Abstract
Background. Interspecies interactions of the nasopharyngeal microbiota are likely to be involved in the pathogenesis of acute otitis media (AOM). Capturing the breadth of microbial interactions requires a detailed description of the microbiota during health and AOM.
Methods. The nasopharyngeal microbiota of 163 infants with (n = 153) or without (n = 10) AOM was characterized using nasopharyngeal swabs and multiplexed pyrosequencing of 16S rRNA. Nasopharyngeal swab specimens were collected during 4 winter seasons from 2004 through 2010 for infants with AOM and during 2010 for controls.
Results. Fifty-eight bacterial families were identified, of which Moraxellaceae, Streptococcaceae, and Pasteurellaceae were the most frequent. Commensal families were less prevalent in infants with AOM than in controls. In infants with AOM, prior exposure to antimicrobials and administration of the heptavalent conjugated pneumococcal polysaccharide vaccine (PCV7) were also associated with reduced prevalence of distinct commensal families (Streptococcaceae and Corynebacteriaceae). In addition, antimicrobial exposure increased the prevalence of Enterobacteriaceae and the abundance of Pasteurellaceae. Other factors, such as age, sex, day care, and a history of recurrent AOM, did not influence the microbiota.
Conclusions. Infants’ nasopharyngeal microbiota undergoes significant changes during AOM and after exposure to antimicrobials and PCV7, which is mainly attributable to reduced prevalence of commensal bacterial families.
Acute otitis media (AOM) is one of the most common pediatric infectious diseases worldwide and a major reason for antibiotic prescription for children in developed countries [1–3]. The 3 main pathogens associated with AOM are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis [4]. The human nasopharynx is the primary site of colonization for these pathogens, and it is the starting point for their ascendance to the middle ear. Numerous bacterial virulence factors have been identified and strategies used by these pathogens to evade host defense mechanisms have been studied [5]. Less is known about the role of the microbial community structure and interspecies interactions for the pathogenesis of AOM [6, 7].
Evidence for interspecies cross-talk has recently been demonstrated by the influence of the heptavalent conjugated pneumococcal polysaccharide vaccine (PCV7) on the colonization prevalence of the AOM pathogens H. influenzae, M. catarrhalis, and Staphylococcus aureus [8–10].
To capture the breadth of interspecies interplays, a detailed description of the microbiota in the healthy and the disease stage is required. Conventional culture has limited value for such purpose, because many bacteria that colonize humans cannot be cultured [11]. There have been significant developments in applying cultural-independent methods to characterize microbial communities in different human body habitats [12–14], including shotgun sequencing of the whole metagenome and amplicon sequencing of selected phylogenetic markers [15]. In a recent study, microbial communities in the upper respiratory tract of children with or without AOM were described [16]. This study included children of a wide age range spanning from 6 months to 6 years and was based on samples taken from nostrils. The study presented here focused on a narrower age range of infants (0–1 year) and applied pyrosequencing of 16S rRNA gene amplicons to nasopharyngeal swab (NPS) specimens from infants with AOM and control infants. The study period spanned the years before and after introduction of PCV7 in 2006 in Switzerland. The main research aim was to characterize the microbiota in infants with AOM and to compare it with those in control infants. In addition, we studied the influence of recent antimicrobial exposure and administration of PCV7 on the nasopharyngeal microbiota in infants with AOM. Such data will serve as basis for further studies on the role of the nasopharyngeal microbiota in the pathogenesis of AOM.
METHODS
Patients and Sample Collection
NPS specimens were collected in an ongoing nationwide surveillance program from consecutive infants <2 years of age who presented with AOM in outpatient practice [17]. The study period included 4 winter seasons: December 2004–February 2005, November 2006–March 2007, November 2007–March 2008, and November 2008–March 2009 [18]. Data on age, sex, place of residence, antimicrobial treatment during the previous 8 weeks, the number of AOM episodes during the previous 12 months, current use of day care, and vaccination with PCV7 were obtained at the time of sample collection. In Switzerland, PCV7 was added to the routine vaccination schedule in 2006. Therefore, season 1 (2004–2005) was designated as the prevaccine season (Table 1). Of a total of 218 NPS specimens collected during the study period, 153 were selected for this study. Twenty-five and 40 NPS specimens were excluded because they did not have sufficient DNA or were lacking clinical information, respectively.
