Abstract
Rocio virus (ROCV) is a highly neuropathogenic mosquito-transmitted flavivirus responsible for an unprecedented outbreak of human encephalitis during 1975–1976 in Sao Paulo State, Brazil. Previous studies have shown an increased number of inflammatory macrophages in the central nervous system (CNS) of ROCV-infected mice, implying a role for macrophages in the pathogenesis of ROCV. Here, we show that ROCV infection results in increased expression of CCL2 in the blood and in infiltration of macrophages into the brain. Moreover, we show, using CCR2 knockout mice, that CCR2 expression is essential for macrophage infiltration in the brain during ROCV infection and that the lack of CCR2 results in increased disease severity and mortality. Thus, our findings show the protective role of CCR2-mediated infiltration of macrophages in the brain during ROCV infection.
Keywords: ROCV, encephalitis, CCL2, macrophages, pathogenesis
Rocio virus (ROCV) is a highly neuropathogenic mosquito-transmitted flavivirus responsible for an unprecedented outbreak of human encephalitis in Brazil. Here, we show that infiltrated macrophages in the brain may be protective in the pathogenesis of ROCV in a mouse model.
In 1975, an unprecedented outbreak of encephalitis occurred in the Ribeira Valley of Sao Paulo State in Brazil, affecting >900 people across 20 municipalities, with a case-fatality rate of 13% (approximately 117 deaths) [1, 2]. Clinical symptoms included fever, vomiting, malaise, severe headache, and photophobia, and signs of encephalitis, including meningeal irritation, consciousness alterations, and motor impairment, appeared later [3]. Most importantly, around 20% of the survivors (approximately 156 people) from the outbreak developed permanent neurological disorders, such as motor impairment and cerebral dysfunctions, affecting speech and visual capabilities [2, 3].
The causative agent was determined to be Rocio virus (ROCV), a member of the Flavivirus genus, which consists of other encephalitic viruses, such as West Nile virus (WNV), Japanese encephalitis virus (JEV), and St. Louis encephalitis virus, within the Flaviviridae family [4–6].
Interestingly, no further human outbreaks of ROCV have been described since the 1975–1976 outbreak. However, numerous serological surveys indicated the possibility of ROCV circulation among asymptomatic humans and native animals in different regions of Brazil [7–11]. The absence of further ROCV outbreaks in humans points to the possibility of cross-protective immunity elicited by prior infections with other circulating flaviviruses within the Brazilian population. Indeed, we have recently shown in a mouse model that a single preexposure to Ilheus virus resulted in complete protection from lethal ROCV challenge [12]. Despite its potential for further geographical spread, little is known about ROCV pathogenesis. In a mouse model of ROCV infection, a high virus titer was detected in the brain, and an elevated number of inflammatory macrophages was found in the central nervous system (CNS) [13, 14], implying a role for macrophages during infection. However, further details on the role of these cells in the pathogenesis of ROCV have not been reported.
The CNS contains resident innate immune sentinels such as microglia that are among the first responders to infection, but peripheral leukocytes, including monocytes, and T cells, are nevertheless recruited to aid in local inflammatory responses. These cells are reported to play a role in neurotropic viral infections, including those due to WNV, coronavirus (mouse hepatitis virus), and herpes simplex virus type 1 [13–17]. In addition, migration of monocytes to the brain is heavily dependent on the interaction between CCL2 and its receptor, CCR2 [18–20]. As such, we hypothesized that CCL2 and CCR2 may play a role in the migration of monocytes to the brain during ROCV infection.
Here, we showed that ROCV infection induces the production of CCL2 in the blood and brain, resulting in increased infiltration of macrophages and CD8+ T lymphocytes into the brain. We also showed, using CCR2 knockout (ie, Ccr2−/−) mice, that CCR2 is required for efficient infiltration of macrophages into the CNS, which is associated with a reduction in disease severity and mortality.
METHODS
Virus and Cells
ROCV (SPH 34675 strain) was passaged on Aedes albopictus (C6/36) cells and titrated by a plaque assay on BHK-21 cells as previously described [21].
