Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) causes severe disease in human with an overall case-fatality rate of >35%. Effective antivirals are crucial for improving the clinical outcome of MERS. Although a number of repurposed drugs, convalescent-phase plasma, antiviral peptides, and neutralizing antibodies exhibit anti-MERS-CoV activity in vitro, most are not readily available or have not been evaluated in nonhuman primates. We assessed 3 repurposed drugs with potent in vitro anti-MERS-CoV activity (mycophenolate mofetil [MMF], lopinavir/ritonavir, and interferon-β1b) in common marmosets with severe disease resembling MERS in humans. The lopinavir/ritonavir-treated and interferon-β1b-treated animals had better outcome than the untreated animals, with improved clinical (mean clinical scores ↓50.9%–95.0% and ↓weight loss than the untreated animals), radiological (minimal pulmonary infiltrates), and pathological (mild bronchointerstitial pneumonia) findings, and lower mean viral loads in necropsied lung (↓0.59–1.06 log10 copies/glyceraldehyde 3-phosphate dehydrogenase [GAPDH]; P < .050) and extrapulmonary (↓0.11–1.29 log10 copies/GAPDH; P < .050 in kidney) tissues. In contrast, all MMF-treated animals developed severe and/or fatal disease with higher mean viral loads (↑0.15–0.54 log10 copies/GAPDH) than the untreated animals. The mortality rate at 36 hours postinoculation was 67% (untreated and MMF-treated) versus 0–33% (lopinavir/ritonavir-treated and interferon-β1b-treated). Lopinavir/ritonavir and interferon-β1b alone or in combination should be evaluated in clinical trials. MMF alone may worsen MERS and should not be used.
Keywords: animal, common marmoset, coronavirus, interferon, Kaletra, lopinavir, MERS, mycophenolate, primate, treatment
Coronaviruses (CoVs) have repeatedly crossed species barriers and caused epidemics with significant socioeconomic impact [1]. Middle East respiratory syndrome coronavirus (MERS-CoV) is a novel zoonotic lineage C βCoV discovered in 2012 [2]. MERS-CoV infection in human, or MERS, commonly manifests as a severe acute respiratory disease, which is often complicated by acute respiratory distress syndrome and multiorgan failure [3, 4]. Clinical deterioration and death occur rapidly and at a few days earlier in MERS than in severe acute respiratory syndrome (SARS) [3]. The case-fatality rate of MERS is the highest among all human CoV infections and exceeds 35% [3, 5]. Effective antiviral treatment, especially when given early to patients at risk of developing severe disease, may improve the clinical outcome of MERS. A number of new drug compounds, such as humanized or human monoclonal antibodies against the S1 subunit receptor-binding domain and antiviral peptides targeting the S2 subunit heptad repeat 2 domain of the MERS-CoV spike glycoprotein, have recently been developed and showed potent anti-MERS-CoV activity in vitro [6–9]. However, their developments are still in the preclinical phase. Another possible treatment option for MERS is convalescent-phase plasma therapy. This has been previously used to treat other severe viral respiratory infections, including SARS and pandemic influenza, with good clinical and virological response [10–12]. However, this is also not readily available, as its preparation requires the recruitment of a large number of convalescent patients with high serum antibody titers. Convalescent-phase plasma with low serum antibody titers may be associated with immune enhancement, which could lead to worsened outcome as observed in in vitro and animal experiments on SARS [13, 14].
In order to find immediately available treatment for MERS before these novel agents and convalescent-phase plasma therapy become available, we and others have applied high-throughput screening to identify existing drugs that may be repurposed to treat patients with MERS [15–17]. Examples of drugs identified using this drug discovery approach included inhibitors of virus entry, clathrin-mediated endocytosis, neurotransmitters, estrogen receptor, kinase signaling, lipid metabolism, protein processing, and DNA synthesis/repair [3, 15–19]. Although all of them exhibit in vitro anti-MERS-CoV activity, only a few are likely clinically relevant, as most are either associated with severe adverse effects or have anti-MERS-CoV half-maximal effective concentrations (EC50) that are not clinically achievable at therapeutic dosages. Previous studies have identified mycophenolate mofetil (MMF), ribavirin, lopinavir, interferon-α, and interferon-β as potential candidates for further in vivo evaluation [15–17, 20]. Treatment with ribavirin and interferon-α has shown some effects in vitro and in MERS-CoV-infected rhesus macaques with mild disease, but the benefits in MERS patients were not obvious [20–22]. In this study, we evaluated the treatment effects of MMF, lopinavir, and interferon-β in MERS-CoV-infected common marmosets. This recently described nonhuman primate model is the animal model which best represents severe human disease among all existing animal models for MERS [3, 23]. Importantly, we found that lopinavir/ritonavir and interferon-β1b, but not MMF, improved the outcome of MERS-CoV-infected common marmosets. The findings provide the basis for future clinical trials for this highly fatal disease.
METHODS
Virus Strain and Titration
MERS-CoV (EMC/2012 strain) was kindly provided by Ron Fouchier (Erasmus Medical Center). The virus was propagated in VeroE6 cells (ATCC) in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum and 100 units/mL penicillin plus 100 µg/mL streptomycin. All experiments involving live MERS-CoV followed the approved standard operating procedures of the biosafety level 3 facility as previously described [24–28]. The 50% tissue culture infectious dose (TCID50) per mL was determined for MERS-CoV in VeroE6 cells as previously described [25].
