Abstract
Objective
The objective of this study was to assess epidemiological and clinical features of human bocavirus (HBoV) coinfection with other viruses.
Method
Children coinfected with HBoV between January 2012 and December 2014 were enrolled and retrospectively reviewed.
Result
A total of 984 patients were stratified into five groups: HBoV infection alone (n = 249), respiratory syncytial virus (RSV) infection alone (n = 649), HBoV coinfection with RSV (n = 28), with human rhinovirus (HRV) (n = 39) and with other virus (n = 19). Length of hospitalization was longer in HBoV coinfection with RSV group than HBoV (9.0 days vs. 7.0 days, p = 0.001), RSV (9.0 days vs. 8.0 days, p = 0.016) infection alone group. Pneumonia was more common in the HBoV coinfection with RSV group compared with the HBoV, RSV infection alone group, respectively (75.0% vs. 44.2%, 31.3%, p < 0.001). HBoV DNA copy numbers (383 000 copies/ml) were positively correlated with the length of hospitalization (r = 0.334, p < 0.001).
Conclusion
HBoV coinfection with RSV increases HBoV infection severity.
Keywords: coinfection, human bocavirus, respiratory syncytial virus, disease severity, acute respiratory infections
INTRODUCTION
Human bocavirus (HBoV) was first identified in 2005 in nasopharyngeal aspirates collected from children with respiratory diseases [1]. Since then, HBoV has been detected worldwide, with prevalence rates between 1.5% and 19% [2–4].
The recent widespread use of molecular methods has markedly increased the ability to identify respiratory viruses in children with acute respiratory infections (ARIs) [5]. Recent studies showed that 10–30% of pediatric patients have coinfection by two or more respiratory viruses [6–8]. Coinfection with other viruses is commonly observed in HBoV-positive patients with ARI, e.g. adenovirus (ADV), picornavirus, respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) [9–12]. Although most HBoV infections are mild and often self-limited [13], the clinical features of HBoV coinfection are not well described, especially regarding the role of specific viral interactions.
RSV is generally considered a well-established pathogen that can cause severe acute respiratory tract infection [14]. Recent data suggest that HBoV also causes severe acute respiratory tract infection as the single causative agent [15]. The relationship between respiratory viral coinfection and disease severity in children remains unclear [16, 17]. The aim of this study was to assess the epidemiology features of both HBoV infection alone and coinfection with other virus, evaluating the impact of RSV coinfection on HBoV in children hospitalized with ARI.
MATERIALS AND METHODS
Study patients and specimen collection
This retrospective descriptive study was conducted over a 36-month period (1 January 2012 to 31 December 2014) on children (<18 years) presenting with ARI (n = 6459) admitted to the Children's Hospital affiliated to Soochow University, China. Clinical diagnoses of ARI included upper respiratory tract infections (URTIs), bronchitis, bronchiolitis, pneumonia and asthma. Diagnostic definitions were as follows: URTI was defined as ARI with no abnormalities on chest X-ray; bronchiolitis was characterized by cough, tachypnea, retraction and expiratory wheezes, often accompanied by rales; bronchitis was used to define a lesser disease with rhonchi and cough, sometimes with wheezing; pneumonia required rales on auscultation or the demonstration of an infiltrate by chest X-ray [18, 19]. Patients with bronchopulmonary dysplasia, genetic syndromes and immunosuppression were excluded from the study.
Nasopharyngeal aspirates were obtained from all children within 24 h of hospital admission; a catheter was inserted into one nostril to a depth of 7–9 cm and withdrawn while applying gentle suction with an electric suction device. Specimens (leukocytes >25 per high-power microscopic field and few epithelial cells <10 per high-power microscopic field) [20] were centrifuged twice, with the pellets stored at −80°C until use.
VIRUS DETECTION
Viral DNA and RNA from nasopharyngeal samples were extracted with DNA-EZ Reagents (Sangon Biotech, Shanghai) and TRIzol Reagent (Life Technologies, Carlsbad, CA, USA), respectively, according to the manufacturers’ protocols. The primers and probes used for HBoV detection were targeted to the NP1 gene, and fluorescent real-time polymerase chain reaction (PCR) was performed for HBoV detection, as described previously [21]. In addition, samples were sent for the detection of hMPV and human rhinovirus (HRV) by reverse-transcription PCR; direct immunofluorescence was used to detect RSV, ADV, parainfluenza virus (PIV) types 1–3, and influenza virus (IV) types A and B [21].