Table 1.
Sociodemographic and Clinical Characteristics of Study Infants With Acute Otitis Media and Healthy Control Infants
| Infants With AOMa |
Controlsb |
|||
| Characteristic | No. | % | No. | % |
| Age, y | ||||
| 0 | 61 | 40 | 5 | 50 |
| 1 | 92 | 60 | 3 | 30 |
| 2 | 0 | 0 | 2 | 20 |
| Sex, female | 74 | 48 | 6 | 60 |
| Antibiotic exposurec | 39 | 26 | 1 | 10 |
| Day care used | 54 | 35 | 0 | 0 |
| Streptococcus pneumoniae carriagee | 80 | 52 | 1 | 10 |
| PCV7 vaccinated | 87 | 57 | 7 | 70 |
| Post–vaccine seasonf | 122 | 80 | 10 | 100 |
| Past AOM episodesg | ||||
| ≤1 episode | 118 | 83 | 0 | 0 |
| ≥2 episodes | 25 | 17 | 0 | 0 |
Abbreviations: AOM, acute otitis media; PCV7, heptavalent conjugated pneumococcal polysaccharide vaccine.
General practitioners contributing the 153 nasopharyngeal swabs of infants with AOM are part of the Swiss Sentinel Surveillance Network [17].
The 10 control infants were recruited at the outpatient clinic of the University Hospital of Berne.
Antibiotic exposure during the 8 wk before swab collection.
Current day care usage: no information available for 10 samples.
S. pneumoniae carriage results derived from culture.
PCV7 was added to the routine vaccine schedule for infants in 2006.
Number of AOM episodes during the past 12 mo; no information available for 10 samples.
In addition to the 153 infants with AOM, NPS specimens were collected from 10 infants without AOM who were seen for various reasons at the outpatient clinic of the University Hospital of Berne in 2010 (Table 1). The diagnoses for which control infants were seen were surgery of the lingual frenulum in 1 infant and a lymphangioma at the neck in 1 infant. Six and 1 infants were part of a human immunodeficiency virus (HIV) and hepatitis C virus exposure study group, respectively, but were HIV and hepatitis C virus negative. Finally, 1 infant presented to the clinic with repeated skin abscesses, but no underlying morbidity was identified.
Approval from the local ethics committee and informed consent were obtained for the collection of samples from controls. The surveillance program is part of the governmental public health surveillance and is exempted from approval by institutional review boards.
16S rRNA Gene–Specific Polymerase Chain Reaction
Handling of swabs and DNA extraction has been previously described [19]. For every 10 samples, we included a negative mock control for which the extraction protocol was performed in an identical manner as for the samples. V3–V5 regions of bacterial 16S rRNA genes were then amplified using the primer pairs 341F/926R [14]. Primer sequences were modified by addition of sequencing adaptors A (forward) or B (reverse) and a 10-mer multiplex identifier (Supplementary Table 1): A-341F (5′-CGT ATC GCC TCC CTC GCG CCA TCA G[NN NNN NNN NN]A CTC CTA CGG GAG GCA GCA G-3′) and B-926R (5′-CTA TGC GCC TTG CCA GCC CGC TCA G[NN NNN NNN NN]C CGT CAA TTC MTT TGA GTT T-3′).
Polymerase chain reaction (PCR) was performed in a total volume of 50 μL with use of 1× Fast Start Taq reaction buffer, 2.0 mM (final) magnesium chloride, 0.2 mM (final) deoxyribonucleotide triphosphate, 1 μM (final) of forward and reverse fusion primers, 3 units of Fast Start Taq Polymerase (Roche), and 10 μL of extracted DNA. PCR cycling conditions included 95°C for 6 minutes; 35 cycles of denaturation at 95°C (30 seconds), annealing at 59°C (30 seconds), and elongation at 71°C (2 minutes); and final at 71°C for 7 minutes. PCR products were purified using Wizard SV PCR clean-up system (Promega). The final elution step was performed using 40 μL double distilled water.