Mice Infection
Wild-type (WT) and Ccr2−/− C57BL/6 female mice (age, 6 weeks) were obtained from the Central Animal Facility at the University of Sao Paulo, Ribeirao Preto. All animal experiments were performed according to the guidelines of the Brazilian College of Animal Experimentation. The Ethics Committee on Animal Experimentation of the Medical School of Ribeirao Preto, University of Sao Paulo, approved this study (permits 022/2015-1 and 006/2017-1). All in vitro and in vivo experiments were performed in a biosafety level 3 facility at the Medical School of Ribeirao Preto, University of Sao Paulo. The C57BL/6 mice were infected intraperitoneally with 2.8 × 106 or 2.8 × 102 plaque-forming unit (PFU)/mouse of ROCV. Signs of encephalitis were monitored and scored from 0 to 5 as previously described [12, 22].
Blood Specimen and Organ Collection
Blood specimens were obtained from the retro-orbital region and collected in a tube containing 3.8% sodium citrate buffer [23]. Mice were perfused intracardially with 20 mL of 0.9% NaCl solution to remove blood from the tissues. Subsequently, the following organs were collected in preweighted tubes: spleen, kidney, liver, brain, lung, heart, spinal cord, and the following regions of the CNS: olfactory bulb, cerebellum, brain stem, hypothalamus, white matter, hippocampus, and cortex. Additionally, the bone marrow from the left femur was removed using 1 mL of saline solution. The organs were homogenized using a tissue homogenizer (UltraStirrer, Biosystems, PR, Brazil) and clarified by centrifugation at 8000 × g for 5 minutes. Supernatants were collected and stored at −80°C. Viral loads in serum and tissue samples were determined by a plaque assay on BHK-21 cells. The limit of detection of the plaque assay was 50 PFU/mL (for serum and bone marrow specimens) and 66 PFU/g for all tissue specimens. For hematological parameter assessments, platelets, red blood cells, and leukocytes were counted and fully described in the Supplementary Methods.
Leukocyte Isolation From CNS Specimens and Flow Cytometry
Leukocytes in the brain were isolated as previously described [14, 24]. Leukocyte purification and quantification of cytokines and chemokines are briefly described in the Supplementary Methods.
Statistical Analysis
All data were analyzed using Prism software (GraphPad Software). Kaplan-Meier survival curves were analyzed by the log-rank test, and clinical scores were analyzed by the Student t test. For viral burden analysis, data were transformed to log10 titers and analyzed by the Mann-Whitney test or 1-way analysis of variance followed by the Dunnett multiple comparisons test.
RESULTS
Characterization of ROCV Dissemination in the Mouse Model
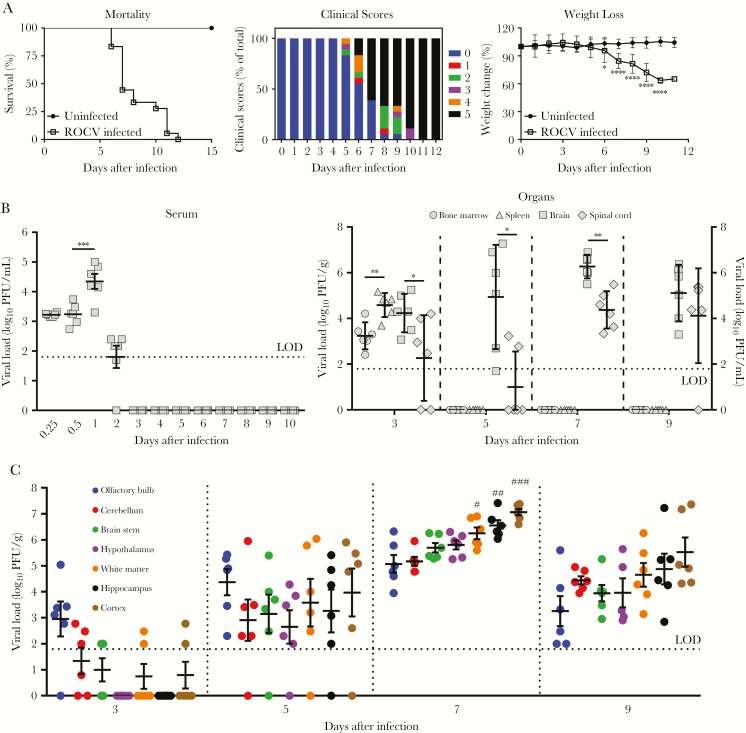
Recently, we showed that C57BL/6 mice are more susceptible than BALB/c mice to ROCV infection, suggesting a more suitable mouse model for viral encephalitis [12]. However, no detailed studies have been performed to characterize ROCV infection in vivo. To address this, C57BL/6 mice were infected intraperitoneally with 2.8 × 106 PFU/mouse of ROCV, which induced significant morbidity, weight loss, and mortality 6–12 days after infection [11]. Consistently, we observed similar results in the present study (Figure 