (0.40, 1.18) | 0.19 |
| • Adjusted OR (95% CI) | 1.0 | 0.63 (0.33, 1.14) | 0.13 |
| Mucosal Healing | |||
| • Proportion (n/N) | 76 / 121 (62.8%) | 200 / 362 (55.2%) | |
| • Unadjusted OR (95% CI) | 1.0 | 0.73 (0.48, 1.11) | 0.15 |
| • Adjusted OR (95% CI) | 1.0 | 0.77 (0.49, 1.20) | 0.25 |
| Golimumab | |||
| Clinical remission | |||
| • Proportion (n/N) | 70 / 221 (31.7%) | 336 / 1032 (32.6%) | |
| • Unadjusted OR (95% CI) | 1.0 | 1.04 (0.77, 1.43) | 0.80 |
| • Adjusted OR (95% CI) | 1.0 | 0.76 (0.49, 1.21) | 0.24 |
| Clinical Response | |||
| • Proportion (n/N) | 92 / 214 (43.0%) | 428 / 1015 (42.2%) | |
| • Unadjusted OR (95% CI) | 1.0 | 0.97 (0.72, 1.31) | 0.83 |
| • Adjusted OR (95% CI) | 1.0 | 0.88 (0.57, 1.35) | 0.55 |
| Mucosal Healing | |||
| • Proportion (n/N) | 93 / 272 (34.2%) | 437 / 1089 (40.1%) | |
| • Unadjusted OR (95% CI) | 1.0 | 1.29 (0.98, 1.71) | 0.07 |
| • Adjusted OR (95% CI) | 1.0 | 1.42 (0.93, 2.18) | 0.11 |
Similar analysis was performed in a cohort of placebo-treated patients from these trials (eTable 6). There was no significant difference in rates of clinical remission, clinical response or mucosal healing in placebo-treated patients who were vs. were not concomitant on 5-ASA in these trials, in the overall analysis and in subgroup analyses (eTables 7–9).
Impact of Concomitant 5-ASA Use on Clinical Response, Mucosal Healing and Biochemical Remission
Similar to the results for clinical remission, concomitant 5-ASA use was not associated with increased odds of achieving clinical response (aOR, 0.89 [0.60–1.33]), mucosal healing (aOR, 1.12 [0.82–1.51]) or biochemical remission (aOR, 0.94 [0.61–1.46]) (Table 2, Figure 1). These results were consistent according to trial design (induction and maintenance, Table 3), drug therapy (infliximab and golimumab, Table 4) and in each included trial individually (eTables 1–5). The magnitude of decline in C-reactive protein was also comparable in both groups (eTable 10). Similar results were noted in the placebo-treated cohort of patients (eTables 7–9).
Systematic literature review
We identified 12 additional trials of infliximab (2 trials – Jiang et al,(14) UC-SUCCESS(15)), golimumab (PURSUIT-J(16)), adalimumab (3 trials – ULTRA 1(17) and 2,(18) Suzuki et al(19)), tofacitinib (3 trials – OCTAVE 1 and 2,(20) Sandborn et al(21)), vedolizumab (GEMINI 1(22)), etrolizumab (Vermeire et al(23)) and ozanimod (TOUCHSTONE(24)). While IPD was available for two trials of infliximab, these were not included in the analysis either due to incomplete data to calculate Mayo score (Jiang et al(14)) or inability to ascertain treatment allocation from available files (UC-SUCCESS(15)). Of the remaining 10 trials, results stratified by concomitant exposure to 5-ASA vs. no exposure was only reported in 2 trials. Individually, in these trials, there was no difference in rates of achieving clinical remission or response in biologic-treated patients on concomitant 5-ASA vs. not on 5-ASA (eTable 11).
DISCUSSION
We performed a post-hoc pooled analyses of IPD of trials of infliximab and golimumab in patients with moderate-severe UC, comparing outcomes in patients who had active disease despite continued use of 5-ASA vs. those who were not on 5-ASA at time of trial entry. In this analysis, we observed no short- or intermediate-term benefit of concomitant 5-ASA in a subset of patients who fail 5-ASA and escalate to anti-TNF therapy for moderate-severe UC. These findings were consistent across individual trials, in trials of induction and maintenance therapy, and in trials of infliximab and golimumab. Due to the short duration of follow-up in clinical trials, we were not able to study the impact of concomitant 5-ASA on longer-term risk of disease-related complications (i.e. beyond 52 weeks) including surgery and development of colorectal neoplasia. In the era of ‘Choosing Wisely’, continuing 5-ASA in patients who failed this therapy and escalated to anti-TNF agents may represent low-value care. Based on annual cost of mesalamine therapy of approximately $1,476 per patient,(10) and with estimated 50% biologic-treated patients concomitantly continued on mesalamine, we estimate this may translate into $73.8 million spent directly on mesalamine therapy per 100,000 biologic-treated patients, annually, without any evidence of efficacy.