454 Titanium Amplicon Sequencing
Samples were pooled using 10 ng of PCR product of each samples, resulting in 16 different pools, including every multiplex identifier once. The amplification results of the mock controls were consistently below detection level and could therefore not have been added to the 454 run. The amplicon libraries were sequenced according to the 454 Titanium Amplicon Sequencing protocols, and a series of quality control steps were applied to the resulting 454 reads (Supplementary Methods). A total of 77 599 forward and 133 761 reverse reads of uniform length of 200 nucleotides and high quality were retained after filtering and have been submitted to National Center for Biotechnology Information Sequence Read Archive (accession number SRA026964.1) (Supplementary Table 1).
Calculation of Richness and Shannon Diversity Indices and Community Comparisons (α and β Diversity)
Pyrotagger was used for the definition of operational taxonomic units (OTUs) based on 97% sequence identity, estimation of chimeras, and taxonomy assignments [20] (Supplementary Methods). Outputs were used to create rarefaction curves and showed flattening of curves after 250 sequences (Supplementary Figure 1). Alpha diversity analysis (including richness and Shannon and Simpson Diversity indices) was performed using Mothur software [21]. Statistically significant differences were received using 2-tailed t test or analysis of variance if variables comprised >3 groups (aov function in R) without excluding outliers.
Differences between relative abundance measures (percentage) were assessed by parametric (t test or analysis of variance) and nonparametric (Mann–Whitney) tests. Fisher exact test was used for the prevalence analysis of bacterial families (Table 2).
Table 2.
Prevalence of Bacterial Families in the Nasopharynx of Infants With Acute Otitis Media and Healthy Controlsa
| Infants With AOM, % |
|||||||||
| Controls |
Antibiotic Exposure |
PCV7 Vaccination |
|||||||
| Organism | No. (%) | All No. (%) | P Valueb | Yes No. (%) | No No. (%) | P Valueb | Yes No. (%) | No No. (%) | P Valueb |
| Total number of study infants | 10 | 153 | 39 | 114 | 87 | 66 | |||
| Moraxellaceae | 9 (90) | 145 (95) | .4 | 37 (95) | 108 (95) | 1 | 81 (93) | 64 (97) | .5 |
| Streptococcaceae | 9 (90) | 113 (74) | .5 | 23 (59) | 90 (79) | .02 | 60 (69) | 53 (80) | .1 |
| Pasteurellaceae | 6 (60) | 91 (60) | 1 | 27 (69) | 64 (56) | .2 | 55 (63) | 36 (55) | .3 |
| Staphylococcaceae | 7 (70) | 29 (19) | .001 | 7 (18) | 22 (19) | 1 | 16 (18) | 13 (20) | .8 |
| Corynebacteriaceae | 8 (80) | 71 (46) | .05 | 9 (23) | 62 (54) | .001 | 33 (38) | 38 (58) | .02 |
| Flavobacteriaceae | 7 (70) | 24 (16) | .0004 | 10 (26) | 14 (12) | .07 | 15 (17) | 9 (14) | .7 |
| Carnobacteriaceae | 5 (50) | 25 (16) | .02 | 10 (26) | 15 (13) | .08 | 15 (17) | 10 (15) | .8 |
| Enterobacteriaceae | 4 (40) | 25 (16) | .08 | 11 (28) | 14 (12) | .03 | 17 (20) | 8 (12) | .3 |
| Comamonadaceae | 6 (60) | 23 (15) | .003 | 7 (18) | 16 (14) | .6 | 16 (18) | 7 (11) | .3 |
| Streptococcaceae with S. pneumoniaec | 1 (10) | 80 (52) | .0002 | 15 (38) | 65 (57) | .04 | 47 (54) | 33 (50) | .048 |
| Streptococcaceae without S. pneumoniaec | 8 (80) | 33 (22) | 8 (21) | 25 (22) | 13 (15) | 20 (30) | |||
Abbreviations: AOM, acute otitis media; PCV7, heptavalent pneumococcal polysaccharide vaccine; S. pneumoniae, Streptoccocus pneumoniae.