Review of medical records
Medical records were reviewed for the following: ① general characteristics such as age, gender, season of infection, duration of symptoms (cough or fever) before admission, birth week, congenital heart disease and history of breastfeeding; ② clinical symptoms and signs, including fever, cough, wheezing, tachypnea, diarrhea and vomiting; ③ laboratory findings such as peripheral leukocyte count, neutrophil count, lymphocyte count, platelet number and C-reactive protein (CRP) levels; ④ disease severity parameters, including requirement for supplemental oxygen, pediatric intensive care unit (PICU) admission, length of hospitalization and need for mechanical ventilation; ⑤ clinical diagnoses, including URTI, bronchitis, bronchiolitis, pneumonia and asthma.
STATISTICAL ANALYSIS
Statistical analysis was performed with PASW version 20.0 (IBM, SPSS, Chicago, IL, USA). Descriptive analyses were performed, with findings reported as absolute frequencies or rates for categorical variables, median (25th–75th) values for quantitative variables with non-normal distribution. Quantitative variables between the two groups were compared by the Mann–Whitney U test; comparison of quantitative variables among the five infections groups was performed using the Kruskal–Wallis test. Comparison of frequency distribution was performed by the chi-square test; Fisher’s exact test was used when at least one expected frequency was <5. Spearman’s rank correlation coefficient was used to evaluate correlations between the number of copies of HBoV DNA and the length of hospitalization. p < 0.05 was considered statistically significant.
RESULTS
Demographic characteristics
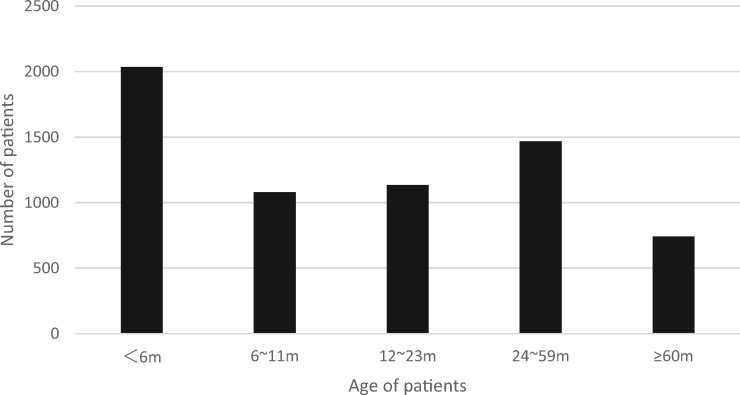
Between 1 January 2012 and 31 December 2014, a total of 6459 patients were admitted for ARI; patient age distribution is shown in Fig. 1. Of these patients, HBoV was positive in 335 cases, indicating a positive rate of 5.2%. Of the HBoV-infected cases, 86 (25.7%) were coinfection with other respiratory viruses, most frequently with HRV (n = 39), followed by RSV (n = 28) (Table 1). Single RSV infection was detected in 649 cases during the same period. Those 984 patients were stratified into five groups: HBoV infection alone (n = 249), RSV infection alone (n = 649), HBoV coinfection with RSV (n = 28), HBoV coinfection with HRV (n = 39) and HBoV coinfection with other virus (n = 19).
Fig. 1.
Distribution of ARI patients stratified by age.
Table 1.
Distribution of HBoV cases by infection type
| Type of infection | Virus | Number of patients (%) | Subtotal (%) | ||
|---|---|---|---|---|---|
| Single infection | HBoV (only) | 249 (74.33) | 249 (74.33) | ||
| Coinfection | HBoV | RSV | 28 (8.36) | 86 (25.67) | |
| HBoV | HRV | 39 (11.64) | |||
| HBoV | HMPV | 1 (0.3) | |||
| HBoV | PIV1 | 2 (0.6) | |||
| HBoV | IAV | 3 (0.9) | |||
| HBoV | ADV | 1 (0.3) | |||
| HBoV | PIV3 | 11 (3.3) | |||
| HBoV | PIV3 | HRV | 1 (0.3) | ||
| Total | 335 (100) | ||||
Note: PIV1, parainfluenza virus 1; IAV, influenza virus A; PIV3, parainfluenza virus 3.
