Abstract
Background. Annually, Saudi Arabia is the host of the Hajj mass gathering. We aimed to determine the Middle East respiratory syndrome coronavirus (MERS-CoV) nasal carriage rate among pilgrims performing the 2013 Hajj and to describe the compliance with the Saudi Ministry of Health vaccine recommendations.
Method. Nasopharyngeal samples were collected from 5235 adult pilgrims from 22 countries and screened for MERS-CoV using reverse transcriptase–polymerase chain reaction. Information regarding the participants' age, gender, country of origin, medical conditions, and vaccination history were obtained.
Results. The mean age of the screened population was 51.8 years (range, 18–93 years) with a male/female ratio of 1.17:1. MERS-CoV was not detected in any of the samples tested (3210 pre-Hajj and 2025 post-Hajj screening). According to the vaccination documents, all participants had received meningococcal vaccination and the majority of those from at-risk countries were vaccinated against yellow fever and polio. Only 22% of the pilgrims (17.5% of those ≥65 years and 36.3% of diabetics) had flu vaccination, and 4.4% had pneumococcal vaccination.
Conclusion. There was no evidence of MERS-CoV nasal carriage among Hajj pilgrims. While rates of compulsory vaccinations uptake were high, uptake of pneumococcal and flu seasonal vaccinations were low, including among the high-risk population.
Keywords: Hajj pilgrimage, MERS-CoV, nasal carriage, screening, vaccination
The Middle East respiratory syndrome coronavirus (MERS-CoV) is a newly emerged respiratory virus with initial high fatality rate among identified cases [1]. MERS-CoVis an enveloped, single-stranded, positive-sense RNA virus in lineage C of the genus Betacoronavirus within the subfamily Coronavirinae [2, 3]. The first case of MERS-CoV was identified in a patient with acute pneumonia and renal failure in Jeddah, Kingdom of Saudi Arabia (KSA) in June 2012 [2]. As of 27 January 2014, the World Health Organization (WHO) has been notified of 180 laboratory-confirmed MERS-CoV cases from 9 countries (France, Germany, Italy, Jordan, KSA, Qatar, Tunisia, United Arab Emirates [UAE], and the United Kingdom [UK] [1], all with a direct or indirect link with the Middle East. Of these cases, 77 patients (42.7%) have died [4].
While the majority of cases now reported have likely acquired infection through human-to-human transmission, the primary sporadic cases in clusters are more likely to have been acquired through contact with nonhuman sources of the virus [1]. Transmission could be through respiratory droplets, or direct or indirect contact [5]. Hospital outbreaks [5–7] and family clusters were described [8]. Travel-associated cases were observed in Europe, notably in UK, France, Germany, and Italy, with secondary cases in close contacts of index cases without a travel history [1].
Seventy-six percent of patients with MERS-CoV infection had at least 1 underlying medical condition, and fatal cases were more likely to have an underlying condition (86.8% among fatal cases vs 42.4% among recovered or asymptomatic cases; P < .001) [1]. In a study of 47 confirmed patients from KSA identified between September 2012 and June 2013, almost all (45/47) cases had at least 1 underlying condition, including diabetes (68%), hypertension (34%), chronic cardiac disease (28%), and chronic renal disease (49%) [9].
The role of asymptomatic carriers in virus transmission and the prevalence among the general population is unknown. Asymptomatic carriage of MERS-CoV has been described [1], including among family contacts [10]. In a study of 7 healthcare workers with MERS-CoV infection, 2 of them were asymptomatic and 5 had mild upper-respiratory-tract symptoms [11]. Asymptomatic carriers of the virus could be a source of transmission and infection, especially among the high-risk population. Hence, the potential of global spread of MERS-CoV is great if asymptomatic pilgrims were to be identified.