Presence of a bacterial family was defined as detection of at least 1 sequence attributed to this family.
Fisher exact test (2 × 2 or 2 × 3) was used as appropriate using absolute sample numbers.
Presence of S. pneumoniae was based on bacterial culture results [18].
Community comparisons were done using nonmetric multidimensional scaling (nMDS) and the adonis function of the vegan package in R (Supplementary Methods). The nMDS analysis is a nonparametric ordination-based method for reducing ecological community data complexity and identifying meaningful relationships among communities [22].
RESULTS
Description of Study Patients
Sociodemographic and clinical characteristics of the 153 infants with AOM and 10 controls included in this study are described in Table 1. Male infants predominated slightly. Approximately one-quarter of infants with AOM had been recently exposed to antimicrobials, approximately one-third attended day care, and approximately half had received PCV7. Streptococcus pneumoniae was detected by culture in more than half of infants with AOM and in 10% of controls.
Richness and Diversity of Bacterial OTUs
The composition of the microbial communities in each of the 163 samples was determined using bidirectional pyrosequencing targeting the variable regions 3–5 of the 16S rRNA gene. Forward sequences were then clustered at 97% sequence identity to identify OTUs. The median number of OTUs from infants with AOM (median, 6; range, 1–61) was significantly lower than for controls (median, 24; range, 2–144; P <.001). The median Shannon Diversity indices, which include both OTU richness and evenness, were 0.69 for infants with AOM and 1.31 for control infants (P = .002). Therefore, in infants with AOM, microbiota comprised fewer OTUs and OTUs were less evenly distributed than in controls.
Prevalence of Bacterial Families
OTUs were grouped into 58 bacterial families, of which Moraxellaceae (present in 154 of 163 NPS specimens), Streptococcaceae (122 of 163), and Pasteurellaceae (97 of 163) were most frequent (Table 2). Putative commensal families were more frequent in controls than in infants with AOM. These included Staphylococcaceae (Fisher exact test, P = .001), Flavobacteriaceae (P = .0004), Carnobacteriaceae (P = .02), and Comamonadaceae (P = .003). Infants with AOM who were recently exposed to antimicrobials less frequently carried Streptococcaceae (P = .02) and Corynebacteriaceae (P = .001), but more frequently carried Enterobacteriaceae (P = .03) than did those without such exposure. In addition, infants with AOM who had received at least 1 dose of PCV7 less frequently carried Corynebacteriaceae (P = .02) than did nonvaccinated infants. A map showing the prevalence of all bacterial families is available (Supplementary Figure 2).
More-detailed analysis of Streptococcaceae was hampered by the fact that 16S sequencing does not discriminate in this family when using a cutoff of 97%. Therefore, culture results for S. pneumoniae were included (Table 2). Most controls (80%) but only approximately one-fifth (21.5%) of infants with AOM had a culture negative for S. pneumoniae but carried other Streptococcaceae (P = .0002). Recent exposure to antimicrobials was associated with a lower prevalence of carriage of Streptococcaceae, including S. pneumoniae (P = .04). Receipt of PCV7 was associated with reduced prevalence of nonpneumococcal Streptococcaceae (P = .048).
Relative Abundance of Bacterial Families
Next, the relative abundance of bacterial families in each sample was determined and means (± standard error of the mean) were calculated and compared between infants with AOM and controls, infants with AOM who had been exposed to antimicrobials or not, and those who had received PCV7 or not (Figure 1A, 1B, and 1C). Moraxellaceae, Streptococcaceae, and Pasteurellaceae tended to be more abundant in infants with AOM than in controls, although these differences did not reach statistical significance (Figure 1A). Only Staphylococcaceae were more abundant in controls than in infants with AOM (P = .0001). Of the bacterial families classified as others, none reached an abundance of >2% in infants with AOM. In the healthy controls, the other families reaching an abundance of >2.0% included Comamonadaceae (4.8%, mainly Comamonas species), Acidaminococcaceae (3.5%, mainly Veillonella species), Enterococcaceae (3.4%, mainly Enterococcus species), Carnobacteriaceae (2.9%, mainly Granulicatella species), and Neisseriaceae (2.6%, mainly Neisseria species).