1A). Next, we determined the dissemination of ROCV in the C57BL/6. ROCV was detected in serum samples on the first and second days after infection, while no infectious virus was detected in serum specimens from 3 days after infection onward (Figure 1B). A significant higher level of infectious virus particles was detected in spleens as compared to bone marrow specimens 3 days after infection (Figure 1B). No infectious virus particles were detected in kidneys, lungs, hearts, and livers (data not shown) throughout the infection, suggesting that ROCV does not have tropism and/or the ability to replicate in these tissues. Strikingly, virus particles in brains and spinal cords were readily detected and persisted throughout the infection (Figure 1B). Infectious ROCV titers in brains were significantly higher than those in spinal cords 3–7 days after infection but not 9 days after infection (Figure 1B). These results indicate that ROCV is able to access the brain earlier than it accesses the spinal cord. To further define the regions within the brain that are permissive to and capable of supporting ROCV multiplication, the virus load was quantified in the following major regions of the brain 3, 5, 7, and 9 days after infection: the olfactory bulb, cerebellum, brain stem, hypothalamus, white matter, hippocampus, and cortex. As illustrated in Figure 1C, 3 days after infection, ROCV was detected in all regions except for the hypothalamus and hippocampus. However, 5, 7, and 9 days after infection, ROCV was found in all brain regions, with variation in the viral load among different regions. The highest virus titers were detected in the cortex, hippocampus, and white matter 7 days after infection, suggesting they are preferential replication sites in the brain.
Figure 1.
Viral burden of Rocio virus (ROCV) replication in C57BL/6 mice. Six-week-old female C57BL/6 mice were inoculated intraperitoneally with 2.8 × 106 plaque-forming units (PFU) of Rocio virus (ROCV; 20 mice/group). A, Survival rates (left), the percentage of the total clinical score (middle), and the percentage change in body weight (right) were determined up to 21 days after infection. B, Viral load in serum (left) and organ (right) specimens. C, Viral load in the major brain regions. Virus titers were determined by the plaque assay on BHK-21 cells and are presented as PFU per gram of tissue (for all tissue specimens) or PFU per milliliter (for serum or bone marrow specimens). #Statistically significant difference as compared to the olfactory bulb (P < .01) and cerebellum (P < .01). ##Statistically significant difference as compared to the olfactory bulb (P < .0001), cerebellum (P < .001), and brain stem (P < .05). ###Statistically significant difference as compared to the olfactory bulb (P < .0001), cerebellum (P < .0001), brain stem (P < .001), and hypothalamus (P < .01). None of the differences among the cortex, hippocampus, and white matter were statistically significant. In panels B and C, the horizontal dotted lines correspond to the limit of detection (LOD) of the plaque assay. *P < .05, **P < .01, ***P < .001, and ****P < .0001, by the Student t test (A), the Mann-Whitney test (B), or 1-way analysis of variance with the Dunnett multiple comparisons test (C).