KSA annually hosts more than 2 million Muslim pilgrims from around 184 countries during the Hajj pilgrimage, making it one of the largest and most culturally and geographically diverse mass gatherings in the world [12]. The presence of elderly pilgrims from across the globe and being in close contact to perform physically exhausting religious rites increases their susceptibility to infections and creates conditions with a potential transmission of respiratory pathogens [13, 14]. Given the predicted population movement out of Saudi Arabia, the potential for worldwide spread of MERS-CoV is a continued concern [15]. To reduce the risk of infectious diseases transmission during the Hajj, the Saudi Ministry of Health (MoH) issues health conditions and recommendations for travelers to KSA for the Hajj pilgrimage, including vaccinations requirement [16]. Recommendations specific to the emergence of MERS-CoV have been issued and at-risk individuals coming for Hajj in 2013 were advised to postpone the performance of the Hajj for their own safety [17].
In order to evaluate the compliance with the Hajj recommendations and to evaluate the possibility of acquiring MERS-CoV during the 2013 Hajj, we undertook this cross-sectional study to screen 2 pilgrims' cohorts (beginning of Hajj cohort and end of Hajj cohort) for MERS-CoV, and we sought to describe the compliance with the Saudi MoH health recommendations.
METHODS
Study Population
The screening was conducted on 5235 adult (>18 years of age) pilgrims performing the 2013 Hajj. The sample size was calculated based on the number of pilgrims attending the 2012 Hajj season with 95% confidence level and 2% error margin. The countries of the study population were preselected to cover a wide range of pilgrims from different continents. The selection reflected the Hajj pilgrims' population, and included countries with MERS-CoV cases, countries with close geographic proximity to KSA, and countries with frequent and significant population movement from and to KSA. These countries included those with the highest annual number of Hajj pilgrims. The individuals chosen for the trail from the selected flights were recruited on a voluntary and random basis (by selecting every 4th pilgrim from the queue at the airport processing area upon arrival or before boarding returning flights) regardless of their age, gender, or medical conditions (excluding children). A standardized data collection form was used to obtain information regarding the participants' age, gender, country of origin, and medical conditions. Vaccination history was determined from the participants' vaccination documents.
Screaming and Processing of the Samples
Nasopharyngeal samples were collected from all participants by trained physicians. Collection, handling, and storage of the samples were done as per the CDC's guidelines for patients under investigation for MERS-CoV [18]. A single nasopharyngeal swab was obtained from each pilgrim using a swab with synthetic fiber with a plastic shaft (Remel). The samples were then immediately placed in viral transport medium, transported on ice to the lab, and stored at −70°C. The screening process was conducted at the Hajj terminal of King Abdulaziz International Airport at 2 time periods: a pre-Hajj screening (which included 3210 pilgrims and was conducted in the period between 29th of September and 9th of October 2013 as the pilgrims were arriving to KSA for the Hajj), and a post-Hajj screening (which involved another 2025 pilgrims and was conducted at the end of the Hajj between the 14th and 26th of October 2013 as pilgrims were returning to their respective countries).
Detection of MERS-CoV in Samples
MERS-CoV was detected in the samples using reverse transcriptase–polymerase chain reaction (RT-PCR) targeting the region upstream of the E gene (upE) and the open reading frame 1a (nsp6 protein) as described previously [19, 20]. Briefly, nucleic acid was purified from a 200 µL volume of sample using Magna Pure LC nucleic acid extraction kit (Roche). Each sample was independently tested with the 2 RT-PCR assays in a 25 µL reaction containing 5 µL RNA, 12.5 µL of 2X buffer (SuperScript® III one-Step RT-PCR with Platinum Taq® [Invitrogen]), 0.4 µL MgCl2 (50 mM), 1 µL forward primer (10 µM), 1 µL reverse primer (10 µM), 1 µL probe (5 µM), 3.1 µL RNAse-free H2O2, and 1 µL SSIII/Platinum Taq enzyme mix (1 U). The RT-PCR reactions were performed in a real-time LightCycler 480 machine (Roche) under the following cycling profile: 1 cycle of 55°C for 20 minutes followed by 1 cycle of 94°C for 3 minutes, then 45 cycles of 94°C for 15 seconds, 45 cycles of 58°C for 30, seconds and a single cycle of 40°C for 30 seconds. A sample was confirmed MERS-CoV positive if both RT-PCR assays were positive as per current recommendations [20].