5-ASA is the most commonly prescribed drug therapy for UC and is a cornerstone of management in patients with mild-moderate disease for both induction and maintenance therapy.(2) However, the benefit of continuing 5-ASA in corticosteroid-dependent patients with UC, who have failed oral 5-ASA and are escalated to immunomodulator or biologic therapy has not been well-studied. In a randomized, observer-blind, maintenance trial of 70 corticosteroid-dependent patients with UC, in clinical, endoscopic and histologic remission on azathioprine and oral olsalazine, Mantzaris and colleagues did not identify any difference in rates of clinical and endoscopic relapse over 2 years in patients receiving combination therapy with azathioprine and olsalazine or azathioprine alone.(25) In this trial, patients assigned to combination therapy experienced higher rates of adverse events, lower compliance and higher cost of therapy, as compared to patients who received azathioprine monotherapy. In a retrospective cohort study of 82 patients with UC in remission on azathioprine followed over a median 4.3y, concomitant therapy with 5-ASA was not associated with lower risk of clinical relapse (surgery or need for rescue therapy).(26) A similar study on concomitant 5-ASA for induction and/or maintenance of remission in biologic-treated patients has not been conducted, and while the practice of continuing 5-ASA is common, our findings based on post-hoc analyses of controlled clinical trials, indicate no benefit to continuing concomitant 5-ASA in these patients. In fact, there was a trend towards lower rates of clinical remission in anti-TNF-treated patients on concomitant 5-ASA, as compared to patients not on concomitant 5-ASA. This may represent residual confounding by severity, even after adjusting for baseline Mayo clinic score and baseline biochemical variables.
One proposed benefit of long-term 5-ASA use is a potential chemoprevention effect against colorectal cancer. Large observational studies and meta-analyses have variably suggested that UC patients treated with 5-ASA have lower risk of developing colorectal cancer.(27–30) Besides disease duration, extent, concomitant primary sclerosing cholangitis, and family history of colorectal cancer, chronically active disease has been consistently identified as a potentially modifiable risk factor for colorectal cancer.(31–33) It is likely that any medication that successfully controls inflammation and maintains remission would reduce the risk of colorectal cancer, as has also been observed with long-term thiopurine use.(34) Hence, 5-ASA may not have an independent biologic chemopreventive effect beyond controlling inflammation. In our analysis, there was no difference in rates of achieving biochemical remission or magnitude of decline in C-reactive protein in anti-TNF-treated patients who were vs. were not concomitantly on 5-ASA. Moreover, even if 5-ASA is believed to have a chemopreventive effect, the estimated cost to prevent one colorectal cancer with 5-ASA would, at minimum, be 153 x annual cost of 5-ASA therapy (~$1476/patient), which is approximately $225,828; true costs may be substantially higher given possible over-estimation of 5-ASA-specific chemopreventive effect.(6) Guidelines do not recommend use of specific medications for the sole indication of cancer prevention in patients with UC.(35) Our study was unable to examine the potential benefit of concomitant 5-ASA for chemoprevention in biologic-treated patients because of the short duration of the follow-up available.
Our study has some limitations. First, we focused primarily on concomitant oral 5-ASA, and not topical 5-ASA. Topical 5-ASA is highly effective for inducing and maintaining remission in patients with distal disease, and is often added in treatment regimens of patients with suboptimally controlled rectal urgency. Hence, the benefit of adding intermittent topical therapy in patients with refractory proctitis despite biologic therapy is unknown. Second, participants in clinical trials may be systematically different than the usual population seen in clinically practice, which may limit generalizability of findings. Moreover, this was a post-hoc analysis of previously conducted studies, and not a randomized trial comparing whether addition of 5-ASA to biologic therapy offers benefit in patients with moderate-severe disease. Patients in these trials had previously failed 5-ASA therapy to be eligible to participate in trials of biologic therapy; it is unclear whether the same findings would apply to treatment-naïve, newly diagnosed patients with severe UC who start on biologic therapy. Third, we did not compare differences in safety outcomes in patients who were, or were not, concomitantly on 5-ASA. However, 5-ASA therapy is safe and well-tolerated, with minimal risk of serious adverse events. Moreover, patients in these trials, if on 5-ASA, were on stable doses for at least 2 weeks prior to trial screening, and hence, had demonstrated tolerability, as opposed to new users who may have higher rates of intolerance. Separate analysis of mesalamine- and sulfasalazine-treated patients was not performed. Fourth, there were differences in trial design for maintenance of remission, with ACT-1 and −2 designed as treat straight-through trials, whereas PURSUIT-M re-randomizing responders to induction therapy and also had an additional feed-in from an open-label cohort. Likewise, there were subtle differences in timing of assessment of outcomes, and none of the trials used a centralized reader for assessing endoscopic remission. However, our results were stable on multiple subgroup analyses, and unlikely to be influenced by these difference. Finally, as mentioned previously, the follow-up in included trials was short, and limited to <1 year. However, in other studies of de-escalation in moderate to severe IBD patients, one year if a sufficient horizon to observe disease relapse.(36)
In conclusion, based on a pooled analysis of clinical trials of infliximab and golimumab in patients with moderate-severe UC, there may be minimal short- and intermediate-term benefit of continuing 5-ASA in patients who have failed these therapies and escalated to anti-TNF agents. Use of 5-ASA solely for its potential chemoprevention effect is not recommended and is unlikely to be cost-effective for this indication. An interventional randomized study of systematic withdrawal of 5-ASA in biologic-treated patients with UC in remission is warranted. It remains unclear whether there may be benefit of adding 5-ASA to biologic therapy in newly diagnosed, treatment-naïve patients with moderate-severe UC.