Common Marmoset Infection Model and Treatment
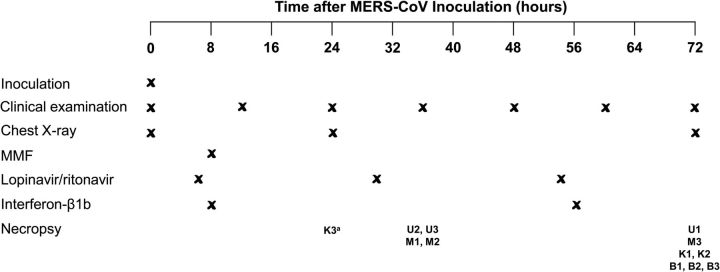
All experiments involving common marmosets were performed as previously described with slight modifications and approved by the Institutional Animal Care and Use Committee [23, 26, 29]. Briefly, 12 healthy male common marmosets (Callithrix jacchus; 3 years old; 230–395 g) were inoculated with 5 × 106 TCID50 of MERS-CoV in 500 µL DMEM intratracheally (Figure 1). The animals were randomly assigned to 1 of 4 treatment groups in a nonblinded manner (Table 1). Each treatment group included 3 animals. They were treated with sham treatment, MMF (CellCept, Roche Pharmaceuticals, Inc), lopinavir/ritonavir (4:1) (Kaletra, Abbot Laboratories), or recombinant interferon-β1b (Betaferon, Bayer Schering Pharma, Germany) using previously described dosages [30–32]. We administered MMF intraperitoneally and interferon-β1b subcutaneously instead of intravenously or orally, respectively, because the small veins of the common marmosets prevented reliable drug delivery through the intravenous route and because critically ill MERS patients usually require parenteral treatment. Lopinavir/ritonavir was only available in oral preparation. We initiated treatment with MMF and interferon-β1b at 8 hours postinoculation (hpi) according to a previously described treatment model using MERS-CoV-infected rhesus macaques [21]. Treatment with lopinavir/ritonavir was started at 6 hpi as lopinavir/ritonavir takes longer to achieve its peak serum concentration (Cmax) compared with MMF and interferon-β1b. The animals were observed twice daily for clinical signs and scored using a previously described clinical scoring system, which included the evaluation of the general appearance, skin and fur appearance, discharge (oral, nasal, and/or ocular), respiratory rate, and food consumption [23]. Other clinical examinations in this study included measurement of body temperature (twice daily) and body weight (0, 24, and 72 hpi), and blood collection for hematology tests (0 hpi and necropsy). Dorsal-ventral and lateral chest X-rays (CXR) were performed on anesthetized animals (0, 24, and 72 hpi) as previously described [29]. Necropsies were scheduled at 72 hpi or when the clinical score was ≥35 as previously described [21, 23]. The necropsied tissues were collected for histopathology, immunohistochemistry, and viral load studies.
Figure 1.
Schedule of MERS-CoV inoculation, examination, treatment, and necropsy of the common marmosets. aK3 died unexpectedly during anesthesia at 24 hpi, which was likely related to the anesthesia procedure, as common marmosets are very small and fragile. Abbreviations: hpi, hours postinoculation; MERS-CoV, Middle East respiratory syndrome coronavirus; MMF, mycophenolate mofetil.
Table 1.
Treatment Groups and Drug Regimens Used in This Study
| Group | Common Marmoset | Treatment Regimen |
|---|---|---|
| 1 | U1, U2, U3 | Untreated (sham treatment with comparable volume per kg body weight of sterile saline) |
| 2 | M1, M2, M3 | CellCept (25 mg/kg of MMF given ip once at 8 hpi) |
| 3 | K1, K2, K3 | Kaletra (12 mg/kg/day of lopinavir + 3 mg/kg/day of ritonavir given orally once daily at 6, 30, and 54 hpi) |
| 4 | B1, B2, B3 | Betaferon (0.267 million IU/kg of interferon-β1b given sc at 8 hpi and at 56 hpi) |
Abbreviations: hpi, hours postinoculation; ip, intraperitoneal; MMF, mycophenolate mofetil; sc, subcutaneous.
Histopathology and Immunohistochemistry
The sections of the necropsied tissues were stained with hematoxylin and eosin for light microscopic examination and immunohistochemically stained with mouse anti-MERS-CoV nucleocapsid protein (1:200) antibody overnight at 4°C for immunofluorescent examination of MERS-CoV nucleocapsid protein expression as described previously [27, 29, 33].
Viral Load Studies
RNA extraction and quantitative real-time reverse transcription–polymerase chain reaction (RT-PCR) were performed on necropsied lung, kidney, liver, spleen, and heart tissues as we previously described [25–27]. Three areas with the most severe macroscopic lesions were obtained from each necropsied organ for viral load studies. Briefly, total RNA and viral RNA were isolated using Trizol (Life Technologies) and Viral RNA Mini kit (QIAGEN), respectively. After RNA quantification, 1 µg RNA was reverse transcribed into complementary DNA (cDNA) using random hexamers. Total viral transcripts were detected using Novel Coronavirus 2012 Real-Time RT-PCR assay (CDC; Catalog #KT0136). The viral RNA were normalized to the messenger RNA expression level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and a spike-in control, enterovirus 71 (strain SZ/HK08–5), using primers 5′-GCTCACTGGCATGGCCTTCCGTGT-3′ and 5′-TGGAGGAGTGGGTGTCGCTGTTGA-3′ (GAPDH), and 5′-CCCCTGAATGCGGCTAATCC-3′ and 5′- ACACGGACACCCAAAGTAGT -3′ (enterovirus 71). For each reaction, equal amounts of cDNA were mixed with FS Universal SYBR Green Master Rox (Roche) plus 5 pmol each of forward and reverse primers. Amplification was done under the condition of 15 seconds at 95°C and 1 minute at 60°C for 55 cycles in a 7900 real-time PCR detection system (ABI).