Ethics
The study was approved by the King Fahad Medical City Institutional Review Board. All participants were recruited on a voluntary basis and gave verbal consent before being included in the study.
RESULTS
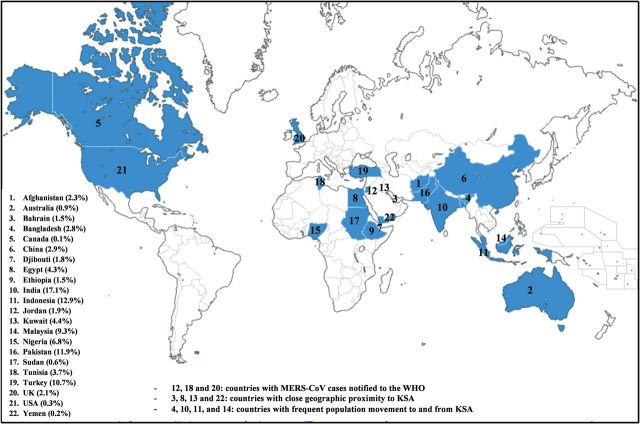
Samples were obtained from 5235 pilgrims (3210 pre-Hajj, 2025 post-Hajj) representing 22 countries (Figure 1) from Asia, Africa, Australia, North America, and Europe. The characteristics of the screened population are summarized in Table 1. The mean age was 51.8 years (range, 18–93 years) with a male/female ratio of 1.17:1. Approximately 15% of the population screened was 65 years of age or more. Most participants were from Asia (78%), followed by Africa (18.7%), then Europe (2.1%). India was the most represented country with 17.1% of the screened population, followed by Indonesia (12.9%), Pakistan (11.9%), and Turkey (10.7%). Pilgrims from Yemen, Afghanistan, China, Ethiopia, Sudan, Djibouti, and Canada were represented in the post-Hajj screening only. Bangladesh, Turkey, India, Indonesia, Malaysia, Pakistan, Egypt, and Nigeria were represented in both pre- and post-Hajj screenings. The screened population included 410 pilgrims from 3 out of the 9 countries with MERS-CoV cases notified to the WHO (Jordan, Tunisia, and UK).
Figure 1.
Percentage contribution from each country and geographical spread of the 5235 screened pilgrims.
Table 1.
Characteristics of the Screened Population
| Number | (Number/Total Number) | % | |
|---|---|---|---|
| Pilgrims screened | |||
| Pre-Hajj | 3210 | 3210/5235 | 61.3 |
| Post-Hajj | 2025 | 2025/5235 | 38.7 |
| Total screened | 5235 | 5235/5235 | 100 |
| Gender | |||
| Males | 2831 | 2831/5235 | 54 |
| Females | 2404 | 2404/5235 | 46 |
| Age (mean = 51.8) | |||
| <18 | 0 | 0/5213 | 0 |
| 18–64 | 4413 | 4413/5213 | 84.6 |
| ≥65 | 800 | 800/5213 | 15.4 |
| Continent | |||
| Asia | 4080 | 4096/5235 | 78 |
| Africa | 979 | 980/5235 | 18.7 |
| Australia | 46 | 48/5235 | 0.9 |
| North America | 19 | 21/5235 | 0.3 |
| Europe | 111 | 112/5235 | 2.1 |
| Vaccination history | |||
| Preflu vaccination | 1149 | 1157/5235 | 22 |
| ≥65 y of age | 140 | 140/800 | 17.5 |
| Diabetes | 4 | 4/11 | 36.3 |
| Prepneumococcal vaccination | 232 | 237/5235 | 4.4 |
| ≥65 y of age | 12 | 12/800 | 1.5 |
| Diabetes | 3 | 3/11 | 27.3 |
| Pre–yellow fever vaccination | 465 | 466/5235 | 8.8 |
| Nigeria | 357 | 357/357 | 100 |
| Sudan | 30 | 30/30 | 100 |
| Ethiopia | 78 | 78/78 | 100 |
| Other countries | 0 | 0 | 0 |
| Prepolio vaccination | 2228 | 2228/5235 | 42.5 |
| Nigeria | 358 | 358/358 | 100 |
| Pakistan | 615 | 615/623 | 98.7 |
| India | 893 | 893/894 | 99.9 |
| Afghanistan | 121 | 121/121 | 100 |
| Other countries | 599 | 599/599 | 100 |
| Premeningococcal vaccination | 5229 | 5229/5229 | 100 |
| ACYW135 | 4861 | 4861/5229 | 93 |
| CSM | 354 | 354/5229 | 6.8 |
| MCV4 | 10 | 10/5229 | 0.2 |
| Underlying health conditions | |||
| Diabetes | 11 | 11/161 | 6.8 |
| Hypertension | 21 | 21/160 | 13.1 |
Abbreviations: ACYW135, quadrivalent vaccine against meningitis; CSM, cerebrospinal meningitis; MCV4, meningococcal conjugate vaccine 4.
