Abstract
Background
Bronchiolitis is associated with a greater risk of developing recurrent wheezing, but with currently available tools, it is impossible to know which infants with bronchiolitis will develop this condition. This preliminary prospective study aimed to assess whether urine metabolomic analysis can be used to identify children with bronchiolitis who are at risk of developing recurrent wheezing.
Methods
Fifty-two infants <1 year old treated in the emergency department at University Hospital of Padova for acute bronchiolitis were enrolled (77% tested positive for respiratory syncytial virus [RSV]). Follow-up visits were conducted for 2 years after the episode of bronchiolitis. Untargeted metabolomic analyses based on mass spectrometry were performed on urine samples collected from infants with acute bronchiolitis. Data modeling was based on univariate and multivariate data analyses.
Results
We distinguished children with and those without postbronchiolitis recurrent wheeze, defined as ≥3 episodes of physician-diagnosed wheezing. Pathway overrepresentation analysis pointed to a major involvement of the citric acid cycle (P < .001) and some amino acids (lysine, cysteine, and methionine; P ≤ .015) in differentiating between these 2 groups of children.
Conclusion
This is the first study showing that metabolomic profiling of urine specimens from infants with bronchiolitis can be used to identify children at increased risk of developing recurrent wheezing.
Keywords: Bronchiolitis, metabolomics, pediatrics, recurrent wheezing, urine, mass spectrometry, citric acid cycle
Bronchiolitis is associated with the risk of developing wheezing disorders. We found that metabolomic profiling of urine specimens from infants with acute bronchiolitis can be used to identify children who are at risk of developing recurrent wheezing and could benefit from early prevention measures.
Bronchiolitis is the most common lower respiratory tract infection in children, and it is associated with a high mortality rate, especially in countries with limited resources [1, 2]. It is also well known that infants with a history of acute bronchiolitis are subsequently much more likely to develop recurrent wheezing and/or asthma [3].
Recently, a birth cohort study that has been ongoing for almost 4 decades showed that respiratory syncytial virus (RSV) infection of the lower respiratory tract during early life is associated with a persistently low trajectory of lung function that represents an important pathway to chronic obstructive pulmonary disease [4].
What is not known is which children will develop wheezing after an episode of bronchiolitis. To date, the severity of bronchiolitis has been considered the main predictor of the likelihood of developing recurrent wheezing or asthma [5].
Recently, an approach called “metabolomics” [6, 7] has revealed the potential to identify the metabolic features of certain conditions, differentiating between phenotypes of the same disease and enabling a better characterization of the pathological mechanisms involved. Metabolomic studies are typically performed on biofluids, and urine samples are among the most often used in medical research, especially studies of children, because they can be collected using simple, noninvasive techniques [8–10].
To the best of our knowledge, no studies have been published on the application of untargeted metabolomics to acute bronchiolitis and the subsequent onset of recurrent wheezing. The main aim of the present preliminary prospective study was to use untargeted metabolomics to analyze urine samples collected from infants with acute bronchiolitis in an effort to elucidate disease pathways and identify metabolic signatures discriminating children who will subsequently develop recurrent wheezing from those who will not.
PATIENTS AND METHODS
Study Subjects
We prospectively and consecutively enrolled all infants <1 year of age with a diagnosis of bronchiolitis who accessed the pediatric emergency department at University Hospital of Padova during a typical RSV season in Italy (December 2013–March 2014). Bronchiolitis was defined as a first episode of lower respiratory tract infection with signs of respiratory distress and crackles on chest auscultation, preceded by a coryzal illness.
Parents were approached for consent if infants admitted to the emergency department had a clinical diagnosis of bronchiolitis but satisfied none of the following exclusion criteria: chronic lung diseases (ie, bronchopulmonary dysplasia, cystic fibrosis, interstitial lung diseases, or neonatal respiratory distress), other chronic conditions (ie, congenital heart diseases and/or immunocompromised state), and/or history of prematurity (ie, birth at <37 weeks of gestational age).
During the same period (December 2013–March 2014), we also enrolled 24 healthy infants <1 year of age who had no history of bronchiolitis and did not satisfy the exclusion criteria listed above. We enrolled these children with the help of local pediatricians.
The study was approved by the Ethics Committee of Padova University and General Hospital (protocol 3112P), and all parents gave their written informed consent to their children’s participation in the study.
Study Design
At the time of recruitment, parents were interviewed with the aid of a structured questionnaire to obtain patients’ clinical histories. In particular, they provided details of the pregnancy, delivery, perinatal history, auxological parameters at birth, vaccinations, ongoing treatments (including vitamins), breastfeeding history and weaning, parents’ smoking habits, number of siblings, day care center attendance, family history of allergies (in parents or siblings), and presence of atopic eczema; details of any surgery, diseases, and previous hospital admissions were also recorded.