Statistical Analysis
Statistical comparison between different groups was performed by the Student t test using GraphPad Prism 6. A P value <.05 was considered statistically significant.
RESULTS
Clinical and Radiological Findings
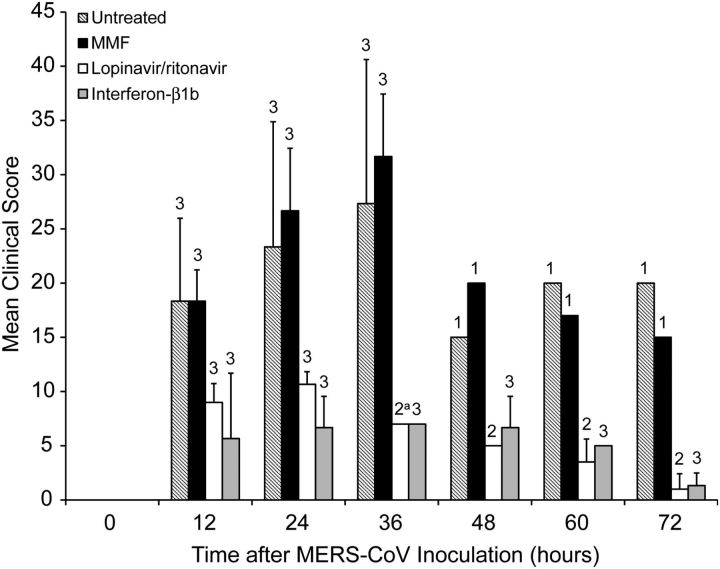
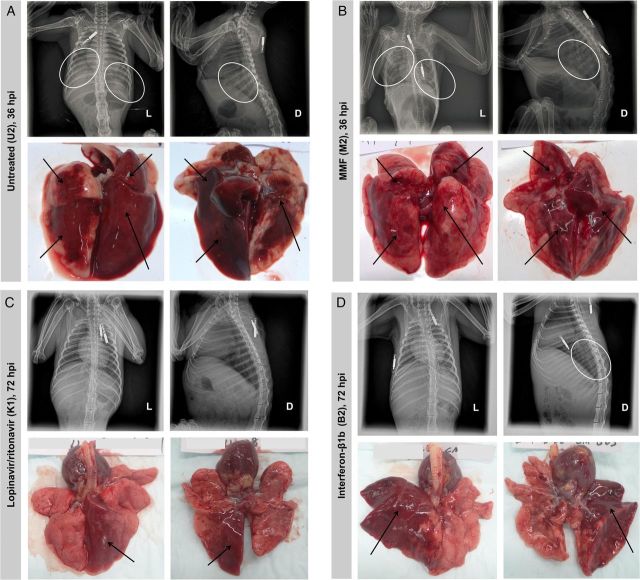
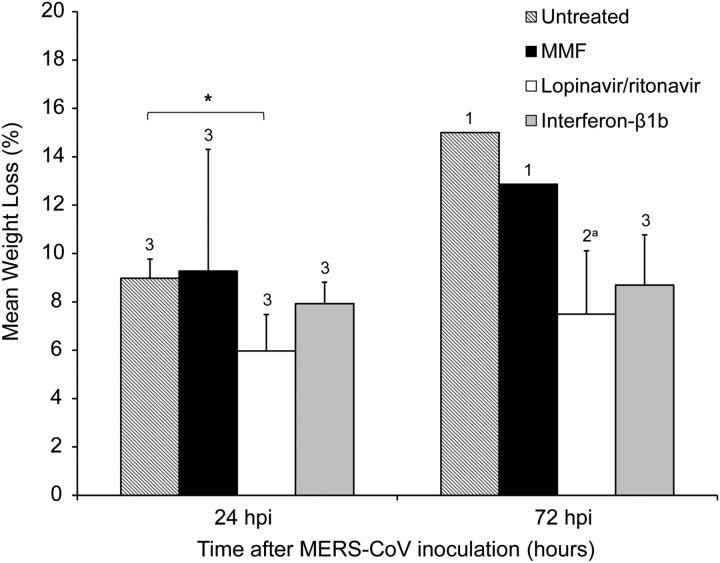
The untreated and MMF-treated animals developed severe disease with increased respiratory rates, reduced movement, and loss of appetite soon after MERS-CoV inoculation. At 12 hpi, the untreated and MMF-treated animals both had a mean clinical score of 18.3 (Figure 2). Their mean clinical scores peaked at 36 hpi, at which point 2 untreated (U2 and U3) and 2 MMF-treated (M1 and M2) animals developed severe dyspnea with cyanosis, barely moved, and had blood-stained oral discharge. They were euthanized at 36 hpi as their clinical scores exceeded 35. The euthanized animals had severe hypothermia (≤34.5°C) at necropsy, which was suggestive of severe disease with shock (Supplementary Data). CXR performed on these 4 animals prior to necropsy showed bilateral interstitial infiltrates, which were indicative of extensive pneumonia (Figure 4A and Figures 4B). The remaining untreated (U1) and MMF-treated (M3) animals survived, but continued to have severe symptoms with clinical scores ≥15 (Figure 2) and temperature ≤37.5°C, which was below their baseline levels, throughout the remaining study period (Supplementary Data). CXR at 24 and 72 hpi showed interstitial infiltrates at bilateral lung bases (data not shown). At 24 hpi, the untreated and MMF-treated animals had 9.0% and 9.3% of weight loss, respectively (Figure 3). At 72 hpi, the weight loss increased to 15.0% and 12.9% in the remaining untreated and MMF-treated animals, respectively. In contrast, none of the lopinavir/ritonavir-treated and interferon-β1b-treated animals developed severe symptoms. However, 1 lopinavir/ritonavir-treated (K3) animal died unexpectedly during anesthesia at 24 hpi, which was likely related to the anesthesia procedure, as common marmosets are very small and fragile. As we could not obtain ethics approval to add another animal in the study, only K1 and K2 were assessed after 24 hpi. The overall mortality rate at 36 hpi was 67% in the untreated and MMF-treated animals, and 0%–33% in the lopinavir/ritonavir-treated and interferon-β1b-treated animals. The mean clinical score of the lopinavir/ritonavir-treated animals peaked at 24 hpi and gradually improved, whereas that of the interferon-β1b-treated animals peaked at 36 hpi and then improved (Figure 2). Throughout the study, their mean clinical scores were consistently lower than those of the untreated (50.9%–95.0%) and MMF-treated (50.9%–93.3%) animals. Their temperature trend grossly correlated with their clinical scores, with the lowest temperature occurring at around 36 hpi, followed by normalization to the baseline level at 72 hpi (Supplementary Data). At 24 hpi, the lopinavir/ritonavir-treated animals had some weight loss (6.0%), which was significantly less than the untreated animals (P = .038) (Figure 3). At 72 hpi, the weight loss slightly increased to 7.5%. The interferon-β1b-treated animals also had mild weight loss of 7.9% and 8.7% at 24 and 72 hpi, respectively. The lopinavir/ritonavir-treated animals had grossly normal CXR appearances, while the interferon-β1b-treated animals only had mild basal interstitial infiltrate at 24 and 72 hpi (Figure 4C and Figures 4D). There were no obvious differences in the mean leukocyte, erythrocyte, and platelet counts obtained at 0 hpi and at necropsy among the 4 treatment groups (data not shown). However, changes at other time points might have been overlooked, as the small size of the animals prevented serial and frequent blood taking as described [23].