Epidemiology of HBoV infection
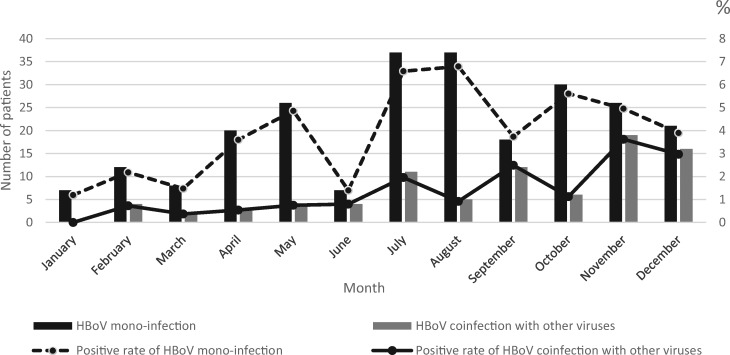
Monthly distribution of HBoV infection is shown in Fig. 2. Infections associated with HBoV occurred throughout the year. Peak incidence of HBoV infection alone appeared in August (6.8%); meanwhile, peak incidence of HBoV coinfection with other viruses was found in November (3.6%). These results indicated that HBoV coinfection with other viruses and HBoV infection alone had different seasons of prevalence.
Fig. 2.
Distribution by month of HBoV infection alone and HBoV coinfection with other viruses.
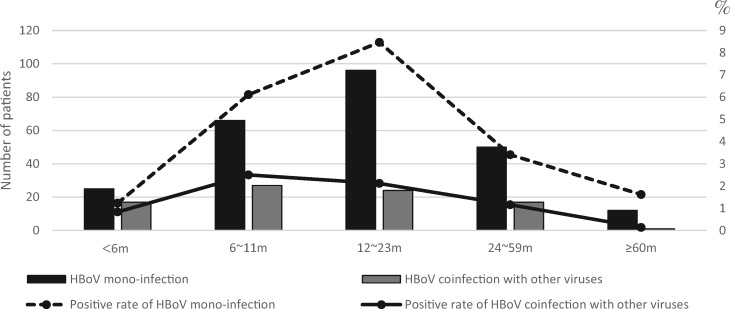
Age distribution of HBoV patients is shown in Fig. 3. Patients of age from 12 to 23 months had the highest rate of HBoV infection alone (8.5%), while those below 6 months showed the lowest infection rate (1.2%). The highest rate for HBoV coinfection with other viruses was found in patients aged from 6 to 11 months (2.5%); patients aged ≥60 months had the lowest infection rate (0.1%). These data revealed that HBoV coinfection with other viruses and HBoV infection alone target different age group.
Fig. 3.
Distribution by age of HBoV infection alone and HBoV coinfection with other virus.
Clinical and laboratory characteristics of children in various infection groups
The demographic and clinical characteristics of the enrolled patients are summarized in Table 2. First, the general characteristics among the five infection groups were compared. Patients with RSV infection alone were younger than the other four groups (all p < 0.05). Symptom duration <7 days before admission was more common in RSV infection alone group than the other four groups (all p < 0.05). Symptom duration ≥14 days before admission was less frequent in RSV infection alone group than HBoV infection alone group, HBoV coinfection with RSV group and HBoV coinfection with HRV group (all p < 0.05). Symptom duration ≥14 days before admission was less frequent in HBoV infection alone group than HBoV coinfection with RSV group (p < 0.05). Gender ratio, prematurity rate, congenital heart disease rate and history of breastfeeding were not significantly different between the groups (all p > 0.05).
Table 2.