Figure 1.
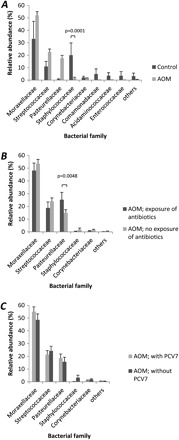
Relative abundance of bacterial families (mean and standard error of the mean) with an abundance of >2% are shown for infants with acute otitis media (AOM; A), compared with controls, in infants with AOM according to recent antibiotic exposure (B), and the vaccination status for the 7-valent conjugated polysaccharide vaccine (PCV7; C). P values derived by Student t test are indicated.
Use of the Mann–Whitney U test revealed statistically significant differences among Staphylococcaceae (P = .003), Comamonadaceae (P = .04), Acidaminococcaceae (P = .003), and Corynebacteriaceae (P = .04). This is comparable to the prevalence analysis (Table 2).
Recent exposure to antimicrobials was associated with an increased abundance of Pasteurellaceae (P = .0048). Furthermore, nonsignificant trends for a lower relative abundance of Moraxellaceae, Streptococcaceae, and Staphylococcaceae were noted in the antimicrobial exposure group (Figure 1B). Administration of PCV7 was not associated with a significant increase or decrease in the abundance of bacterial families (Figure 1C).
Microbial Community Comparisons
To further evaluate the influence of clinical factors, pairwise distances between microbial communities per sample were measured. Prevalence-based (unweighted) and abundance-based (weighted) distance matrices were used as input, and the amount of variance that each clinical variable (AOM, antimicrobial exposure, and administration of PCV7) contributed was calculated.
In the unweighted analysis, the nasopharyngeal bacterial communities of controls and infants with AOM differed significantly from each other (P < .005) (Figure 2A). To exclude a selection bias by time, this analysis was also performed after excluding all samples collected in the prevaccine season, but a similar difference was received (P < .05; data not shown). Recent exposure to antimicrobials had the strongest influence on the microbiota of infants with AOM (P < .005) (Figure 2B). In addition, administration of PCV7 (P < .05) (Figure 2C) and sampling in the prevaccine versus postvaccine season (P < .05; data not shown) had a slight but statistically significant influence on the microbial community. Factorial analysis of variance showed that the antimicrobials and PCV7 acted independently from each other. Furthermore, age, sex, number of AOM episodes during the previous 12 months and day care use had no influence in the univariate analysis (Supplementary Table 2). Use of weighted distance matrices again revealed a significant difference between infants with AOM and controls (P < .05), but for none of the other factors studied (not shown). This indicates that differences between microbiota were based more on discrepancies related to prevalence of bacterial families (unweighted analysis) than on their relative abundance (weighted analysis).
Figure 2.
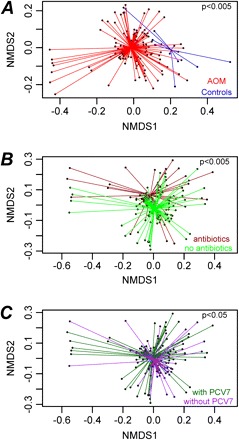
Comparison of bacterial communities by nonmetric multidimensional scaling (nMDS) ordination plots. Shown are the results for infants with acute otitis media (AOM), compared with control infants (A), infants with AOM with or without recent antibiotic exposure (B), and receipt or not of the 7-valent conjugated polysaccharide vaccine (PCV7; C). Unweighted unifrac distance matrices were used as input, and each data point in the nMDS plot represents the bacterial community identified from a single nasopharyngeal sample. Comparison using F tests using the adonis function of the vegan package yielded the F values for A, 9.5 (P < .005); for B, 9.9 (P < .005); and for C, 5.4 (P < .05). P values are not shown as absolute values, because multiple calculations yielded slight variations
Colonization Density
Colonization density was estimated by measuring the DNA concentration of the PCR amplification product. Significantly lower DNA quantities were observed in the nasopharynx of controls (median value, 12 ng/μL), compared with infants with AOM (38 ng/μL; P = .005). In addition, DNA quantities were lower in microbial communities containing no S. pneumoniae but other Streptococcaceae (32 ng/μL), compared with those with a culture positive for S. pneumoniae (39 ng/μL) and those without any Streptococcaceae (42 ng/μL; P = .048). These results suggest an inverse relationship between colonization density and OTU richness. No statistically significant differences were found when stratifying DNA concentrations for the clinical variables age, sex, recent antibiotic exposure, and PCV7 vaccination.