A nasopharyngeal aspirate was collected and tested for respiratory viruses (ie, RSV, human rhinovirus [HRV], adenovirus, bocavirus, enterovirus, influenza virus A and B, parainfluenza virus types 1–3, metapneumovirus, coronavirus, and parechovirus), using polymerase chain reaction analysis. A urine sample was collected from all infants, using a sterile bag, within 24 hours of their arrival in the emergency department. Urine samples were stored at −80°C until metabolomic analysis.
A clinical follow-up was performed 6 months, 12 months, and 2 years after the episode of bronchiolitis to monitor any history of wheezing. In addition, information on any wheezing occurring after the episode of bronchiolitis was obtained by interviewing the family pediatricians of all enrolled children (in Italy, 90% of children <6 years old are routinely followed up by family pediatricians). Recurrent wheezing was defined as ≥3 episodes of wheezing diagnosed by a pediatrician during the 2-year follow-up after the acute episode of bronchiolitis.
Metabolomic Analysis
The urine samples were analyzed at the Mass Spectrometry Laboratory, Department of Women’s and Children’s Health, Padova University Hospital, Fondazione Istituto di Ricerca Pediatrica Città della Speranza. The sample preparation method and the chromatographic and mass spectrometry conditions were as described in a previous work [9, 11] and elsewhere in this article (Supplementary Materials). Briefly, the metabolic profile of the urine was acquired using a Waters Acquity Ultra-Performance Liquid Chromatography system coupled to a Waters Q-TOF Synapt G2 mass spectrometer (Waters, Milford, MA). The diluted samples were separated by a C18 column (Acquity HSS T3; dimensions, 2.1 mm × 100 mm; pore diameter, 1.7 µm; Waters). The electrospray source was operated in positive and negative ionization modes with a capillary voltages of 3 kV and 1.5 kV, respectively. Data were collected in centroid mode, with a mass scan range of 20–1200 m/z and a resolution of 20000. All operations were controlled with MassLynx 4.1 (Waters). Quality control samples and standard solution samples were used to test for reproducibility and accuracy during the analysis and were injected at regular intervals throughout the sequence, together with blank samples (water and 0.1% formic acid). The samples were randomly injected in triplicate to prevent any spurious classification deriving from the position of the samples in the sequence.
Statistical Analysis and Metabolite Identification
Raw data were converted into 2 data sets with the MarkerLynx software. The Rt_mass variables were filtered by excluding all variables with >20% of values missing, and the remaining missing values (4% of the data) were imputed with the minimum value of their related variable. Thus, probabilistic quotient normalization was used to correct the data for the effect of the reduction in the performance of the detector during the analytical session and for the different dilution of the collected samples. Data were log transformed to get a normal distribution centered around a mean value.
Multivariate data analyses were based on projection methods, and univariate data analyses were based on the t test with a false-discovery rate; receiver operating characteristic (ROC) curve analysis was also performed. Specifically, exploratory data analysis was performed using principal component analysis (PCA), and projection to latent structures discriminant analysis based on variable influence on projection selection (VIP-based PLS-DA) [12] was used to identify differences between the groups under investigation. Multivariate projection methods linearly combine the measured variables to obtain the components (known as latent variables) that provide a data representation where the effects of the factors included in the experimental design can be easily investigated. Because, in our untargeted approach, most of the measured variables are not relevant for the aim of the study, variable selection was used to improve model robustness, and a procedure called stability selection was implemented to simplify model interpretation. In this procedure, stability selection [13] based on Monte Carlo sampling (200 random subsamples) was used to pinpoint the subset of relevant variables characterizing the groups being investigated. The relevant variables were those selected in >50% of the submodels generated during the stability selection procedure. Moreover, the group of samples excluded during Monte-Carlo sampling has been used to estimate the predictive power of the models. Seven-fold full cross-validation was used to determine the number of components to use, whereas permutation analysis of the class response was used to avoid overfitting. Each PLS-DA model was characterized by the area under the ROC curve (AUC) in fitting the training data, during cross-validation, and in predicting the samples excluded during stability selection (AUCpred).
The relevant variables selected in light of the multivariate data analysis were merged with those obtained from the univariate data analysis. Thus, variables were identified by searching in the Human Metabolome Database and the METLIN metabolite database. The set of compounds identified was investigated by means of pathway overrepresentation analysis (Supplementary Materials).
RESULTS
Patients’ Characteristics
A total of 52 infants with acute bronchiolitis were recruited (median age, 46 days; range, 14–350 days), of whom 39 (75%) were <90 days old, and 35 (67%) were male.
Urine samples and nasopharyngeal aspirates were collected from all patients, and 41 patients (79%) were hospitalized. As for the etiology of their bronchiolitis, aspirates from 40 children (77%) tested positive for RSV, those from 5 (10%) tested positive for HRV, those from 3 (6%) tested positive for other viruses (coronavirus and influenza virus), and those from 4 (8%) tested negative for viruses.