General characteristics of children in various infection groups
| Characteristics | HBoV single infection (n = 249) | RSV single infection (n = 649) | HBoV with RSV coinfection (n = 28) | HBoV with HRV coinfection (n = 39) | HBoV with other virus coinfection (n = 19) | p |
|---|---|---|---|---|---|---|
| Male [n (%)] | 162 (65.1) | 420 (64.7) | 16 (57.1) | 27 (69.2) | 11 (57.9) | 0.836 |
| Age, median (25th–75th) (months) | 14.0 (9.0–24.0) | 4.0 (2.0–10.0)a | 13.0 (5.7–27.0)b | 11.0 (8.0–19.0)b | 11.0 (6.0–19.0)b | <0.001 |
| Premature [n (%)] | 17 (6.8) | 55 (8.5) | 2 (7.1) | 1 (2.6) | 0 (0.0) | 0.564 |
| Congenital heart disease [n (%)] | 5 (2.0) | 21 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.776 |
| Breastfeeding [n (%)] | 208 (8.5) | 571 (88.0) | 24 (85.7) | 30 (76.9) | 15 (78.9) | 0.11 |
| Duration of symptoms before admission (days) | ||||||
| <7 [n (%)] | 109 (43.8) | 389 (59.9)a | 5 (17.9)ab | 15 (38.5)b | 7 (36.8)b | <0.001 |
| 7–14 [n (%)] | 65 (26.1) | 145 (22.3) | 9 (32.1) | 7 (17.9) | 6 (31.6) | 0.386 |
| ≥14 [n (%)] | 75 (30.1) | 115 (17.7)a | 14 (50.0)ab | 17 (43.6)b | 6 (31.6) | <0.001 |
p < 0.05, p values obtained by comparison with patients of the HBoV single infection group at baseline.
p < 0.05, p values obtained by comparison with patients of the RSV single infection group at baseline.
Other virus = hMPV, PIV1, IAV, ADVs and PIV3.
Next, clinical symptoms and signs were compared among the five infection groups (Table 3). We found that fever was more common in HBoV coinfection with RSV group than RSV infection alone group and HBoV coinfection with HRV group (both p < 0.05), fever was also more common in HBoV infection alone group than RSV infection alone group and HBoV coinfection with HRV group (both p < 0.05). Cough was more common in RSV infection alone group than HBoV infection alone group (p < 0.05). Wheezing was less common in HBoV infection alone group than RSV infection alone group and HBoV coinfection with HRV group (both p < 0.05), and wheezing was also less common in HBoV coinfection with other virus group than RSV infection alone group, HBoV coinfection with RSV group and HBoV coinfection with HRV group (all p < 0.05). Tachypnea was common in RSV infection alone group than HBoV infection alone group (p < 0.05).
Table 3.
Clinical symptoms and signs of children in various infection groups
| Characteristics | HBoV single infection (n = 249) | RSV single infection (n = 649) | HBoV with RSV coinfection (n = 28) | HBoV with HRV coinfection (n = 39) | HBoV with other virus coinfection (n = 19) | p |
|---|---|---|---|---|---|---|
| Fever [n (%)] | 164 (65.9) | 217 (33.4)a | 20 (71.4)b | 16 (41.0)ac | 11 (57.9)b | <0.001 |
| Cough [n (%)] | 241 (96.8) | 646 (99.5)a | 28 (100.0) | 39 (100.0) | 18 (94.7) | 0.008 |
| Wheezing [n (%)] | 112 (45.0) | 420 (64.7)a | 16 (57.1) | 26 (66.7)a | 5 (26.3)b,c,d | <0.001 |
| Tachypnea [n (%)] | 34 (13.7) | 131 (20.2)a | 2 (7.1) | 6 (15.4) | 1 (5.3) | 0.049 |
| Diarrhea or vomiting [n (%)] | 20 (8.0) | 62 (9.6) | 6 (21.4) | 7 (17.9) | 1 (5.3) | 0.079 |
p < 0.05, p values obtained by comparison with patients of the HBoV single infection group at baseline.
p < 0.05, p values obtained by comparison with patients of the RSV single infection group at baseline.
p < 0.05, p values obtained by comparison with patients of the HBoV with RSV coinfection group at baseline.
p < 0.05, p values obtained by comparison with patients of the HBoV with HRV coinfection group at baseline.
Other virus = hMPV, PIV1, IAV, ADVs and PIV3.