According to the vaccination documents, all participants received meningococcal vaccination; 93% were vaccinated with the recommended quadrivalent vaccine (serogroups A, C, W-135, and Y). A minority of pilgrims (6.8%), all from Nigeria, were immunized against cerebrospinal meningitis (CSM, meningococcal serogroup “A”) without further details on the type of vaccine used. Ten pilgrims, 9 of whom were from the United States, were vaccinated with the meningococcal conjugate vaccines MCV4. Only 22% of the pilgrims screened had flu vaccination, including 17.5% of those ≥65 years of age and 36.3% of the diabetics. The overall vaccination rate for yellow fever was 8.8% (among all pilgrims), but 100% for pilgrims from countries that are required to have such vaccination (Nigeria, Sudan, and Ethiopia). Approximately 43% of all pilgrims and 99.5% of those from at-risk countries (Pakistan, India, Nigeria, or Afghanistan) received polio vaccination. Only 4.4% of all pilgrims, 1.5% of those ≥65 years old and 27.3% of those with diabetes, had pneumococcal vaccination. No information on the type of pneumococcal or polio vaccines could be obtained from the pilgrims' vaccination documents. Data on underlining health conditions was available for only 3% of the screened population. Nevertheless, 13.1% had hypertension and 6.8% were diabetic.
MERS-CoV was not detected in any of the 5235 (3210 pre-Hajj, 2025 post-Hajj) nasopharyngeal samples tested.
DISCUSSION
We screened 5235 pilgrims attending the 2013 Hajj season for nasal carriage of the emerging respiratory virus, MERS-CoV. These included pilgrims from 3 out of the 9 countries with MERS-CoV cases notified to the WHO (Jordan, Tunisia, and UK) in addition to a number of countries with close geographic proximity to KSA (Bahrain, Yemen, Kuwait, and Egypt) and with frequent and significant population movement to and out of KSA (Bangladesh, Pakistan, India, Malaysia, and Indonesia). The screened population was over-represented by Asian countries (78%), and 60% of the pilgrims were ≥50 years of age, which is representative of the general Hajj pilgrims population. No samples were positive for the presence of the MERS-CoV virus by the 2 RT-PCR assays.
These results show that among the population screened, there was a lack of MERS-CoV nasal carriage. Moreover, the lack of nasal carriage of the virus among the post-Hajj cohort suggests that there were no events of MERS-CoV acquisition in the cohort once the pilgrims have been in KSA, performed the Hajj, and were in close contact with other pilgrims and the local population. These results are in accordance with a previous cohort survey conducted during the 2012 Hajj, which showed lack of nasal carriage of MERS-CoV among French pilgrims returning from Hajj [21]. A cohort of 154 French Hajj pilgrims were systematically sampled with nasal swabs prior to returning to France, and screened for MERS-CoV using the same RT-PCR assay used in our study. Despite a high rate of respiratory symptoms (83.4%), including 41.0% influenza-like illness, no case of MERS-CoV nasal carriage was detected [21]. Although our cohort represents a small proportion of the overall Hajj pilgrim's population (>1.9 million in 2013), the results showed a lack of nasal carriage of MERS-CoV among pilgrims. In addition, the results showed the lack of transmission of the virus among pilgrims.