Two years after patients received a diagnosis of bronchiolitis, they could be divided into 2 different groups, one comprising 17 children with recurrent wheezing (they had experienced ≥3 episodes of wheezing during the 2-year follow-up), hereafter referred to as the “recurrent wheezing group,” and the other consisting of 24 children with no history of wheezing after their episode of bronchiolitis, hereafter referred to as the “no wheezing group.” The median ages of the 2 groups did not differ significantly (P = .40, by the Mann-Whitney test). Eleven children who experienced 1 or 2 episodes of wheezing during the 2-year follow-up were not included in the comparison between the wheezing and nonwheezing groups.
Table 1 shows the characteristics and metadata for these 2 subgroups. The severity of bronchiolitis was assessed using the Respiratory Syncytial Virus Network scale, based on feeding intolerance, medical intervention, respiratory difficulty, respiratory frequency, apnea, general condition, and fever [14]. No significant differences emerged in the metadata between the recurrent wheezing and the no wheezing groups (Table 1).
Table 1.
Metadata of the Enrolled Patients
| Characteristic | No Wheezing Group | Recurrent Wheezing Group | P a | Patients With 1 or 2 Wheezing Episodes |
|---|---|---|---|---|
| Male sex | 13 (54) | 14 (82) | .10 | 8 (72) |
| Cesarean section delivery | 6 (25) | 7 (41) | .32 | 2 (18) |
| RSV positivity | 18 (75) | 13 (76) | 1.00 | 9 (82) |
| Family history of allergy | 9 (38) | 8 (47) | .51 | 5 (45) |
| Atopic eczema (dermatitis) | 4 (17) | 6 (35) | .27 | 1 (9) |
| ≥1 sibling | 14 (58) | 15 (88) | .08 | 10 (91) |
| ≥1 parent smoker | 9 (38) | 5 (29) | .74 | 7 (64) |
| Breastfed | 20 (83) | 13 (76) | .70 | 11 (100) |
| Severe bronchiolitis | 4 (17) | 6 (35) | .27 | 3 (27) |
| Nursery attendance | 1 (4) | 1 (6) | 1.00 | 0 (0) |
Data are no. (%) of patients.
Abbreviation: RSV, respiratory syncytial virus.
aBy the Fisher exact test, for the comparison between no wheezing group and the recurrent wheezing group.
A total of 24 healthy infants (median age, 60 days; range, 22–349 days) with no history of bronchiolitis were included in the final analysis. Seventeen (75%) were <90 days old, and 14 (58%) were male. The median age did not differ from that in the acute bronchiolitis group (P = .27, by the Mann-Whitney test). None developed acute bronchiolitis or recurrent wheezing during the 2-year follow-up.
Metabolomic Analysis
Mass spectrometry of the urine samples led to the generation of 2 data sets: a NEG data set including 786 Rt_mass variables and a POS data set comprising 802 Rt_mass variables, obtained by processing the raw data from all 3 groups acquired by negative and positive ionization modes, respectively. The exploratory data analysis based on PCA did not identify any outliers at the observation level on the basis of the Hotelling’s T2 test and the DModX test (significance level, 5%). The 2 data sets were combined and investigated using multivariate and univariate data analyses.
Bronchiolitis Group Versus Healthy Controls
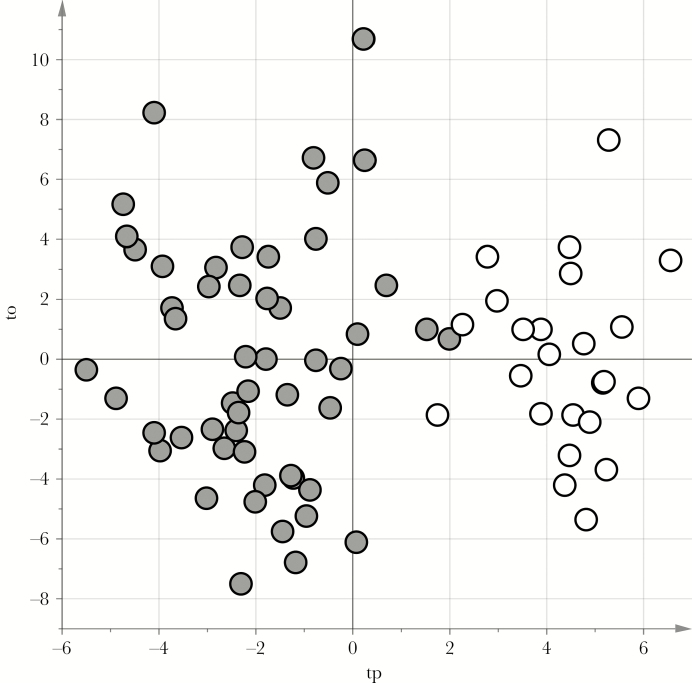
As a first step in the data analysis, the metabolic profile of urine specimens from the control group was compared to that of urine specimens from the 52 children with bronchiolitis. We obtained a reliable PLS-DA model capable of distinguishing between the 2 groups. It showed an A of 2 components, an AUC of 1.00 (P < .01), an AUC calculated by 7-fold full cross-validation of 0.99 (P < .01), and a median AUCpred calculated by means of stability selection of 0.98 (5th percentile, 0.92). The score scatterplot of the model is shown in Figure 1 (the model underwent posttransformation according to the methods of Stocchero and Paris [15]).