Figure 2.
Mean clinical scores of MERS-CoV-infected common marmosets at different time points after virus inoculation. Necropsies of the common marmosets were scheduled at 72 hpi or when the clinical score was ≥35. The number of animals remaining in the experiment is indicated above the bar at each time point. Student t test was performed for comparison when all 3 animals in each group were available. aK3 died unexpectedly during anesthesia at 24 hpi, which was likely related to the anesthesia procedure, as common marmosets are very small and fragile. Abbreviations: hpi, hours postinoculation; MERS-CoV, Middle East respiratory syndrome coronavirus; MMF, mycophenolate mofetil.
Figure 4.
Representative radiological findings and macroscopic pathology of the lungs of MERS-CoV-infected common marmosets. Dorsal-ventral and lateral chest X-rays were performed immediately prior to necropsy. The circles represent areas of interstitial infiltration indicative of pneumonia. The arrows represent gross lesions in the necropsied lungs. A, Untreated common marmoset (U2) at 36 hpi. B, MMF-treated common marmoset (M2) at 36 hpi. C, Lopinavir/ritonavir-treated common marmoset (K1) at 72 hpi. D, Interferon-β1b-treated common marmoset (B2) at 72 hpi. Abbreviations: D, dorsal side; hpi; hours postinoculation; L, left side; MERS-CoV, Middle East respiratory syndrome coronavirus; MMF, mycophenolate mofetil.
Figure 3.
Serial measurements of body weight of MERS-CoV-infected common marmosets. Mean percentage of weight loss at 24 and 72 hpi, compared with the baseline body weight at 0 hpi. The number of animals remaining in the experiment is indicated above the bar at each time point. aK3 died unexpectedly during anesthesia at 24 hpi, which was likely related to the anesthesia procedure, as common marmosets are very small and fragile. *P < .05. Abbreviations: hpi, hours postinoculation; MERS-CoV, Middle East respiratory syndrome coronavirus; MMF, mycophenolate mofetil.
Histopathology and Immunohistochemistry
Macroscopically, the necropsied lungs of the untreated and MMF-treated animals showed extensive, multilobar hemorrhagic lesions. The lungs were firm and fluid-filled with fluid leak (Figure 4A and Figures 4B). In contrast, the gross lesions in the necropsied lungs of the lopinavir/ritonavir-treated and interferon-β1b-treated animals were confined to 1–2 lobes (Figure 4C and Figures 4D). Corroborating with the mild symptoms and normal CXR findings in the lopinavir/ritonavir-treated animals, the macroscopic lesions in their lungs were the mildest. Microscopically, the necropsied lungs of the untreated and MMF-treated animals showed severe, multifocal to coalescing acute bronchointerstitial pneumonia (Figure 5A and Figures 5C). Pulmonary alveoli were infiltrated by a large amount of inflammatory cells, consisting predominantly of macrophages, neutrophils, and lymphocytes. The alveolar interstitium was thickened with edema, fibrin, and hemorrhage. Abundant MERS-CoV nucleocapsid protein expression was detected in immunohistochemical staining (Figure 5B and Figures 5D). In contrast, the necropsied lungs of the lopinavir/ritonavir-treated and interferon-β1b-treated animals showed mild to moderate bronchointerstitial pneumonia confined to 1–2 lobes, with a small amount of inflammatory cell infiltrates, edema, and hemorrhage (Figure 5E and Figures 5G). Immunohistochemical staining showed scarce and focal MERS-CoV nucleocapsid protein expression (Figure 5F and Figures 5H). The histopathological changes in the necropsied extrapulmonary tissues of the 4 treatment groups were similar and considered to be nonspecific changes associated with hypoxic damage or incidental findings in common marmosets as described (data not shown) [23].