Given the predicted population movement out of Saudi Arabia, the potential for worldwide spread of MERS-CoV exists according to Khan and colleagues [15]. By contrast, Breban and colleagues [22] calculated that the risk of MERS-CoV to have pandemic potential does not exceed 5%, but they did not take into account the effect of the Hajj mass gathering in their scenario. Memish et al [23] showed that circulation of the MERS-CoV in Saudi Arabia is much lower than it was feared, announced, or predicted, and that no significant rise in detection rates could be observed along 1 year. In addition, mass gatherings in KSA during the pilgrimages of 2012 and 2013 were associated neither with an increased number of cases, nor with reported clusters of cases [24], suggesting poor or moderate interhuman transmission. Our results support the latter and that MERS-CoV in its current form may not have the pandemic potential as those of other respiratory viruses, including that of severe acute respiratory syndrome coronavirus [22].
Due to the MERS-CoV situation, the Saudi MoH recommended that elderly people (>65 years), people with chronic diseases (eg, heart disease, kidney disease, respiratory disease, and diabetes), and pilgrims with immune deficiency (congenital and acquired), malignant disease, and terminal illnesses, as well as pregnant women and children (<12 years), postpone the performance of the 2013 Hajj for their own safety. This advice was also endorsed by the US CDC for pilgrims traveling to Saudi Arabia for the 2013 Hajj. In our study, 15.3% of the population was ≥65 years old, and although data on underling health conditions were available for a minority of the cohort and only related to diabetes and hypertension, 18.7% had at least 1 of these 2 conditions. Both are disorders for which the Saudi MoH recommended potential participants postpone doing the Hajj in 2013. Although our results cannot be extrapolated to all Hajj pilgrims, they suggest that despite the MoH recommendations, a proportion of the pilgrims were unaware or did ignore this advice. This is not without precedent; a study of a cohort of 167 French pilgrims participating in the 2012 Hajj season found that 39% were over 65 years of age and 59% had at least 1 risk factor mentioned in the MERS-CoV MoH recommendations [21]. In 2013, in preparation of the Hajj, a similar study (360 French cohort) reported that 30.8% were over 65 years of age, and nearly half had at least 1 disorders for which the Saudi MoH recommended potential participants postpone doing the Hajj [25]. In the latter study, nearly 65% of the respondents were aware of the ongoing MERS epidemic in the KSA and 35.3% were aware of the Saudi MoH recommendations for at-risk pilgrims to postpone performing the Hajj in 2013. Among 179 at-risk individuals, none decided to cancel their participation in the Hajj, even after advice during pretravel consultation [25].
In addition to the MERS-CoV recommendations, the Saudi MoH has a set of other health conditions for travelers to KSA for Hajj [16]. Seasonal influenza vaccination is recommended for international pilgrims before arrival into KSA, particularly those at increased risk of severe influenza diseases, including pregnant women, children <5 years of age, the elderly, and individuals with underlying health conditions (such as HIV/AIDS, asthma, diabetes, and chronic heart or lung diseases) [16]. The rate of seasonal influenza vaccination was low in our study, including among the high-risk population. Only 22% of the screened population (17.5% of those ≥65 years and 36.3% of those with diabetes) was vaccinated.
In accordance with the International Health Regulations 2005 [26], yellow fever vaccination is mandatory for all travelers arriving from countries or areas at risk of yellow fever. Travelers should show evidence of vaccination at least 10 days and at most 10 years before arrival to KSA. Three countries at risk of yellow fever transmission (as defined by the International Travel and Health 2012 [27]) were represented in our cohort population: Nigeria, Sudan, and Ethiopia. Although the overall yellow fever vaccination rate was low (8.8%), all (465/465) pilgrims from these 3 high-risk countries were vaccinated against yellow fever [16].