Figure 1.
Score scatterplot of the projection to latent structures discriminant analysis (PLS-DA) model for patients with bronchiolitis versus healthy controls. tp and to are the predictive and nonpredictive latent variables, respectively, obtained by posttransformation of the PLS-DA model. Gray circles represent urine samples from the bronchiolitis group, and white circles represent those from the control group. The samples from the bronchiolitis group lie in the region with a negative tp value, while those from the control group show positive tp values.
Recurrent Wheezing Group Versus the No Wheezing Group
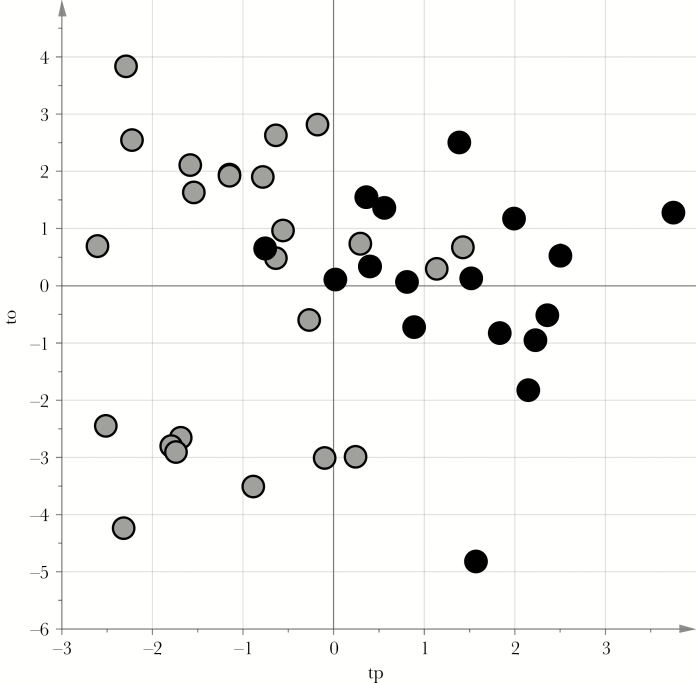
The differences in the metabolic profiles obtained for the urine samples from the 17 children with recurrent wheezing and the 24 children with no wheezing were investigated using VIP-based PLS-DA with stability selection. We obtained a reliable PLS-DA model capable of distinguishing between the 2 groups, showing an A of 2 components, an AUC of 0.94 (P < .01), an AUC calculated by 7-fold full cross-validation of 0.87 (P < .01), and a median AUCpred calculated by stability selection of 0.75 (5th percentile, 0.50). The score scatterplot of the model after posttransformation [15] is shown in Figure 2. The variables earmarked by the stability selection procedure were merged with those highlighted by the t test with a false-discovery rate (P < .05; q < .15) and ROC analysis (P < .05 for the AUC), obtaining a set of 82 relevant variables to identify (Supplementary Table 1). Twenty of these variables showed a specificity of >0.70, and 55 showed a sensitivity of >0.70. On searching the available online metabolite databases, we found a plausible identification for a subset of these variables, comprising 30 putative markers, which are listed in Table 2.
Figure 2.
Score scatterplot of the projection to latent structures discriminant analysis (PLS-DA) model for the no wheezing and recurrent wheezing groups. tp and to are the predictive and nonpredictive latent variables, respectively, obtained by posttransformation of the PLS-DA model. Gray circles represent urine samples from the no wheezing group, and black circles represent those from the recurrent wheezing group. The samples from the no wheezing group lie in the region with negative tp values, while those from the recurrent wheezing group show positive tp values.
Table 2.