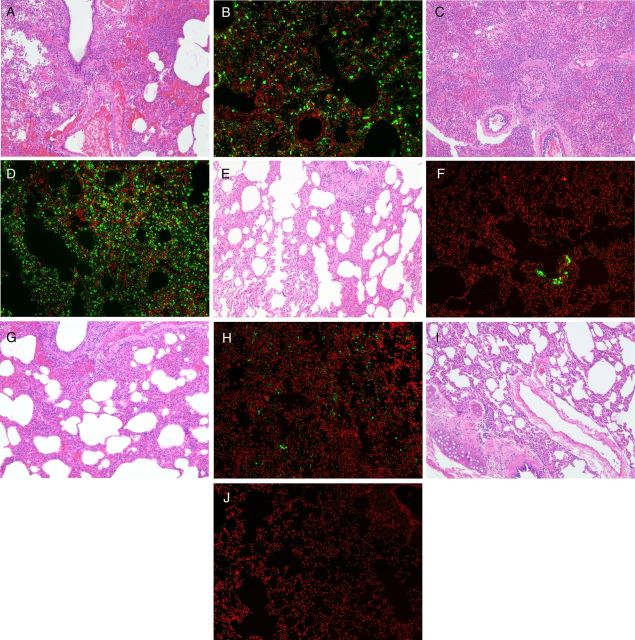
Figure 5.
Representative photomicrographs of histopathology examination and immunohistochemical staining of necropsied lung tissues of MERS-CoV-infected common marmosets. Severe acute bronchointerstitial pneumonia centered on terminal bronchioles, with thickened alveolar interstitium and alveoli being filled with large amount of inflammatory cell infiltrate, edema, and hemorrhage in hematoxylin and eosin (H&E)–stained necropsied lung tissue of untreated (A) and MMF-treated (C) animals collected at 36 hpi (magnification, 100×). Abundant expression of MERS-CoV nucleocapsid protein was detected by mouse anti-MERS-CoV nucleocapsid protein (1:200) antibody (green) overlaid with counterstaining by propidium iodide (red) in the necropsied lung tissues of the untreated (B) and MMF-treated (D) animals (magnification, 100×). These pathological changes were seen extensively in multiple lobes of the necropsied lungs of the untreated and MMF-treated animals. Mild acute bronchointerstitial pneumonia with small amount of inflammatory cell infiltrate and preserved histological architecture in H&E-stained necropsied lung tissue of lopinavir/ritonavir-treated (E) animals collected at 72 hpi (magnification, 100×). Mild to moderate acute bronchointerstitial pneumonia with moderate amount of inflammatory cell infiltrate and hemorrhage in H&E-stained necropsied lung tissue of interferon-β1b-treated (G) animals collected at 72 hpi (magnification, 100×). Small amount of MERS-CoV nucleocapsid protein expression was detected in the necropsied lung tissues of the lopinavir/ritonavir-treated (F) and interferon-β1b-treated (H) animals (magnification, 100×). These pathological changes were only seen in lesions which were confined to 1–2 lobes of the necropsied lungs of the lopinavir/ritonavir-treated and interferon-β1b-treated animals. H&E-stained necropsied lung tissue (I) (magnification, 100×) without MERS-CoV nucleocapsid protein expression (J) of an uninfected control animal. Abbreviations: hpi; hours postinoculation; MERS-CoV, Middle East respiratory syndrome coronavirus; MMF, mycophenolate mofetil.
Viral Load Studies
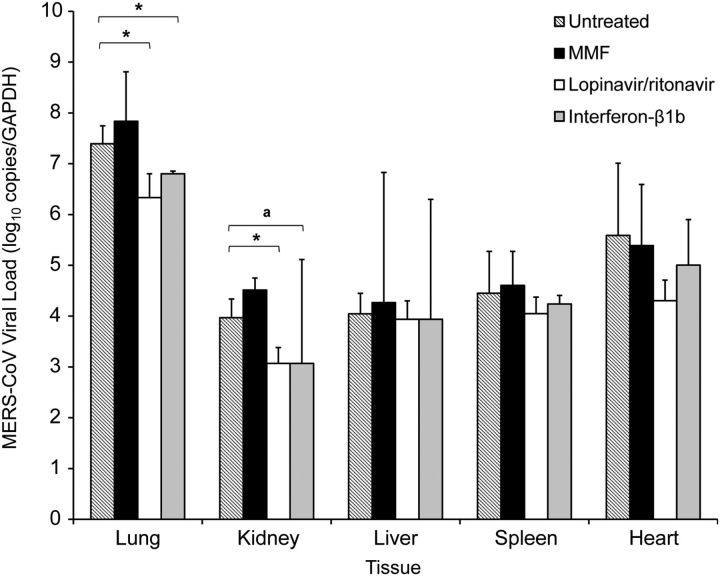
The mean viral load of necropsied lungs was highest in the MMF-treated animals and lowest in the lopinavir/ritonavir-treated animals (Figure 6). The mean viral load of the necropsied lungs of the MMF-treated animals was 0.44 log10 copies/GAPDH higher than that of the untreated animals. The mean viral loads of the necropsied lungs of lopinavir/ritonavir-treated and interferon-β1b-treated animals were significantly lower than that of the untreated animals by 1.06 (P = .036) and 0.59 (P = .048) log10 copies/GAPDH, respectively. The MMF-treated animals also had the highest mean viral loads in the kidney, liver, and spleen, which were 0.15–0.54 log10 copies/GAPDH higher than those of the untreated animals. In contrast, the mean viral loads in all extrapulmonary tissues of the lopinavir/ritonavir-treated and interferon-β1b-treated animals were consistently lower than those of the untreated animals by 0.11–1.29 log10 copies/GAPDH. The mean viral load in the necropsied kidney tissues of the lopinavir/ritonavir-treated animals was significantly lower than that of the untreated animals by 0.90 log10 copies/GAPDH (P = .032). Notably, 2 of the 3 interferon-β1b-treated animals actually had undetected viral loads in necropsied kidney tissues, which accounted for a large standard deviation value and apparent lack of statistically significant difference, despite a 0.90 log10 copies/GAPDH reduction as compared to the untreated animals.