Furthermore, laboratory findings among the five infection groups were compared (Table 4). Peripheral leukocyte count was higher in patients with HBoV infection alone group than RSV infection alone group (p < 0.05), and peripheral leukocyte count was also higher in HBoV coinfection with HRV group than HBoV infection alone group, HBoV coinfection with RSV group and HBoV coinfection with other virus group (all p < 0.05). Neutrophil count was lower in RSV infection alone group than HBoV infection alone group, HBoV coinfection with RSV group and HBoV coinfection with HRV group (all p < 0.05). Platelet amounts were lower in HBoV infection alone group than RSV infection alone group and HBoV coinfection with HRV group (both p < 0.05). CRP was lower in RSV infection alone group than HBoV infection alone group and HBoV coinfection with RSV group (both p < 0.05).
Table 4.
Laboratory findings of children in various infection groups
| Characteristics | HBoV single infection (n = 249) | RSV single infection (n = 649) | HBoV with RSV coinfection (n = 28) | HBoV with HRV coinfection (n = 39) | HBoV with other virus coinfection (n = 19) | p |
|---|---|---|---|---|---|---|
| Peripheral leukocyte count, median (25th–75th) × 109/l | 9.6 (7.2–13.2) | 8.7 (6.6–11.4)a | 8.9 (6.2–12.6) | 10.6 (9.1–14.1)abc | 8.1 (4.9–10.6) | <0.001 |
| Neutrophil count, median (25th–75th) (%) | 45.1 (28.5–62.1) | 30.8 (20.8–46.5)a | 47.9 (38.2–66.7)b | 38.4 (29.8–56.3)b | 41.6 (23.8–63.9) | <0.001 |
| Lymphocyte count, median (25th–75th) (%) | 44.5 (30.4–61.8) | 58.7 (44.5–69.1)a | 44.1 (24.8–53.6)b | 50.6 (35.3–57.6)b | 53.1 (27.3–60.2) | <0.001 |
| Platelet number, median (25th–75th) × 109/l | 346.0 (267.5–436.5) | 383.5 (304.2–472.0)a | 375.0 (284.7–493.2) | 446.0 (297.2–495.2)a | 403.0 (287.0–489.0)b | <0.001 |
| CRP, median (25th–75th) (mg/dl) | 3.0 (0.4–11.0) | 0.8 (0.2–3.7)a | 3.2 (0.2–9.4)b | 0.8 (0.2–4.5) | 1.3 (0.2–6.8) | <0.001 |
p < 0.05, p values obtained by comparison with patients of the HBoV single infection group at baseline.
p < 0.05, p values obtained by comparison with patients of the RSV single infection group at baseline.
p < 0.05, p values obtained by comparison with patients of the HBoV with RSV coinfection group at baseline.
Other virus = hMPV, PIV1, IAV, ADVs and PIV3.
To assess differences in disease severity among the five infection groups (Table 5), (i) requirement for supplemental oxygen, (ii) PICU admission, (iii) length of hospitalization and (iv) the need for mechanical ventilation were compared. Median length of hospitalization in patients with HBoV infection alone was shorter than patients with RSV infection alone [7.0 (6.0–9.0) days vs. 8.0 (7.0–9.0) days, p = 0.002] and HBoV coinfection with RSV [7.0 (6.0–9.0) days vs. 9.0 (7.2–10.0) days, p = 0.001]. Patients with RSV infection alone also had a shorter length of hospitalization than patients with HBoV coinfection with RSV [8.0 (7.0–9.0) days vs. 9.0 (7.2–10.0) days, p = 0.016]. However, requirement for supplemental oxygen, PICU admission and the need for mechanical ventilation were not significantly different among the groups (all p > 0.05). No patients died during hospitalization.
Table 5.