ROCV Infection Induces Monocytosis, Leukopenia and Lymphopenia in the Peripheral Blood
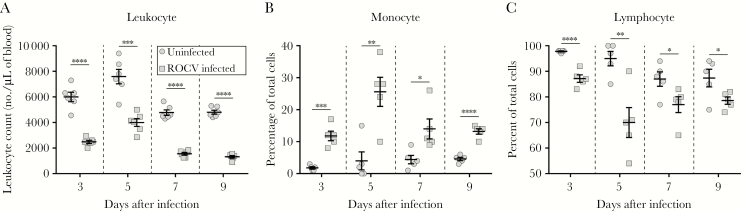
Previously, it was reported that levels of multiple leukocytes, such as CD8+ T lymphocytes, macrophages, neutrophils, and CD45R+ B lymphocytes, were elevated in response to ROCV infection in the CNS of BALB/c mice [13, 14]. However, no leukocyte population was assessed in blood specimens in an animal model. Here, we found that ROCV infection caused leukopenia 3–9 days after infection (Figure 2A). Furthermore, detailed analysis revealed an increased percentage of monocytes (Figure 2B) and a reduction in the percentage of lymphocytes (Figure 2C), suggesting a role for monocytes during ROCV infection in C57BL/6 mice. Additionally, levels of hematocrit, platelets, and erythrocytes between the ROCV-infected and naive mice were assessed and shown to be consistently similar in both groups (Supplementary Figure 1A–C). Last, no significant differences in hepatic tissue damage between naive and ROCV-infected mice was observed by measuring the levels of alanine transaminase and aspartate transaminase in the animal sera (Supplementary Figure 1D and 1E).
Figure 2.
Leukocyte, monocyte, and lymphocyte levels in blood of Rocio virus (ROCV)–infected mice. Six-week-old female C57BL/6 mice were inoculated intraperitoneally with 2.8 × 106 plaque-forming units (PFU) of ROCV and had blood specimens collected at various time points after inoculation, to determine the leukocyte (A), monocyte (B), and lymphocyte (C) levels. Scatter plots comprise data for 5–6 mice per group at each time point. *P < .05, **P < .01, ***P < .001, and ****P < .0001, by the Mann-Whitney test, for comparison of infected and uninfected mice.
ROCV Infection Induces Infiltration of Macrophages and CD8+ T Cells Into the CNS
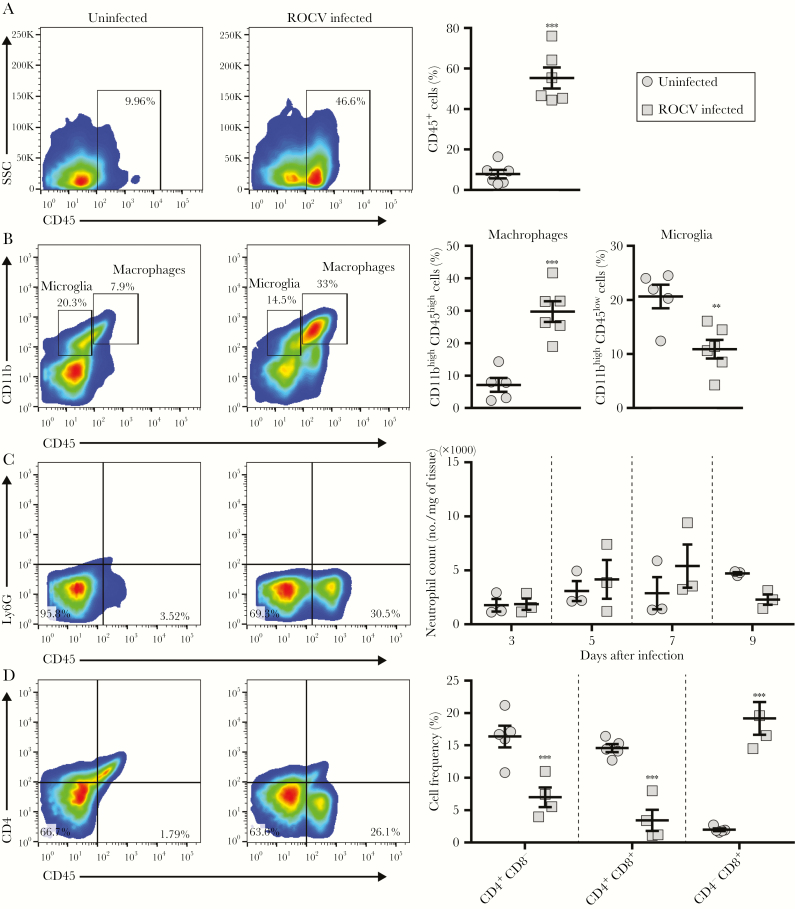
Next, we sought to identify the types of leukocytes in the CNS during ROCV infection. Leukocytes isolated from brains of infected and uninfected animals were phenotyped by flow cytometry (Figure 3). Leukocyte counts were significantly increased in brains from ROCV-infected animals as compared to brains from uninfected animals (Figure 3). Consistent with this finding, histopathologic analysis of ROCV-infected brains showed infiltration of mononuclear leukocytes into the perivascular space, as well as the neuropil (Supplementary Figure 2A and 2B). In association with leukocyte infiltration, there were occasionally multifocal areas of neuropilar rarefaction suggestive of edema (Supplementary Figure 2C). To distinguish between infiltrated macrophages