Figure 6.
Mean viral loads with standard deviation values in different tissues of MERS-CoV-infected common marmosets collected at the time of necropsy. *P <.05. aTwo of the 3 interferon-β1b-treated animals had undetected viral loads in necropsied kidney tissues, which accounted for a large standard deviation value and apparent lack of statistically significant difference from the mean viral load of the untreated animals. Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MERS-CoV, Middle East respiratory syndrome coronavirus; MMF, mycophenolate mofetil.
DISCUSSION
As in most other epidemics caused by emerging viruses, the de novo development of novel anti-MERS-CoV drugs has lagged behind the rapid expansion of the MERS epidemic [3]. The recent large healthcare-associated outbreak involving more than 180 infected patients and over 5000 close contacts in South Korea reemphasizes the urgent need to find effective anti-MERS-CoV treatment. Drug repurposing is an especially advantageous approach, as repurposed drugs usually have well-known pharmacokinetics, pharmacodynamics, side effects, and dosing regimens. These data would facilitate drug use in patients with severe MERS, who often have comorbidities or are complicated by multiorgan dysfunction. However, the lack of suitable animal models has been a major obstacle to evaluating countermeasures for MERS in the past 3 years. BALB/c mice, Syrian hamsters, and ferrets are not susceptible to MERS-CoV infection, while MERS-CoV-infected rhesus macaques only develop very mild and self-limiting disease [3, 21, 34–36]. Intranasal administration of adenoviral vectors encoding the human dipeptidyl peptidase-4 (DPP4) receptor has been used to transduce and render BALB/c and C57BL/6 mice susceptible to MERS-CoV infection [37]. Using this animal model, the treatment effects of polyinosinic-polycytidylic acid and adoptive transfer of serum samples containing anti-MERS-CoV spike glycoprotein antibodies in accelerating virus clearance from the lungs were demonstrated [37]. However, the infection was relatively mild and confined to the respiratory tract. The DPP4-transgenic mouse model with more severe pulmonary and disseminated extrapulmonary disease was definitely better, but it was still not a nonhuman primate model [38]. Recently, the common marmoset model, which mimicked severe, disseminated MERS-CoV human infection, was established [23]. This is the first study to report on the use of this new nonhuman primate model for evaluating the treatment effects of repurposed drugs for MERS.
Consistent with the findings in the original description of the common marmoset model for MERS, the untreated animals in our study developed severe disease after MERS-CoV inoculation [23]. Comparatively, our untreated animals developed symptoms and died slightly earlier than those described in Falzarano's report [23]. This might be related to the different ages of the animals (our study, 3 years; Falzarano's study, 2–6 years) and routes of virus inoculation. Instead of combined intranasal (8 × 105 TCID50), intratracheal (2 × 106 TCID50), conjunctival (4 × 105 TCID50), and oral (2 × 106 TCID50) inoculation (total inoculum: 5.2 × 106 TCID50), we used intratracheal inoculation (5 × 106 TCID50), which likely resulted in more direct delivery of the virus to the lower respiratory tract with faster disease onset. Nonetheless, the overall clinical, radiological, pathological, and virological findings of our untreated animals were otherwise similar to those described previously and mimicked severe MERS in humans.
We have previously demonstrated the potent in vitro anti-MERS-CoV activity of MMF [15]. However, the immunosuppressive effects of MMF have so far limited its use in MERS patients. Compared to ribavirin, which has doubtful effects in MERS patients, MMF exhibited a much lower EC50 (MMF, 0.17 µg/mL; ribavirin, 10–40 µg/mL) that was markedly below its peak serum level (10–50 µg/mL) at routine clinical dosages [15]. It was therefore postulated that a less immunosuppressive dose of MMF could be used to treat MERS. Our primate study showed that a single dose of MMF with long half-life did not improve and might have worsened MERS-CoV infection in common marmosets. This may partially explain why renal transplant recipients on MMF still developed severe or fatal MERS [39, 40].