According to the Saudi MoH health regulations, proof of polio vaccination at least 6 weeks prior to departure to KSA is mandatory for all travelers arriving from polio-endemic countries and reestablished transmission countries, namely, Afghanistan, Chad, Nigeria, and Pakistan, as well as from recently endemic countries at high risk of reimportation of poliovirus (ie, India) [16]. The overall polio vaccination rate was 42.5%, but 99.5% of all pilgrims from high-risk countries (Afghanistan, Pakistan, India, and Nigeria) were vaccinated against polio, according to their vaccination documents.
Vaccination with the quadrivalent ACYW135 vaccine against meningitis is required for all pilgrims coming to KSA regardless of their country of origin. Travelers should show evidence of vaccination no more than 3 years and no less than 10 days before arrival to KSA. In addition, pilgrims from the African meningitis belt are automatically administered ciprofloxacin tablets (500 mg) chemoprophylaxis at port of entry to KSA to lower the rate of carriers [16]. All pilgrims screened were vaccinated against meningitis according to their documents, 93% of whom were vaccinated with the recommended quadrivalent vaccine for serogroups A, C, W-135, and Y.
Pneumococci are estimated to cause 1.6 million deaths annually [28]. Pneumococcal vaccines are widely available, and vaccination is recommended for all adults aged ≥65 years and for adults at high risk who are aged 19–64 years and are immunocompromised [29]. Of note, a considerable percentage of Hajj pilgrims have preexisting illnesses or are elderly, both important risk factors for pneumococcal infection [29, 30]. Nevertheless, the rate of pneumococcal vaccination among our cohort was low, including among those ≥65 years of age.
Our study has some limitations. Although the cohort screened was large, it only represented a relatively small percentage of the total number of pilgrims attending the 2013 Hajj; hence, the results cannot be extrapolated to all the pilgrims' population. The use of upper-respiratory-tract swabs instead of lower-respiratory specimens may have influenced the results. The MERS-CoV load in upper-respiratory-tract specimens is generally lower than in the lower-respiratory specimens, though data are limited. The data on vaccination are based on documentations provided by the pilgrims, and without a possibility of verifying the authenticity of these documents, the rate of vaccination in our study may be an overestimation. In addition, due to the pilgrims' age and language barriers, data on underlining health conditions were difficult to obtain and was available for a limited number of subjects only.
Nevertheless, our study investigated a much larger and geographically more diverse cohort than previous studies [21, 25], and found a lack of MERS-CoV nasal carriage among pilgrims arriving to perform the 2013 Hajj and among those returning to their respective countries at the end of the pilgrimage. This suggests low carriage and transmission rate of the virus, although further investigations are warranted. Adherence to the Saudi MoH vaccination recommendations for travelers to the Hajj was variable. While the rates of compulsory vaccinations against meningitis, polio, and yellow fever were high for the targeted populations, uptake of pneumococcal and flu seasonal vaccinations were low, including among the high-risk population. International collaboration between the Saudi MoH and the different countries through the collaborative center for mass gathering and with input from WHO is being formulated to enhance the compliance with the recommended vaccinations.
Notes
Financial support. This work was supported by the Saudi Ministry of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.The Who Mers-Cov Research Group - State of Knowledge and Data Gaps of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Humans. PLoS Curr. 2013;5 doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.van Boheemen S, de Graaf M, Lauber C, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3:ii. doi: 10.1128/mBio.00473-12. e00473–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Middle East respiratory syndrome coronavirus (MERS-CoV)–update. 2014. http://www.who.int/csr/don/2014_01_27mers/en/index.html. Accessed 2 February 2014.