and resident microglia, CD45 was used as a differentiating marker. We observed an increased percentage of macrophages (CD11bhigh CD45high cells) in brains from infected mice as compared to those from uninfected mice (Figure 3B). The level of neutrophils (Ly6G+CD45+ cells) was analyzed by flow cytometry (Figure 3C) and by myeloperoxidase activity assays (Figure 3C), and there was no difference between infected and uninfected animals. Intriguingly, we found that the percentages of CD4+CD8− and CD4+CD8+ T cells were significantly lower, whereas the percentage of CD4−CD8+ T cells was significantly higher in brains from infected animals as compared to those from uninfected animals (Figure 3D). Taken together, these data suggest that macrophages and cytotoxic T cells may play important roles in ROCV-induced encephalitis.
Figure 3.
Characterization of the immune cell profile in the brain after Rocio virus (ROCV) infection. Six-week-old female C57BL/6 mice (n = 3–6) were inoculated intraperitoneally with 2.8 × 106 plaque-forming units (PFU) of ROCV or medium. Brains were collected 7 days after infection, and the total leukocyte population was purified. Flow cytometry plots indicate gating strategies and percentages of total leukocytes (CD45+); A), macrophages (CD11bhighCD45high) and microglia (CD11bhighCD45low); B), neutrophils (Ly6G+CD45+) cells in left 2 panels and myeloperoxidase levels in the right panel; C), and T cells (CD4+/CD8+); D). **P < .01 and ***P < .001, by the Mann-Whitney test, for comparison of infected and uninfected mice.
ROCV Infection Promotes CCL2 Production Systemically and in the Brain
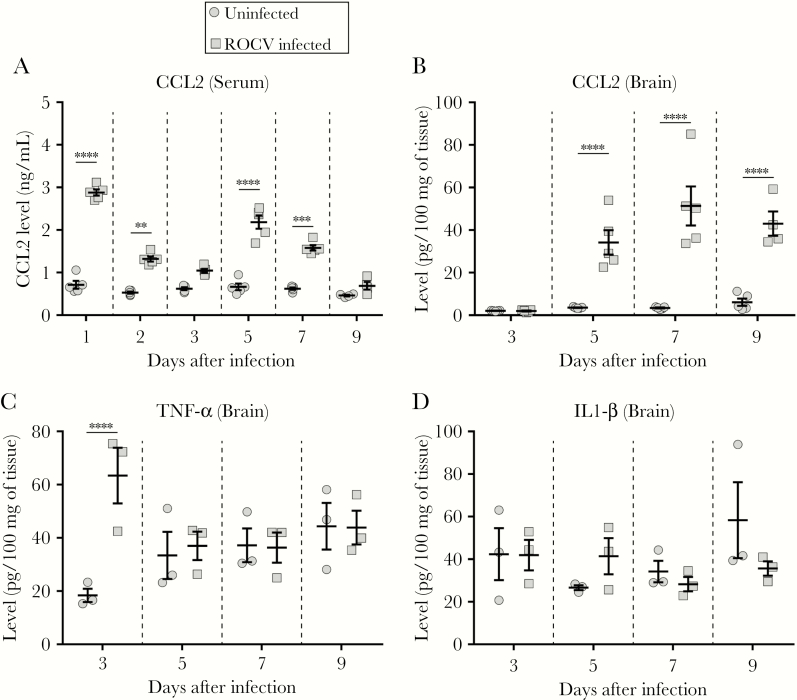
As we observed a significant increase in the number of monocytes and macrophages in brains from ROCV-infected mice, we hypothesized that inflammatory chemokines and/or cytokines could play an important role for recruitment of these cells into the brain. CCL2 has been reported to be crucial for the migration of monocytes across the blood-brain barrier during WNV infection [25, 26]. Therefore, to evaluate the role of CCL2 in response to ROCV infection, levels of CCL2, tumor necrosis factor α (TNF-α), and interleukin 1β (IL-1β) were quantified in blood specimens, livers, and brains from infected mice. We found that CCL2 levels were significantly increased in serum specimens from infected animals 1–7 days after infection as compared to those from uninfected animals (Figure 4A). Interestingly, a progressive increase in CCL2 levels 5, 7 and 9 days after infection (Figure 4B) was also observed in brains from infected mice as compared to those