Lopinavir is a protease inhibitor, which may inhibit the 3C-like protease of MERS-CoV and modulate apoptosis in human cells [17, 41]. Kaletra is a ritonavir-boosted lopinavir preparation, which is commonly used as an anti–human immunodeficiency virus medication. Lopinavir exhibits anti-MERS-CoV activity with an EC50 of 8.0 µM in vitro, which is below its Cmax achieved after a single 500 mg oral dose of Kaletra (400 mg lopinavir/100 mg ritonavir) [17, 42]. We have previously demonstrated the in vivo treatment effects of lopinavir/ritonavir in SARS patients. Compared to control SARS patients who received ribavirin for 21 days, patients who additionally received lopinavir/ritonavir 500 mg twice daily for 14 days had milder disease with less diarrhea, recurrence of fever, lymphopenia, nosocomial infections, nasopharyngeal viral load, fecal RT-PCR positivity rate, and 21-day adverse outcome [43]. Most patients tolerated a short course of lopinavir/ritonavir well, although some experienced gastrointestinal upset, deranged liver function, headache, blurred vision, anemia, and asymptomatic bradycardia [43]. A MERS patient who received combinational lopinavir-ritonavir, pegylated interferon, and ribavirin had resolution of viremia after 2 days of treatment [44]. Our present study showed that treatment with lopinavir/ritonavir improved the clinical, radiological, and pathological features in MERS-CoV-infected common marmosets. The lopinavir/ritonavir-treated animals had the lowest mean viral loads in lung and most extrapulmonary tissues at necropsy. These findings provided further support for the use of lopinavir/ritonavir in patients with severe MERS.
MERS-CoV attenuates interferon response to evade the host's innate immune system [45]. MERS-CoV is 50–100 times more sensitive to pegylated interferon-α than SARS-CoV in vitro [46]. Therefore, treatment of MERS with interferons was considered and evaluated in vitro and in a small number of patients. In Vero/Vero6 cells, interferon-β1b (Betaferon) exhibited a lower EC50:Cmax ratio than interferon-α2a, -α2b, and -β1a [15, 47]. This might partially explain why the treatment effects of interferon-α2a, -α2b, and -β1a on the survival rates of a small cohort of MERS patients were not obvious [22, 48–50]. In this study, we showed that MERS-CoV-infected common marmosets treated with interferon-β1b had less severe disease and lower mean viral loads in necropsied lung and extrapulmonary tissues compared with untreated animals. Notably, as the lesions in the lopinavir/ritonavir-treated and interferon-β1b-treated animals were confined to 1–2 lobes, the overall mean viral load difference between these animals and the untreated animals were likely underestimated. Interferon-β1b is commonly used to treat patients with multiple sclerosis and is usually well tolerated [32]. Rarely, monocloncal gammopathy patients treated with interferon-β1b may develop systemic capillary leak syndrome [32]. Other uncommon side effects include hypersensitivity, pancreatitis, depression, cytopenia, cardiomyopathy, and liver and thyroid dysfunction [32]. Given the good safety profile of interferon-β1b, and our in vitro and in vivo results, its use should be considered in clinical trials with MERS patients who have no contraindication for interferon therapy.
We did not evaluate the effects of combinational treatment with 2 or more drugs due to the difficulty in getting approval for using more primates. Nevertheless, the findings in this nonhuman primate study are encouraging for conducting clinical trials with MERS patients using monotherapy or combinational treatment with these repurposed drugs. The in vivo anti-MERS-CoV effects and the lack of drug interactions between lopinavir/ritonavir and interferon-β1b make this an attractive combination in future clinical trials. In contrast, it is still uncertain if the combination of interferon-β1b and/or lopinavir/ritonavir with a lower dose of MMF may provide better viral load suppression to overcome the deleterious effects of the latter drug. Notably, the combination of MMF and interferon-β1b showed synergistic effects in MERS-CoV inhibition in vitro [15]. If this combination is shown to be effective in common marmosets, it may become an alternative treatment option in patients who are intolerant to interferon-β1b and/or lopinavir/ritonavir. Other potentially effective treatment regimens such as the combination of ribavirin with interferon-β1b and/or lopinavir/ritonavir should also be evaluated in the common marmoset model. Although the use of high-dose ribavirin is limited by its severe side effects, low-dose ribavirin combined with interferon-β1b and/or lopinavir/ritonavir may be synergistic and should still be considered. Finally, the shorter median time interval between symptom onset and death in MERS than in SARS patients emphasizes the importance of early use of these antiviral combinations in MERS. This is because MERS-CoV not only dampens the host innate interferon response, but also effectively suppresses the adaptive immune response by infection of CD3+ lymphocytes and induction of apoptosis via the both extrinsic and intrinsic pathways in a high percentage of peripheral blood and splenic lymphocytes [26]. Failure to control MERS-CoV replication during the early phase of disease may therefore lead to an impaired adaptive immune response with poor outcome.
Supplementary Material
Notes
Acknowledgments. We are grateful to Hua Zhu and Yanfeng Xu for their technical support.