Supplementary Material
Study Highlights.
What is already known?
5-aminosalicylates are the first-line therapy for patients with mild-moderate ulcerative colitis (UC)
5-ASA is frequently continued in patients who are escalated to biologic therapy for moderate-severe disease
The benefit of continuing 5-ASA in patients who fail and escalate to biologic therapy is unclear
What this study adds?
Based on post-hoc analysis of trials of infliximab and golimumab for moderate-severe UC, concomitant 5-ASA is not associated with higher rates of clinical remission and mucosal healing
These results are seen in trials of induction and maintenance therapy
Continuing 5-ASA in anti-TNF-treated patients with moderate-severe UC represents low-value care
Funding:
This project is supported by the American College of Gastroenterology and the Crohn’s and Colitis Foundation. The project was also partially supported by the National Institutes of Health, Grant UL1TR001442. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures: Siddharth Singh: Research support from Pfizer and AbbVie, consulting fees from AbbVie, outside the submitted work; Parambir Dulai: Research support from Pfizer, and has received research support, travel support and served as a consultant for Takeda outside the submitted work; Vipul Jairath: Consulting fees from AbbVie, Sandoz, Takeda, Janssen, Robarts Clinical Trials; speakers fees from Takeda, Janssen, Shire, Ferring; Mathurin Fumery: Lecture/consultant fees from Abbvie, MSD, Takeda, Ferring, Pfizer and Boehringer; Brian Feagan: Received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, AbbVie, Novartis Pharmaceuticals, Centocor Inc., Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, and Wyeth Pharmaceuticals Inc.; consulting fees from Millennium Pharmaceuticals, Merck, Centocor Inc., Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging Inc., Salix Pharmaceuticals, Novonordisk, GSK, Actogenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma Inc., and Sigmoid Pharma; and speakers bureaux fees from UCB, AbbVie, and J&J/Janssen; William Sandborn: Grant support and consulting fees from Pfizer, Prometheus Laboratories, AbbVie, Boehringer Ingelheim, Takeda, Atlantic Pharmaceuticals, Janssen, Bristol-Myers Squibb, Genentech, and Nutrition Science Partners, and personal fees from Kyowa Hakko Kirin, Millennium Pharmaceuticals, Celgene Cellular Therapeutics, Santarus, Salix Pharmaceuticals, Catabasis Pharmaceuticals, Vertex Pharmaceuticals, Warner Chilcott, Gilead Sciences, Cosmo Pharmaceuticals, Ferring Pharmaceuticals, Sigmoid Biotechnologies, Tillotts Pharma, AM-Pharma BV, Dr. August Wolff, Avaxia Biologics, Zyngenia, Ironwood Pharmaceuticals, Index Pharmaceuticals, Nestle, Lexicon Pharmaceuticals, UCB Pharma, Orexigen, Luitpold Pharmaceuticals, Baxter Healthcare, Ferring Research Institute, Amgen, Novo Nordisk, Mesoblast Inc., Shire, Ardelyx Inc., Actavis, Seattle Genetics, MedImmune (AstraZeneca), Actogenix NV, Lipid Therapeutics Gmbh, Eisai, Qu Biologics, Toray Industries Inc., Teva Pharmaceuticals, Eli Lilly, Chiasma, TiGenix, Adherion Therapeutics, Immune Pharmaceuticals, Celgene, Arena Pharmaceuticals, Ambrx Inc., Akros Pharma, Vascular Biogenics, Theradiag, Forward Pharma, Regeneron, Galapagos, Seres Health, Ritter Pharmaceuticals, Theravance, Palatin, Biogen Idec, and the University of Western Ontario (owner of Robarts Clinical Trials) outside the submitted work; James Proudfoot and Ronghui Xi have nothing to declare.