DISCUSSION
This study used high-throughput sequencing for the analysis of the nasopharyngeal microbiota in infants with AOM and control infants, taking into account additional host factors, such as prior exposure to antimicrobials and PCV7 vaccination status. Strengths of this analysis are a homogeneous study population restricted to infants <2 years of age, sampling from the nasopharynx, a large sample size, and the possibility to study the influence of clinical variables.
Microbial community comparisons showed statistically significant differences between infants with AOM and control infants. The healthy infant microbiota was characterized by the presence of low prevalent bacterial families and nonpneumococcal Streptococcaceae. In infants with AOM, however, the classical AOM pathogens predominated over rare, putative commensal bacterial families. This finding is in line with previous reports using culture- and nonculture- based methods to examine the respiratory tract microbiota [16, 23]. They suggest that a richer and more diverse microbiota may protect against AOM. This is supported by reports about an inhibitory effect of α-hemolytic streptococci on colonization with AOM pathogens and, thereby, protection against recurrence of AOM [24, 25]. Little is known about the epidemiology and biology of the low prevalent bacterial families living in the nasopharynx, apart from being involved in isolated cases of AOM, opportunistic infections, or bloodstream infections associated with dental procedures [26–28].
A recent study investigated the microbiota in healthy infants 18 months of age during the winter and the summer season [29]. Comparable to the controls in our study, a complex, diverse, and highly variable microbiota was found with relatively lower proportions of the 3 AOM pathogens (Moraxellaceae, Streptococcaceae, and Pasteurellaceae), compared with our infants with AOM. This confirms the validity of our control group and shows that, independent of the geographical site, the same bacterial families are residing in the nasopharynx of infants.
The modulating effect of antibiotic exposure on the human microbial ecosystem has been well recognized [30]. In this study, recent antimicrobial treatment was the strongest factor inducing bacterial community changes in the nasopharyngeal microbiota in infants with AOM. Community structure changed toward reduced prevalence of commensal families (Streptococcaceae, including pneumococci and Corynebacteriaceae), increased prevalence of Enterobacteriaceae, and greater relative abundance of Pasteurellaceae. The latter observation has been reported also in earlier culture-based studies [31, 32]. Therefore, antimicrobials change the microbiota toward an unhealthy status with lesser presence of commensals. In general, effects of antibiotic exposure on the microbiota can be expected to depend largely on the prevalence of resistance genes. They may therefore overrule interspecies relationships and may lead to complex and unpredictable changes of the microbiota.
Broad use of PCV7 has been paralleled by shifts of pneumococcal serotype distribution and, unexpectedly, changes in the prevalence of pathogens residing in the nasopharynx on an individual and population level [10]. In our study, having received vaccination with PCV7 and having had samples obtained after the introduction of PCV7 changed the community structure of the microbiota toward reduced prevalence of commensals (Streptococcaceae excluding pneumococci and Corynebacteriaceae). Therefore, unexpectedly, the results indicate that PCV7 drives the microbiotia toward a composition with lower presence of commensals. The mechanisms mediating the influence of PCV7 are not clear, but it may be that pneumococcal serotypes that have emerged under the selection pressure of PCV7 inhibit colonization by commensal Streptococcaceae more than the PCV7 serotypes did.