Financial support. This work was supported by the donations of Respiratory Virus Research Foundation Limited; Larry Chi-Kin Yung; the Hui Hoy and Chow Sin Lan Charity Fund Limited; and the Providence Foundation Limited in memory of the late Dr Lui Hac Minh; and funding from the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease of the Department of Health, the Hong Kong Health and Medical Research Fund (14131392); Hong Kong Research Grants Council (N_HKU728/14); the Theme-based Research Scheme (T11/707/15) of the Research Grants Council, Hong Kong Special Administrative Region; and the National Science and Technology Major Projects of Infectious Diseases, China (2012ZX10004501-004). The funding sources had no role in study design; data collection, analysis, or interpretation; or writing of the report.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chan JF, To KK, Tse H, Jin DY, Yuen KY. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol 2013; 21:544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814–20. [DOI] [PubMed] [Google Scholar]
- 3. Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev 2015; 28:465–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan JF, Li KS, To KK, Cheng VC, Chen H, Yuen KY. Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic? J Infect 2012; 65:477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang L, Wang N, Zuo T et al. Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci Transl Med 2014; 6:234ra59. [DOI] [PubMed] [Google Scholar]
- 7. Lu L, Liu Q, Zhu Y et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun 2014; 5:3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao J, Lu G, Qi J et al. Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the S protein of Middle East respiratory syndrome coronavirus. J Virol 2013; 87:13134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Du L, Zhao G, Yang Y et al. A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in Middle East respiratory syndrome coronavirus spike protein. J Virol 2014; 88:7045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soo YO, Cheng Y, Wong R et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect 2004; 10:676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng Y, Wong R, Soo YO et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005; 24:44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hung IF, To KK, Lee CK et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 2011; 52:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang ZY, Werner HC, Kong WP et al. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc Natl Acad Sci USA 2005; 102:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weingartl H, Czub M, Czub S et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol 2004; 78:12672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan JF, Chan KH, Kao RY et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect 2013; 67:606–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dyall J, Coleman CM, Hart BJ et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 2014; 58:4885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Wilde AH, Jochmans D, Posthuma CC et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother 2014; 58:4875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kindrachuk J, Ork B, Hart BJ et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother 2015; 59:1088–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Q, Xia S, Sun Z et al. Testing of Middle East respiratory syndrome coronavirus replication inhibitors for the ability to block viral entry. Antimicrob Agents Chemother 2015; 59:742–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Falzarano D, de Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci Rep 2013; 3:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falzarano D, de Wit E, Rasmussen AL et al. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med 2013; 19:1313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Omrani AS, Saad MM, Baig K et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 2014; 14:1090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Falzarano D, de Wit E, Feldmann F et al. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLOS Pathog 2014; 10:e1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chan JF, Choi GK, Tsang AK et al. Development and evaluation of novel real-time RT-PCR assays with locked nucleic acid probes targeting the leader sequences of human pathogenic coronaviruses. J Clin Microbiol 2015; 53:2722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou J, Chu H, Li C et al. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J Infect Dis 2014; 209:1331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu H, Zhou J, Wong BH et al. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates both the extrinsic and intrinsic apoptosis pathways [Epub ahead of print] J Infect Dis 2015; 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan JF, Chan KH, Choi GK et al. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J Infect Dis 2013; 207:1743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chan KH, Chan JF, Tse H et al. Cross-reactive antibodies in convalescent SARS patients’ sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. J Infect 2013; 67:130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yao Y, Bao L, Deng W et al. An animal model of MERS produced by infection of rhesus macaques with MERS coronavirus. J Infect Dis 2014; 209:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishimatsu M, Suzuki H, Akiyama H, Miura T, Hayami M, Ido E. Construction of a novel SHIV having an HIV-1-derived protease gene and its infection to rhesus macaques: a useful tool for in vivo efficacy tests of protease inhibitors. Microbes Infect 2007; 9:475–82. [DOI] [PubMed] [Google Scholar]
- 31. Genentech USA, Inc. Product Information: CellCept®. http://www.gene.com/download/pdf/cellcept_prescribing.pdf Accessed 10 June 2015.
- 32. Bayer Australia Limited. Product Information: Betaferon® Single use pack drug. http://www.bayerresources.com.au/resources/uploads/PI/file9314.pdf Accessed 10 June 2015.
- 33. Chu H, Zhou J, Wong BH et al. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology 2014; 454–455:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raj VS, Smits SL, Provacia LB et al. Adenosine deaminase acts as a natural antagonist for dipeptidyl peptidase 4-mediated entry of the Middle East respiratory syndrome coronavirus. J Virol 2014; 88:1834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Wit E, Prescott J, Baseler L et al. The Middle East respiratory syndrome coronavirus (MERS-CoV) does not replicate in Syrian hamsters. PLOS One 2013; 8:e69127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coleman CM, Matthews KL, Goicochea L, Frieman MB. Wild-type and innate immune-deficient mice are not susceptible to the Middle East respiratory syndrome coronavirus. J Gen Virol 2014; 95(Pt 2):408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao J, Li K, Wohlford-Lenane C et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci USA 2014; 111:4970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Agrawal AS, Garron T, Tao X et al. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol 2015; 89:3659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Faure E, Poissy J, Goffard A et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLOS One 2014; 9:e88716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant 2015; 15:1101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rizza SA, Badley AD. HIV protease inhibitors impact on apoptosis. Med Chem 2008; 4:75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abbott Laboratories. Product Information: Kaletra®. http://hivdb.stanford.edu/pages/linksPages/LPV_RTV_PI.pdf Accessed 10 June 1015.
- 43. Chu CM, Cheng VC, Hung IF et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004; 59:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spanakis N, Tsiodras S, Haagmans BL et al. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents 2014; 44:528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lau SK, Lau CC, Chan KH et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol 2013; 94:2679–90. [DOI] [PubMed] [Google Scholar]
- 46. de Wilde AH, Raj VS, Oudshoorn D et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-alpha treatment. J Gen Virol 2013; 94:1749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hart BJ, Dyall J, Postnikova E et al. Interferon-beta and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol 2014; 95:571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis 2014; 20:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khalid M, Al Rabiah F, Khan B, Al Mobeireek A, Butt TS, Al Mutairy E. Ribavirin and interferon (IFN)-alpha-2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus (MERS-CoV): a preliminary report of two cases. Antivir Ther 2015; 20:87–91. [DOI] [PubMed] [Google Scholar]
- 50. Shalhoub S, Farahat F, Al-Jiffri A et al. IFN-alpha2a or IFN-beta1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother 2015; 70:2129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.