Putative Markers Characterizing the Recurrent Wheezing Group With Respect to the No Wheezing Group, Based on Merging the Results of Multivariate and Univariate Data Analysis
| Data Seta | RetentionTime (min) | Mass to Charge Ratio | Typeb | Area Under the ROC Curve, Median (95% CI) | P c | Putatively Annotated Compound |
|---|---|---|---|---|---|---|
| POS | 0.5317 | 205.1539 | No wheeze > wheeze | 0.76 (.61–.90) | >.01 | 3-hydroxy-N6,N6,N6-trimethyl-L-lysine |
| POS | 0.6604 | 204.0857 | No wheeze > wheeze | 0.69 (.51–.86) | .01 | N2-acetyl-L-aminoadipate |
| POS | 0.5315 | 189.1593 | No wheeze > wheeze | 0.73 (0.57–0.89) | .01 | 7,8-diaminononanoate |
| NEG | 0.7488 | 191.0192 | No wheeze > wheeze | 0.72 (0.56–0.87) | .01 | Isocitrate |
| NEG | 0.7517 | 111.0083 | No wheeze > wheeze | 0.72 (0.56–0.87) | .01 | 3-furoic acid |
| POS | 0.654 | 310.1133 | No wheeze > wheeze | 0.71 (0.55–0.87) | .04 | N-acetylneuraminic acid |
| NEG | 1.04 | 160.061 | No wheeze > wheeze | 0.69 (0.52–0.86) | .02 | L-2-aminoadipic acid |
| POS | 0.758 | 163.0417 | No wheeze > wheeze | 0.76 (0.60–0.92) | .02 | 1,2-dihydroxy-3-keto-5-methylthiopentene |
| NEG | 1.3627 | 103.0397 | No wheeze > wheeze | 0.71 (0.54–0.87) | .02 | (S)-3-hydroxybutyric acid |
| POS | 0.5053 | 128.0695 | No wheeze > wheeze | 0.67 (0.50–0.84) | .02 | D-1-piperidine-2-carboxylic acid |
| POS | 0.7558 | 181.0526 | No wheeze > wheeze | 0.71 (0.54–0.87) | .02 | 5-methylthioribose |
| POS | 0.5872 | 114.0655 | No wheeze > wheeze | 0.70 (0.53–0.86) | .03 | Creatinine |
| POS | 0.5137 | 198.0861 | No wheeze > wheeze | 0.69 (0.51–0.86) | .03 | N-acetyl-L-histidine |
| NEG | 0.7499 | 173.0087 | No wheeze > wheeze | 0.67 (0.50–0.84) | .03 | Aconitic acid |
| NEG | 0.9233 | 129.0189 | No wheeze > wheeze | 0.69 (0.52–0.85) | .04 | Itaconic acid |
| NEG | 0.9225 | 111.0083 | No wheeze > wheeze | 0.69 (0.52–0.85) | .04 | 2-furoic acid |
| NEG | 4.0527 | 137.0602 | No wheeze > wheeze | 0.70 (0.52–0.87) | .04 | 2-(4-hydroxyphenyl)ethanol |
| POS | 1.496 | 146.0804 | No wheeze > wheeze | 0.68 (0.51–0.84) | .04 | N-butyryl glycine |
| NEG | 0.9221 | 191.0192 | No wheeze > wheeze | 0.68 (0.51–0.84) | .05 | Citric acid |
| NEG | 0.6123 | 165.04 | No wheeze > wheeze | 0.71 (0.53–0.88) | .05 | Ribonic acid |
| NEG | 0.9215 | 87.0082 | No wheeze > wheeze | 0.69 (0.52–0.85) | .05 | Pyruvate |
| NEG | 0.9238 | 173.0087 | No wheeze > wheeze | 0.68 (0.51–0.85) | .07 | Dehydroascorbic acid |
| POS | 0.7051 | 204.1226 | No wheeze > wheeze | 0.38 (0.19–0.57) | .08 | Acetylcarnitine |
| NEG | 0.8199 | 145.0137 | Wheeze > no wheeze | 0.75 (0.59–0.90) | .01 | Oxoglutaric acid |
| NEG | 3.1389 | 143.0352 | Wheeze > no wheeze | 0.76 (0.60–0.91) | .01 | (E)-2-methylglutaconic acid |
| POS | 0.5865 | 156.0417 | Wheeze > no wheeze | 0.66 (0.49–0.83) | .01 | N-methylethanolamine phosphate |
| POS | 4.275 | 281.1128 | Wheeze > no wheeze | 0.70 (0.53–0.86) | .02 | L-beta-aspartyl-L-phenylalanine |
| POS | 4.0403 | 246.1696 | Wheeze > no wheeze | 0.65 (0.48–0.82) | .04 | Pivaloylcarnitine |
| POS | 0.8652 | 258.1071 | Wheeze > no wheeze | 0.64 (0.46–0.81) | .04 | 5-methylcytidine |
Abbreviations: CI, confidence interval; ROC, receiver operating characteristic curve.
aVariables were acquired in positive (POS) or negative (NEG) ionization modes.
bVariables with higher levels in the no wheezing group are described as “no wheeze > wheeze,” and variables with higher levels in the recurrent wheezing group are described as “wheeze > no wheeze.”
cBy the t test (variables with a P value of <.05 had a q value of <.15).
The set of putative markers was investigated using pathway overrepresentation analysis to see which human metabolic pathways showed a different arrangement between the children who subsequently developed recurrent wheezing and those who did not. A different metabolic arrangement emerged for the citric acid cycle (P < .001), lysine biosynthesis (P = .003), lysine degradation (P = .009), and cysteine and methionine metabolism (P = .015). Moreover, we obtained a reliable PLS-DA model capable of distinguishing between the no wheezing group and healthy children, with an A of 2 components, an AUC of 1.00 (P < .01), an AUC calculated by 7-fold full cross-validation of 0.94 (P < .01), and a median AUCpred calculated by stability selection of 0.98 (5th percentile, 0.83).