In this study, no influence of age, history of recurrent AOM, sex, or day care use on the microbiota was observed in infants with AOM. The lack of an association with age can be explained by the narrow age range studied. Sociodemographic differences between Switzerland and other countries may be the reason that day care had no measurable influence on the microbiota. A potentially important factor could be concurrent infection with a respiratory virus. In a preliminary study, we analyzed 81 samples from infants with AOM collected from 2004 through 2006 for the presence of RNA viruses, including human rhinovirus, respiratory syncytial virus, coronavirus, and bocavirus. The presence of any of these viruses did not have an effect on the microbiota, either on the prevalence or the abundance of bacterial families (data not presented). In agreement with our preliminary data, no evidence for an association between seasonal shifts in microbiota and the presence of respiratory viruses was found in healthy infants in the Netherlands [29]. However, additional studies are needed to explore the role of viruses in the microbiota in more detail.
Our study has limitations. Samples from infants with AOM were restricted to those collected during winter seasons because AOM incidence peaks during winter, and there was no information about diet, number of siblings, or parental smoking, which may all have an influence on the microbiota. The results from our study may therefore not apply to the summer season, as has been shown for healthy controls [29]. The number of controls was small, and samples were collected during a relatively short period after the introduction of PCV7 in a selected geographic region, compared with samples for infants with AOM. However, the results obtained for our control infants resembled closely those described in a recent Dutch study, as discussed above [29]. Another limitation of our study is the lack of high-quality quantitative data on colonization density, because the quantity of bacterial DNA in NPS specimens is very small, which impedes a reliable quantification of the microbiota directly in the sample. However, taking the DNA concentration of the PCR amplification product as a surrogate for colonization density has been described as a reasonable approach [18]. Our results suggest that colonization density is lower in bacterial communities of increased richness, such as the healthy microbiota in the controls. Increased quantities of nontypeable H. influenzae, S. pneumoniae, and M. catarrhalis have been observed in nasopharyngeal secretions during AOM, compared with the healthy status [33]. Therefore, the pathogenesis of AOM may include a disturbance of the interspecies balance with ensuing overgrowth of an AOM pathogen.
In conclusion, our study is the first to characterize the nasopharyngeal microbiota of infants with and without AOM with use of molecular techniques. Key findings are the characterization of the microbiota in infants with AOM as a flora of reduced richness and evenness, but higher colonization density, compared with healthy controls. In infants with AOM, recent antimicrobial exposure and PCV7 influenced community structure of the microbiotia toward lower prevalence of commensal bacterial families (especially Streptococcaceae and Corynebacteriaceae).
Supplementary Material
Notes
Financial support.
This study was supported in part by the Swiss National Science Foundation (grant number 3200-067998 to K. M.; MD-PhD scholarship 323500-119214 to S. B.).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Cripps AW, Otczyk DC, Kyd JM. Bacterial otitis media: a vaccine preventable disease? Vaccine. 2005;23:2304–10. doi: 10.1016/j.vaccine.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Paradise JL, Rockette HE, Colborn DK, et al. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–33. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- 4.Vergison A. Microbiology of otitis media: a moving target. Vaccine. 2008;26(Suppl 7):G5–10. doi: 10.1016/j.vaccine.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 6.Armbruster CE, Hong W, Pang B, et al. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. MBio. 2010;1:e00102–10. doi: 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 2005;1:e1. doi: 10.1371/journal.ppat.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J. 2004;23:824–8. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 9.Block SL, Hedrick J, Harrison CJ, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829–33. doi: 10.1097/01.inf.0000136868.91756.80. [DOI] [PubMed] [Google Scholar]