Disease severity of children in various infection groups
| Characteristics | HBoV single infection (n = 249) | RSV single infection (n = 649) | HBoV with RSV coinfection (n = 28) | HBoV with HRV coinfection (n = 39) | HBoV with other virus coinfection (n = 19) | p |
|---|---|---|---|---|---|---|
| Requirement for supplemental oxygen [n (%)] | 27 (10.8) | 86 (13.3) | 1 (3.6) | 5 (12.8) | 4 (21.1) | 0.358 |
| Length of hospitalization, median (25th–75th) (days) | 7.0 (6.0–9.0) | 8.0 (7.0–9.0)a | 9.0 (7.2–10.0)ab | 8.0 (6.0–10.0) | 7.0 (7.0–10.0) | 0.002 |
| Admission to the PICU [n (%)] | 14 (5.6) | 34 (5.2) | 0 (0.0) | 2 (5.1) | 2 (10.5) | 0.571 |
| Need for mechanical ventilation [n (%)] | 1 (0.4) | 5 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.99 |
p < 0.05, p values obtained by comparison with patients of the HBoV single infection group at baseline.
p < 0.05, p values obtained by comparison with patients of the RSV single infection group at baseline.
Other virus = hMPV, PIV1, IAV, ADVs and PIV3.
Finally, clinical diagnosis among the three etiological groups was compared (Table 6). We found that pneumonia was more common in the HBoV coinfection with RSV group compared with the HBoV infection alone group (75.0% vs. 44.2%, p = 0.002), RSV infection alone group (75.0% vs. 31.3%, p < 0.001) and HBoV coinfection with HRV group (75.0% vs. 48.7%, p = 0.031). Pneumonia was also more common in HBoV infection alone group than RSV infection alone group (44.2% vs. 31.3%, p < 0.001); meanwhile, bronchiolitis was common in RSV infection alone group than the other four groups (all p < 0.05).
Table 6.
Clinical diagnosis of children in various infection groups
| Characteristics | HBoV single infection (n = 249) | RSV single infection (n = 649) | HBoV with RSV coinfection (n = 28) | HBoV with HRV coinfection (n = 39) | HBoV with other virus coinfection (n = 19) | p |
|---|---|---|---|---|---|---|
| URTI [n (%)] | 4 (1.6) | 7 (1.1) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0.394 |
| Bronchitis [n (%)] | 61 (24.5) | 46 (7.1)a | 3 (10.7) | 9 (23.1)b | 4 (21.1)b | <0.001 |
| Bronchiolitis [n (%)] | 70 (28.1) | 382 (58.9)a | 4 (14.3)b | 11 (28.2)b | 4 (21.1)b | <0.001 |
| Pneumonia [n (%)] | 110 (44.2) | 203 (31.3)a | 21 (75.0)b | 19 (48.7)bc | 9 (47.4) | <0.001 |
| Asthma [n (%)] | 4 (1.6) | 11 (1.7) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0.598 |
p < 0.05, p values obtained by comparison with patients of the HBoV single infection group at baseline.
p < 0.05, p values obtained by comparison with patients of the RSV single infection group at baseline.
p < 0.05, p values obtained by comparison with patients of the HBoV with RSV coinfection group at baseline.
Other virus = hMPV, PIV1, IAV, ADVs and PIV3.
HBoV DNA copy and disease severity
We further evaluated the correlation between the HBoV DNA copy number and the length of hospitalization. We found that HBoV DNA copy numbers [383 000 (4430–10 000 000) copies/ml] were positively correlated with the duration of hospitalization (r = 0.334, p < 0.001).
DISCUSSION
This retrospective descriptive study was conducted between 1 January 2012 and 31 December 2014, during which 6459 inpatients presenting with ARI had a viral diagnostic test performed. This large amount of data allowed the identification of epidemiological features of HBoV infection alone and coinfection with other viruses, evaluating the impact of RSV coinfection on HBoV in children hospitalized with ARI.
HBoV was first identified in 2005 [1]. Since then, it has been detected worldwide, with rates between 1.5% and 19% [2–4]. In the present study, the overall detection rate of HBoV in ARI patients was 5.2%, a value similar to that found in other regions of the world.
Seasonal prevalence of HBoV infections vary among reports [1, 22, 23], likely because of differences in climates, recruitment criteria, study periods and other factors. In this study, the seasonal patterns of HBoV infection alone peaked in August, in agreement with our previous findings [24]. However, peak incidence of HBoV coinfection with other viruses was found in November, which has not been reported previously. The reason may be that HRV and RSV, the most frequently co-detected viruses in this research, are prevalent in November in Suzhou City [21, 25].