DISCUSSION
The correlation between acute bronchiolitis and preschool wheezing has been demonstrated abundantly in the medical literature [3]. Many studies have suggested that children with an episode of RSV-related bronchiolitis in infancy are more likely to have recurrent wheeze [3, 16], especially if their bronchiolitis was severe [17]. Followed up over time, these children have spirometric values consistent with reduced airway patency at school age [4, 16, 18]. A similar association has been found for HRV-related bronchiolitis, too [19].
The mechanism connecting bronchiolitis with long-term respiratory problems is not fully understood [20]. Some authors have suggested a causal role of bronchiolitis in asthma, since preventing RSV infection with monoclonal antibodies seems to reduce the risk of recurrent wheezing [21, 22]. Others have hypothesized a preexisting altered lung function that predisposes to both bronchiolitis and subsequent recurrent wheezing [23, 24]. Whatever the mechanism involved, it remains unclear which children with a history of acute bronchiolitis in infancy will later develop recurrent wheezing or asthma. Given that asthma is now the most common chronic respiratory disease in childhood, identifying which children with a history of bronchiolitis would benefit from early treatment and close follow-up programs is an important goal in pediatrics.
When metabolomics was applied to asthmatic children in an effort to improve our understanding of the pathogenesis of asthma and better characterize its different phenotypes [25], it led to the identification of specific metabolic patterns in these individuals [26–29]. Although viruses are known to be capable of reprogramming metabolic pathways, very few published studies have applied metabolomics to bronchiolitis. It was recently demonstrated that analysis of the metabolomic profiles of urine and nasopharyngeal aspirate can differentiate healthy children from those with viral respiratory tract infections [10] and can discriminate between different degrees of disease severity [10, 30, 31]. The metabolome of nasopharyngeal aspirates obtained from infants with bronchiolitis also revealed its potential for distinguishing between infants infected with HRV as opposed to RSV [32].
The possible correlation between the metabolomic picture developing during acute bronchiolitis and any subsequent onset of recurrent wheezing had never been investigated. We demonstrated that the process of acute bronchiolitis severely disrupts the metabolic profile, comparison with the profile of healthy children (Figure 1). We also identified differences in the metabolic profiles of infants with acute bronchiolitis that could distinguish between those who would from those who would not subsequently develop recurrent wheezing, and pathway analysis revealed a major role for the citric acid cycle in discriminating between them (P < .001).
Isocitrate, citric acid, and oxoglutaric acid, which are central metabolites of the citric acid cycle, appeared to be critical in the separation between the 2 groups. The citric acid cycle is a key metabolic pathway occurring in the matrix of the mitochondrion that generates most of the energy produced in cellular respiration. How this cycle may influence the pathophysiology of respiratory tract diseases is not clear, but its alteration may be a sign of disrupted cellular energy metabolism, reflecting a condition of stress and increased anaerobic glycolysis [33]. This could explain why inflammatory conditions such as asthma are characterized by such metabolic alterations [29]. Changes in the citric acid cycle induced by RSV infection had already been demonstrated in previous studies [32]. What our study adds is that these perturbations discriminate children who will develop recurrent wheezing from those who will not.
We also found evidence of an altered metabolism of lysine, cysteine, and methionine, pointing to a possible influence of the amino acid pathways, as already suggested elsewhere [32].
Another 2 metabolites found to be disrupted, isobutyrylglycine and N-butyrylglycine, are minor metabolites of fatty acids. A reduction in short-chain fatty acid levels has been reported in children with asthma: in a study conducted in Canada, infants at risk of asthma exhibited lower levels of fecal acetate, which is associated with a transient gut microbial dysbiosis [34]. Short-chain fatty acids are derived from bacterial fermentation and are therefore profoundly influenced by gut microbiota. A recent case-control study also identified a specific fecal microbiota profile associated with a higher likelihood of developing bronchiolitis [35]. The emergence of different fatty acid metabolites in children with and those without recurrent wheezing suggests that a different composition of the gut microbiota might be associated with the onset of this condition. Recent studies on the possible mechanism connecting gut microbiota and respiratory tract disease point to a gut-mediated lung immunity, and authors have postulated the existence of a gut-lung axis.
Our finding of an altered N-acetylneuraminic acid profile suggests an involvement of bacteria in the pathogenic processes of bronchiolitis, since higher levels of N-acetylneuraminic acid in the intestinal mucosa can influence the outgrowth of some bacteria because it serves as a source of nutrients [36].
These findings may contribute to a better understanding of the mechanisms behind recurrent wheezing after acute bronchiolitis, but it is important to bear in mind that children with and those without recurrent wheezing are distinguishable on the basis of their overall metabolic profile, rather than from differences in specific metabolites. From a clinical standpoint, our data may provide the basis for the early identification of children likely to develop recurrent wheezing/asthma, who could benefit from targeted therapies or prevention measures.