from uninfected mice. Expression of TNF-α in brains from infected mice was increased only at 3 days after infection (Figure 4C). No differences were observed between infected and uninfected mice in the level of IL-1β in brains (Figure 4D); levels of CCL2, TNF-α, and IL-1β in livers (Supplementary Figure 3A–C; and levels of CCL2, TNF-α, and IL-1β in lungs (Supplementary Figure 3D–F). These data suggest that the recruitment of macrophages into the brain could be driven by upregulation of CCL2.
Figure 4.
Cytokine and chemokine levels in peripheral blood specimens and brains obtained from C57BL/6 mice after Rocio virus (ROCV) infection. Six-week-old female C57BL/6 mice were inoculated intraperitoneally with 2.8 × 106 plaque-forming units (PFU) of ROCV. Blood and tissue specimens were collected at various time points after inoculation (from 3–6 mice per group at each time point). The level of CCL2 was determined in serum specimens (A), while levels of CCL2 (B), tumor necrosis factor α (TNF-α; C), and interleukin 1β (IL-1β; D) were assessed in brains. **P < .01, ***P < .001, and ****P < .0001, by the Mann-Whitney test.
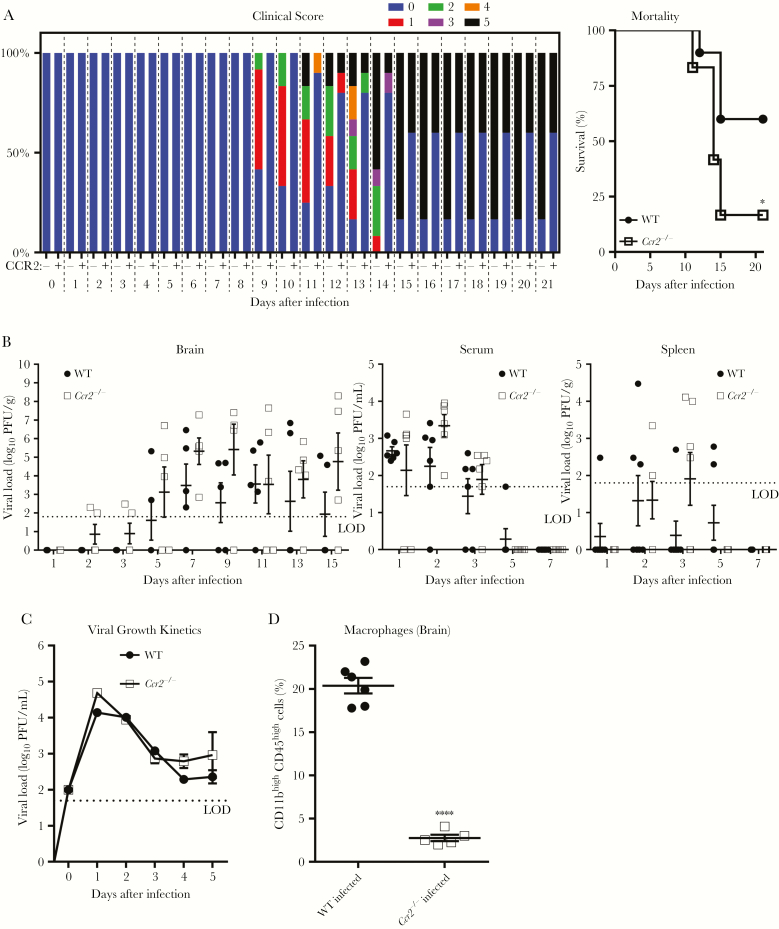
CCR2 Is Required for Efficient Infiltration of Macrophages Into the CNS to Control Disease Severity Caused by ROCV Infection
Given the well-documented role of CCL2 and its corresponding receptor, CCR2, in recruitment of macrophages in response to infections [18, 19, 25], we next investigated the role of CCR2 in the ROCV-induced encephalitis. By contrasting WT and Ccr2−/− C57BL/6 mice, we showed that Ccr2−/− mice displayed an increased disease severity (Figure 5A) and enhanced mortality rate (Figure 5A), compared with WT mice. These effects appeared not to be associated with the increased viral load, as both WT and Ccr2−/− mice showed similar virus titers in CNS specimens (Figure 5B), serum specimens (Figure 5B), and spleen specimens (Figure 5B). No virus was detected in livers, kidneys, lungs, bone marrow specimens, and hearts from either WT or Ccr2−/− mice (data not shown), indicating that CCR2 deficiency did not alter viral tropism. Further analysis using macrophages derived from bone marrow specimens from WT and Ccr2−/− C57BL/6 mice demonstrated that CCR2 did not promote nor inhibit ROCV infection (Figure 5C). These data provide evidence that the lack of CCR2 does not result in an increased viral burden, indicating that CCR2 must protect by some mechanism other than directly limiting viral replication. To reinforce our findings, we investigated the role of CCR2 in the infiltration of macrophages during ROCV infection. We observed that the percentage of infiltrated macrophages in brains from infected Ccr2−/− C57BL/6 mice was significantly lower than in brains from WT mice (Figure 5D), suggesting that a decreased percentage of macrophages infiltrated across the blood-brain barrier, which correlates with the increased morbidity and mortality seen during ROCV infection.