As shown above, patients of age from 12 to 23 months had the highest rate of HBoV infection alone, corroborating most previous studies [26, 27]. However, the highest rate of HBoV coinfection with other viruses was recorded in patients of age from 6 to 11 months, which has not been previously reported. This might be because patients <6 months are likely protected from viral infections by maternal antibodies [28]; meanwhile, patients ≥6 months, because of waning levels of maternally acquired antibodies, are susceptible to many viruses.
In the present study, more patients had symptom duration ≥14 days before admission in the HBoV coinfection with RSV group compared with HBoV infection alone group (50.0% vs. 30.1%, p = 0.033) and RSV infection alone group (50.0% vs. 17.7%, p < 0.001), which was not observed in the previous studies. This indicated that patients with HBoV coinfection with RSV often present slower disease progression.In addition, patients with HBoV infection alone or HBoV coinfection with RSV had higher Neutrophil count and CRP than patients with RSV infection alone, which may indicate the strong inflammatory reaction.
The present study showed that patients with HBoV coinfection with RSV had higher rates of pneumonia than patients with HBoV infection alone and RSV infection alone, and median length of hospitalization in patients with HBoV coinfection with RSV coinfection was longer than that of the HBoV infection along group [9.0 (7.2–10.0) days vs. 7.0 (6.0–9.0) days, p = 0.001], which is consistent with the study of Calvo [29]; this indicates that HBoV patients with RSV coinfection present higher disease severity. It was shown that cloned HBoV1 inhibits Sendai virus-induced interferon production in vitro [30], suggesting that it may also affect the host responses against other viruses in vivo, for example RSV.
We further evaluated the correlation between the HBoV DNA copy number and the length of hospitalization. We found that HBoV DNA copy numbers [383 000 (4430–10 000 000) copies/ml] were positively correlated with the duration of hospitalization (r = 0.334, p < 0.001), which is consistent with our research before [24].
Taken together, patients with HBoV coinfection with RSV often present slow disease progression are prone to suffer pneumonia and have high disease severity, compared with individuals with HBoV infection alone or RSV infection alone.
The present study has potential limitations. First, a diagnostic test for bacterial pathogens was not performed, and disease severity may be overrepresented. Second, only inpatients with ARI were enrolled, and results are not generalizable to outpatients. Third, the pediatric early warning system can be used to assess the severity of disease [31], and it includes the following parameters: heart rate, respiratory rate, systolic blood pressure, pulses, O2 saturation, capillary refill, level of consciousness measured with the Glasgow Coma Scale, oxygen therapy, bolus fluid and temperature. However, because of the retrospective design of the present study, some parameters were missed in the medical records, and further prospective study is potential needed.
In conclusion, these findings suggested that HBoV coinfection with other viruses and HBoV infection alone has different epidemiological characteristics, and RSV increases the disease severity of HBoV infection.
ACKNOWLEDGEMENTS
The authors are grateful to all technicians of the Diagnostic Microbiology Laboratory, the Children’s Hospital of Soochow University, for technical contributions.
ETHICS STATEMENT
This study was approved by the Ethics Committee of Soochow University, and written informed consent was obtained from the parents.
FUNDING
This work was supported by a grant from the National Natural Science Foundation of China (grant number 81573167), Science and Technology Project of Jiangsu (grant number BE2017657) and Livelihood science and Technology of Suzhou (grant number SYS201640).