The strengths of the present study include the criteria for selecting patients, which are based on a strict definition of bronchiolitis in infants <1 year old, thus avoiding the risk of overlaps between bronchiolitis and wheezing; the clear identification of wheezing episodes, based on a pediatrician’s diagnosis, rather than on parents’ reports; and the use of urine specimens for the metabolomic analysis, which can be collected noninvasively—a fundamental consideration for pediatric populations. From a statistical standpoint, a robust procedure for internal model validation was used to support our findings.
Potential limitations of our study mainly concern the small sample size and the brief follow-up period. This aspect may have influenced some results of the study, such as the lack of association between bronchiolitis severity and respiratory outcome, as reported in larger studies [17]. Noteworthy, most of the children included (77%) had RSV bronchiolitis. Further studies including more children with non-RSV bronchiolitis, particularly HRV bronchiolitis, are needed to evaluate whether the metabolomic results are dependent on the virus involved in the acute infection.
It is also important to emphasize that our interpretation of which specific metabolites might be related to the onset of recurrent wheezing is still only speculative. Further studies will be needed to better characterize the biochemical structure of the metabolites that emerged and their role in the onset of recurrent wheezing. Moreover, the present study did not include an independent validation cohort, but these preliminary data represent a first step in improving our understanding of the biological complexity of bronchiolitis and wheezing diseases.
In addition to further investigations to possibly corroborate our preliminary findings, it would be interesting to see whether the basal urinary metabolomic profile (before an episode of acute bronchiolitis) reveals any associations with the subsequent onset of recurrent wheezing and, thus, to establish whether the responsibility lies with the virus-induced bronchiolitis or a preexisting predisposing condition.
In conclusion, the use of metabolomics to evaluate urine samples from infants with acute bronchiolitis (caused by RSV in 77%) enabled us to differentiate children who subsequently developed recurrent wheezing from those who did not. Although these findings demand external validation, they may contribute to a better understanding of the pathogenic mechanisms behind the inception of wheezing disorders after acute bronchiolitis. The children who developed recurrent wheezing were characterized by disruptions in the citric acid cycle and amino acid metabolism, but further investigations are needed to clarify the possible role of these changes. For now, the most interesting result emerging from our study remains the characteristic overall metabolomic profile associated with the onset of recurrent wheezing after acute bronchiolitis. This finding can pave the way to the development of a noninvasive tool for the early identification of infants at high risk of wheezing who could benefit from targeted therapies or prevention measures.
Supplementary Material
Notes
Disclaimer. The funding source had no role in the design of the study, the collection, analysis and interpretation of the data, the writing of the report, or the decision to submit the article for publication.
Financial support. This work was supported by the Bando Ricerca Pediatrica 2012–2014, Fondazione Cassa di Risparmio di Padova e Rovigo.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mazur NI, Martinón-Torres F, Baraldi E, et al. Respiratory Syncytial Virus Network (ReSViNET) Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med 2015; 3:888–900. [DOI] [PubMed] [Google Scholar]
- 2. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med 2015; 191:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berry CE, Billheimer D, Jenkins IC, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med 2016; 194:607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu S, Hartert TV, Everard ML, et al. Predictors of asthma following severe respiratory syncytial virus (RSV) bronchiolitis in early childhood. Pediatr Pulmonol 2016; 51:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carraro S, Giordano G, Reniero F, Perilongo G, Baraldi E. Metabolomics: a new frontier for research in pediatrics. J Pediatr 2009; 154:638–44. [DOI] [PubMed] [Google Scholar]
- 7. Nicholson JK, Wilson ID. Opinion: understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov 2003; 2:668–76. [DOI] [PubMed] [Google Scholar]
- 8. Atzei A, Atzori L, Moretti C, et al. Metabolomics in paediatric respiratory diseases and bronchiolitis. J Matern Fetal Neonatal Med 2011; 24(Suppl 2):59–62. [DOI] [PubMed] [Google Scholar]
- 9. Carraro S, Bozzetto S, Giordano G, et al. Wheezing preschool children with early-onset asthma reveal a specific metabolomic profile. Pediatr Allergy Immunol 2018; 29:375–82. [DOI] [PubMed] [Google Scholar]