- 10.Revai K, McCormick DP, Patel J, Grady JJ, Saeed K, Chonmaitree T. Effect of pneumococcal conjugate vaccine on nasopharyngeal bacterial colonization during acute otitis media. Pediatrics. 2006;117:1823–9. doi: 10.1542/peds.2005-1983. [DOI] [PubMed] [Google Scholar]
- 11.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–2. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–41. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM. Microbial communities of the upper respiratory tract and otitis media in children. MBio. 2011;2:e00245–10. doi: 10.1128/mBio.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muhlemann K, Matter HC, Tauber MG, Bodmer T. Nationwide surveillance of nasopharyngeal Streptococcus pneumoniae isolates from children with respiratory infection, Switzerland, 1998–1999. J Infect Dis. 2003;187:589–96. doi: 10.1086/367994. [DOI] [PubMed] [Google Scholar]
- 18.Brugger SD, Frey P, Aebi S, Hinds J, Muhlemann K. Multiple colonization with S. pneumoniae before and after introduction of the seven-valent conjugated pneumococcal polysaccharide vaccine. PLoS One. 2010;5:e11638. doi: 10.1371/journal.pone.0011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugger SD, Hathaway LJ, Muhlemann K. Detection of Streptococcus pneumoniae strain cocolonization in the nasopharynx. J Clin Microbiol. 2009;47:1750–6. doi: 10.1128/JCM.01877-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunin V, Hugenholtz P. PyroTagger: a fast, accurate pipeline for analysis of rRNA amplicon pyrosequence data. Open J. 2010:1–8. [Google Scholar]
- 21.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price LB, Liu CM, Melendez JH, et al. Community analysis of chronic wound bacteria using 16S rRNA gene-based pyrosequencing: impact of diabetes and antibiotics on chronic wound microbiota. PLoS One. 2009;4:e6462. doi: 10.1371/journal.pone.0006462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1997;175:1440–5. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 24.Tano K, Olofsson C, Grahn-Hakansson E, Holm SE. In vitro inhibition of S. pneumoniae, nontypable H. influenzae and M. catharralis by alpha-hemolytic streptococci from healthy children. Int J Pediatr Otorhinolaryngol. 1999;47:49–56. doi: 10.1016/s0165-5876(98)00174-8. [DOI] [PubMed] [Google Scholar]
- 25.Roos K, Hakansson EG, Holm S. Effect of recolonisation with “interfering” alpha streptococci on recurrences of acute and secretory otitis media in children: randomised placebo controlled trial. BMJ. 2001;322:210–2. doi: 10.1136/bmj.322.7280.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper GR, Staples ED, Iczkowski KA, Clancy CJ. Comamonas (Pseudomonas) testosteroni endocarditis. Cardiovasc Pathol. 2005;14:145–9. doi: 10.1016/j.carpath.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Bahrani-Mougeot FK, Paster BJ, Coleman S, Ashar J, Barbuto S, Lockhart PB. Diverse and novel oral bacterial species in blood following dental procedures. J Clin Microbiol. 2008;46:2129–32. doi: 10.1128/JCM.02004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudley JP, Sercarz J. Enterococcus faecalis and otitis media with effusion. How to treat. Int J Pediatr Otorhinolaryngol. 1990;20:123–6. doi: 10.1016/0165-5876(90)90077-5. [DOI] [PubMed] [Google Scholar]
- 29.Bogaert D, Keijser B, Huse S, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One. 2011;6:e17035. doi: 10.1371/journal.pone.0017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis. 2001;1:101–14. doi: 10.1016/S1473-3099(01)00066-4. [DOI] [PubMed] [Google Scholar]
- 31.Varon E, Levy C, De La Rocque F, et al. Impact of antimicrobial therapy on nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, and Branhamella catarrhalis in children with respiratory tract infections. Clin Infect Dis. 2000;31:477–81. doi: 10.1086/313981. [DOI] [PubMed] [Google Scholar]
- 32.Samuelson A, Freijd A, Jonasson J, Lindberg AA. Turnover of nonencapsulated Haemophilus influenzae in the nasopharynges of otitis-prone children. J Clin Microbiol. 1995;33:2027–31. doi: 10.1128/jcm.33.8.2027-2031.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faden H, Stanievich J, Brodsky L, Bernstein J, Ogra PL. Changes in nasopharyngeal flora during otitis media of childhood. Pediatr Infect Dis J. 1990;9:623–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