- 5.Assiri A, McGeer A, Perl TM, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–16. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memish ZA, Al-Tawfiq JA, Assiri A. Hospital-associated Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;369:1761–2. doi: 10.1056/NEJMc1311004. [DOI] [PubMed] [Google Scholar]
- 7.Guery B, Poissy J, el ML, et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–72. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–94. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 9.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–61. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omrani AS, Matin MA, Haddad Q, Al-Nakhli D, Memish ZA, Albarrak AM. A family cluster of Middle East Respiratory Syndrome Coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17:e668–72. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369:884–6. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
- 12.Memish ZA, Al-Rabeeah AA. Public health management of mass gatherings: the Saudi Arabian experience with MERS-CoV. Bull World Health Organ. 2013;91:899–899A. doi: 10.2471/BLT.13.132266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautret P, Parola P, Brouqui P. Relative risk for influenza like illness in French Hajj pilgrims compared to non-Hajj attending controls during the 2009 influenza pandemic. Travel Med Infect Dis. 2013;11:95–7. doi: 10.1016/j.tmaid.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Alzeer AH. Respiratory tract infection during Hajj. Ann Thorac Med. 2009;4:50–3. doi: 10.4103/1817-1737.49412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan K, Sears J, Hu VW, et al. Potential for the international spread of Middle East respiratory syndrome in association with mass gatherings in Saudi Arabia. PLoS Curr. 2013 doi: 10.1371/currents.outbreaks.a7b70897ac2fa4f79b59f90d24c860b8. 5. pii: ecurrents.outbreaks.a7b70897ac2fa4f79b59f90d24c860b8. doi: 10.1371/currents.outbreaks.a7b70897ac2fa4f79b59f90d24c860b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Memish ZA, Al-Rabeeah AA. Health conditions of travellers to Saudi Arabia for the pilgrimage to Mecca (Hajj and Umra) for 1434 (2013) J Epidemiol Glob Health. 2013;3:59–61. doi: 10.1016/j.jegh.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kingdom of Saudi Arabia Ministry of Health. Health Regulations for travellers to Saudi Arabia for Umrah & Hajj—1434 (2013) 2013. http://www.moh.gov.sa/en/Hajj/Pages/Healthregulations.aspx. Accessed 2 February 2014.
- 18.CDC. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Patients Under Investigation (PUIs) for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) 2014. http://www.cdc.gov/coronavirus/mers/guidelines-clinical-specimens.html. Accessed 2 February 2014.
- 19.Corman VM, Eckerle I, Bleicker T, et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17:ii. doi: 10.2807/ese.17.39.20285-en. 20285. [DOI] [PubMed] [Google Scholar]
- 20.Corman VM, Muller MA, Costabel U, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17:ii. doi: 10.2807/ese.17.49.20334-en. 20334. [DOI] [PubMed] [Google Scholar]
- 21.Gautret P, Charrel R, Belhouchat K, et al. Lack of nasal carriage of novel corona virus (HCoV-EMC) in French Hajj pilgrims returning from the Hajj 2012, despite a high rate of respiratory symptoms. Clin Microbiol Infect. 2013;19:E315–7. doi: 10.1111/1469-0691.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breban R, Riou J, Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet. 2013;382:694–9. doi: 10.1016/S0140-6736(13)61492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Screening for Middle East Respiratory Syndrome Coronavirus Infection in Hospital patients and their Health care Worker and Family Contacts: a prospective descriptive study. Clin Microbiol Infect. 2014 doi: 10.1111/1469-0691.12562. . [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rashid H, Azeem MI, Heron L, Haworth E, Booy R, Memish ZA. Has Hajj-associated MERS-CoV transmission occurred? The case for effective post-Hajj surveillance for infection. Clin Microbiol Infect. 2014;20:273–6. doi: 10.1111/1469-0691.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautret P, Benkouiten S, Salaheddine I, et al. Hajj pilgrims knowledge about Middle East respiratory syndrome coronavirus, August to September 2013. Euro Surveill. 2013;18:20604. doi: 10.2807/1560-7917.es2013.18.41.20604. [DOI] [PubMed] [Google Scholar]
- 26.WHO. International Health Regulations. 2005. http://www.who.int/ihr. Accessed 2 February 2014.
- 27.WHO. International Travel and Health. 2012. http://www.who.int/ith. Accessed 2 February 2014.
- 28.WHO. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2012;61:816–9. [PubMed] [Google Scholar]
- 30.Rashid H, Abdul Muttalif AR, Mohamed Dahlan ZB, et al. The potential for pneumococcal vaccination in Hajj pilgrims: expert opinion. Travel Med Infect Dis. 2013;11:288–94. doi: 10.1016/j.tmaid.2013.06.001. [DOI] [PubMed] [Google Scholar]