- 10. Adamko DJ, Saude E, Bear M, Regush S, Robinson JL. Urine metabolomic profiling of children with respiratory tract infections in the emergency department: a pilot study. BMC Infect Dis 2016; 16:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bozzetto S, Pirillo P, Carraro S, et al. Metabolomic profile of children with recurrent respiratory infections. Pharmacol Res 2017; 115:162–7. [DOI] [PubMed] [Google Scholar]
- 12. Chong I-G, Jun C-H. Performance of some variable selection methods when multicollinearity is present. Chemom Intell Lab Syst 2005; 78:103–12. [Google Scholar]
- 13. Arredouani A, Stocchero M, Culeddu N, et al. ; D.E.S.I.R. Study Group Metabolomic profile of low-copy number carriers at the salivary α-amylase gene suggests a metabolic shift toward lipid-based energy production. Diabetes 2016; 65:3362–8. [DOI] [PubMed] [Google Scholar]
- 14. Justicia-Grande AJ, Pardo-Seco J, Cebey-López M, et al. ; Respiratory Syncytial Virus network (ReSVinet) Development and validation of a new clinical scale for infants with acute respiratory infection: the ReSVinet scale. PLoS One 2016; 11:e0157665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stocchero M, Paris D. Post-transformation of PLS2 (ptPLS2) by orthogonal matrix: a new approach for generating predictive and orthogonal latent variables. J Chemom 2016; 30:242–51. [Google Scholar]
- 16. Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999; 354:541–5. [DOI] [PubMed] [Google Scholar]
- 17. Carroll KN, Wu P, Gebretsadik T, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol 2009; 123:1055–61, 1061.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65:1045–52. [DOI] [PubMed] [Google Scholar]
- 19. Midulla F, Pierangeli A, Cangiano G, et al. Rhinovirus bronchiolitis and recurrent wheezing: 1-year follow-up. Eur Respir J 2012; 39:396–402. [DOI] [PubMed] [Google Scholar]
- 20. Rossi GA, Colin AA. Infantile respiratory syncytial virus and human rhinovirus infections: respective role in inception and persistence of wheezing. Eur Respir J 2015; 45:774–89. [DOI] [PubMed] [Google Scholar]
- 21. Blanken MO, Rovers MM, Molenaar JM, et al. ; Dutch RSV Neonatal Network Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–9. [DOI] [PubMed] [Google Scholar]
- 22. Mochizuki H, Kusuda S, Okada K, Yoshihara S, Furuya H, Simões EAF; Scientific Committee for Elucidation of Infantile Asthma Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. six-year follow-up study. Am J Respir Crit Care Med 2017; 196:29–38. [DOI] [PubMed] [Google Scholar]
- 23. Chawes BL, Poorisrisak P, Johnston SL, Bisgaard H. Neonatal bronchial hyperresponsiveness precedes acute severe viral bronchiolitis in infants. J Allergy Clin Immunol 2012; 130:354–61.e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turner SW, Young S, Landau LI, Le Souëf PN. Reduced lung function both before bronchiolitis and at 11 years. Arch Dis Child 2002; 87:417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turi KN, Romick-Rosendale L, Ryckman KK, Hartert TV. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J Allergy Clin Immunol 2018; 141:1191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carraro S, Giordano G, Reniero F, et al. Asthma severity in childhood and metabolomic profiling of breath condensate. Allergy 2013; 68:110–7. [DOI] [PubMed] [Google Scholar]
- 27. Carraro S, Rezzi S, Reniero F, et al. Metabolomics applied to exhaled breath condensate in childhood asthma. Am J Respir Crit Care Med 2007; 175:986–90. [DOI] [PubMed] [Google Scholar]
- 28. Fitzpatrick AM, Park Y, Brown LA, Jones DP. Children with severe asthma have unique oxidative stress-associated metabolomic profiles. J Allergy Clin Immunol 2014; 133:258–61.e251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saude EJ, Skappak CD, Regush S, et al. Metabolomic profiling of asthma: diagnostic utility of urine nuclear magnetic resonance spectroscopy. J Allergy Clin Immunol 2011; 127:757–64.e1–6. [DOI] [PubMed] [Google Scholar]
- 30. Hasegawa K, Stewart CJ, Celedón JC, Mansbach JM, Tierney C, Camargo CA Jr. Circulating 25-hydroxyvitamin D, nasopharyngeal airway metabolome, and bronchiolitis severity. Allergy 2018; 73:1135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stewart CJ, Mansbach JM, Wong MC, et al. Associations of nasopharyngeal metabolome and microbiome with severity among infants with bronchiolitis. A multiomic analysis. Am J Respir Crit Care Med 2017; 196:882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stewart CJ, Hasegawa K, Wong MC, et al. Respiratory syncytial virus and rhinovirus bronchiolitis are associated with distinct metabolic pathways. J Infect Dis 2018; 217:1160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oexle H, Gnaiger E, Weiss G. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim Biophys Acta 1999; 1413:99–107. [DOI] [PubMed] [Google Scholar]
- 34. Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015; 7:307ra152. [DOI] [PubMed] [Google Scholar]
- 35. Hasegawa K, Linnemann RW, Mansbach JM, et al. The fecal microbiota profile and bronchiolitis in infants. Pediatrics 2016; 138:e20160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juge N, Tailford L, Owen CD. Sialidases from gut bacteria: a mini-review. Biochem Soc Trans 2016; 44:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.