References
- 1. Allander T, Tammi MT, Eriksson M, et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 2005;102:12891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allander T. Human bocavirus. J Clin Virol 2008;41:29–33. [DOI] [PubMed] [Google Scholar]
- 3. Lindner J, Karalar L, Schimanski S, et al. Clinical and epidemiological aspects of human bocavirus infection. J Clin Virol 2008;43:391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tozer SJ, Lambert SB, Whiley DM, et al. Detection of human bocavirus in respiratory, fecal, and blood samples by real-time PCR. J Med Virol 2009;81:488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang L, Zong ZY, Liu YB, et al. PCR versus serology for diagnosing Mycoplasma pneumoniae infection: a systematic review & meta-analysis. Indian J Med Res 2011;134:270–80. [PMC free article] [PubMed] [Google Scholar]
- 6. Macao P, Dias A, Azevedo L, et al. Acute bronchiolitis: a prospective study. Acta Med Port 2011;24:407–12. [PubMed] [Google Scholar]
- 7. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015;372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franz A, Adams O, Willems R, et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol 2010;48:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naghipour M, Cuevas LE, Bakhshinejad T, et al. Human bocavirus in Iranian children with acute respiratory infections. J Med Virol 2007;79:539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manning A, Russell V, Eastick K, et al. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis 2006;194:1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007;44:904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arden KE, McErlean P, Nissen MD, et al. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol 2006;78:1232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jartti T, Hedman K, Jartti L, et al. Human bocavirus-the first 5 years. Rev Med Virol 2012;22:46–64. [DOI] [PubMed] [Google Scholar]
- 14. Hasegawa K, Jartti T, Mansbach JM, et al. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis 2015;211:1550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moesker FM, van Kampen JJ, van der Eijk AA, et al. Human bocavirus infection as a cause of severe acute respiratory tract infection in children. Clin Microbiol Infect 2015;21:964.e961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. da Silva ER, Pitrez MC, Arruda E, et al. Severe lower respiratory tract infection in infants and toddlers from a non-affluent population: viral etiology and co-detection as risk factors. BMC Infect Dis 2013;13:41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scotta MC, Chakr VC, de Moura A, et al. Respiratory viral coinfection and disease severity in children: a systematic review and meta-analysis. J Clin Virol 2016;80:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McIntosh K. CommentaryMcIntosh K, Chao RK, Krause HE, Wasil R, Mocega HE, Mufson MA. Coronavirus infection in acute lower respiratory tract disease of infants. J Infect Dis 1974; 130: 502-7. J Infect Dis 2004;190:1033–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Denny FW, Clyde WA Jr. Acute lower respiratory tract infections in nonhospitalized children. J Pediatr 1986;108:635–46. [DOI] [PubMed] [Google Scholar]
- 20. Lim WS, Baudouin SV, George RC, et al. Pneumonia guidelines committee of the BTSSoCC (2009) BTS guidelines for the management of community acquired pneumonia in adults: update. Thorax 2009;64:1–55. [DOI] [PubMed] [Google Scholar]
- 21. Chen ZR, Ji W, Wang YQ, et al. Etiology of acute bronchiolitis and the relationship with meteorological conditions in hospitalized infants in China. J Formos Med Assoc 2014;113:463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pozo F, Garcia-Garcia ML, Calvo C, et al. High incidence of human bocavirus infection in children in Spain. J Clin Virol 2007;40:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi EH, Lee HJ, Kim SJ, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis 2006;43:585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang W, Yin F, Zhou W, et al. Clinical significance of different virus load of human bocavirus in patients with lower respiratory tract infection. Sci Rep 2016;6:20246.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun H, Sun Q, Jiang W, et al. Prevalence of rhinovirus in wheezing children: a comparison with respiratory syncytial virus wheezing. Braz J Infect Dis 2016;20:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tran DN, Nguyen TQ, Nguyen TA, et al. Human bocavirus in children with acute respiratory infections in Vietnam. J Med Virol 2014;86:988–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahn JG, Choi SY, Kim DS, Kim KH.. Human bocavirus isolated from children with acute respiratory tract infections in Korea, 2010-2011. J Med Virol 2014;86:2011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Endo R, Ishiguro N, Kikuta H, et al. Seroepidemiology of human bocavirus in Hokkaido prefecture, Japan. J Clin Microbiol 2007;45:3218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calvo C, Garcia-Garcia ML, Pozo F, et al. Respiratory syncytial virus coinfections with rhinovirus and human bocavirus in hospitalized children. Medicine 2015;94:e1788.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Z, Zheng Z, Luo H, et al. Human bocavirus NP1 inhibits IFN-beta production by blocking association of IFN regulatory factor 3 with IFNB promoter. J Immunol 2012;189:1144–53. [DOI] [PubMed] [Google Scholar]
- 31. Duncan H, Hutchison J, Parshuram CS.. The pediatric early warning system score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care 2006;21:271–8. [DOI] [PubMed] [Google Scholar]