REFERENCES
- 1.Fumery M, Singh S, Dulai PS, et al. Natural History of Adult Ulcerative Colitis in Population-based Cohorts: A Systematic Review. Clin Gastroenterol Hepatol 2018;16:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harbord M, Eliakim R, Bettenworth D, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis 2017;11:769–784. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85–95. [DOI] [PubMed] [Google Scholar]
- 4.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:96–109. [DOI] [PubMed] [Google Scholar]
- 5.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 6.Andrews JM, Travis SP, Gibson PR, et al. Systematic review: does concurrent therapy with 5-ASA and immunomodulators in inflammatory bowel disease improve outcomes? Aliment Pharmacol Ther 2009;29:459–69. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Horin S, Andrews JM, Katsanos KH, et al. Combination of corticosteroids and 5-aminosalicylates or corticosteroids alone for patients with moderate-severe active ulcerative colitis: A global survey of physicians’ practice. World J Gastroenterol 2017;23:2995–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen GC, Boland K, Afif W, et al. Modified Delphi Process for the Development of Choosing Wisely for Inflammatory Bowel Disease. Inflamm Bowel Dis 2017;23:858–865. [DOI] [PubMed] [Google Scholar]
- 9.Dulai PS, Singh S, Ohno-Machado L, et al. Population Health Management for Inflammatory Bowel Disease. Gastroenterology 2018;154:37–45. [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein JH, Waljee AK, Jeter JM, et al. Cost effectiveness of ulcerative colitis surveillance in the setting of 5-aminosalicylates. Am J Gastroenterol 2009;104:2222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut 2002;51:536–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumholz HM, Waldstreicher J. The Yale Open Data Access (YODA) Project--A Mechanism for Data Sharing. N Engl J Med 2016;375:403–5. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Fumery M, Sandborn WJ, et al. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther 2018;47:162–175. [DOI] [PubMed] [Google Scholar]
- 14.Jiang XL, Cui HF, Gao J, et al. Low-dose Infliximab for Induction and Maintenance Treatment in Chinese Patients With Moderate to Severe Active Ulcerative Colitis. J Clin Gastroenterol 2015;49:582–8. [DOI] [PubMed] [Google Scholar]
- 15.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400. [DOI] [PubMed] [Google Scholar]
- 16.Hibi T, Imai Y, Senoo A, et al. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study-(PURSUIT-J study). J Gastroenterol 2017; doi: 10.1007/s00535-017-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65. [DOI] [PubMed] [Google Scholar]
- 18.Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011;60:780–7. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol 2014;49:283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 21.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012;367:616–24. [DOI] [PubMed] [Google Scholar]
- 22.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 23.Vermeire S, O’Byrne S, Keir M, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet 2014;384:309–18. [DOI] [PubMed] [Google Scholar]
- 24.Sandborn WJ, Feagan BG, Wolf DC, et al. Ozanimod Induction and Maintenance Treatment for Ulcerative Colitis. N Engl J Med 2016;374:1754–62. [DOI] [PubMed] [Google Scholar]
- 25.Mantzaris GJ, Sfakianakis M, Archavlis E, et al. A prospective randomized observer-blind 2-year trial of azathioprine monotherapy versus azathioprine and olsalazine for the maintenance of remission of steroid-dependent ulcerative colitis. Am J Gastroenterol 2004;99:1122–1128. [DOI] [PubMed] [Google Scholar]
- 26.Campbell S, Ghosh S. Effective maintenance of inflammatory bowel disease remission by azathioprine does not require concurrent 5-aminosalicylate therapy. Eur J Gastroenterol Hepatol 2001;13:1297–301. [DOI] [PubMed] [Google Scholar]
- 27.Bonovas S, Fiorino G, Lytras T, et al. Systematic review with meta-analysis: use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017;45:1179–1192. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen GC, Gulamhusein A, Bernstein CN. 5-Aminosalicylic acid is not protective against colorectal cancer in inflammatory bowel disease: A meta-analysis of non-referral populations. Am J Gastroenterol 2012;107:1298–1304. [DOI] [PubMed] [Google Scholar]
- 29.Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology 2012;143:550–563. [DOI] [PubMed] [Google Scholar]
- 30.Dulai PS, Sandborn WJ, Gupta S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer Prev Res (Phila) 2016;9:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi CR, Al Bakir I, Ding NJ, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut 2017; doi: 10.1136/gutjnl-2017-314190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ananthakrishnan AN, Cheng SC, Cai T, et al. Serum inflammatory markers and risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:1342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004;126:451–9. [DOI] [PubMed] [Google Scholar]
- 34.Lu MJ, Qiu XY, Mao XQ, et al. Systematic review with meta-analysis: thiopurines decrease the risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2018;47:318–331. [DOI] [PubMed] [Google Scholar]
- 35.Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis 2017;11:649–670. [DOI] [PubMed] [Google Scholar]
- 36.Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012;142:63–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