Figure 5.
Effect of CCR2 on controlling disease severity and macrophage infiltration into the brain after Rocio virus (ROCV) infection. Wild-type (WT) and CCR2 knockout (ie, Ccr2−/−) C57BL/6 female mice (age, 6 weeks) were inoculated intraperitoneally with 2.8 × 102 plaque-forming units (PFU) of ROCV (12 mice/group). A, Percentage of the total clinical score (left) and survival rate (right) were determined up to 21 days after infection. B, Viral load in the brain (left), serum (middle), and spleen (right) were determined at different time points. No differences in viral load in the brain, serum, and spleen at different time points were statistically significant, by the Mann-Whitney test. C, Viral growth kinetics in bone marrow–derived macrophages from WT or Ccr2−/− mice. Macrophages were infected at multiplicity of infection of 1 with ROCV, and cell supernatants were harvested 0, 1, 2, 3, 4, and 5 days after infection. No differences in viral load at different time points were statistically significant, by 2-way analysis of variance with the Tukey multiple comparisons test. D, Percentage of infiltrated macrophages (CD11bhighCD45high) in the brains of ROCV-infected WT and Ccr2−/− mice (5–6 mice/group). Virus titers were determined by a plaque assay on BHK-21 cells and are presented as PFU per gram of tissue or PFU per milliliter (for serum specimens). In scatter plots, each data point represents an individual mouse, and data from 3–6 mice per group at each time point were analyzed. Dotted lines denote the assay limits of detection. *P < .05, by the log-rank (Mantel-Cox) test; ****P < .0001, by the Mann-Whitney test.
DISCUSSION
In this study, we characterized ROCV infection, a potentially emerging mosquito-transmitted flavivirus, using an immunocompetent C57BL/6 mouse model. The increased expansion of arthropod vectors raises the possibility of ROCV spreading to new geographic areas. Several serological surveys indicated the possibility of ROCV circulation among asymptomatic humans, horses, and wild animals in different regions of Brazil [7–11]. The pathogenesis of ROCV still remains poorly understood, and a suitable animal model mimicking human disease for ROCV has not been well described and characterized. In this study, to characterize in vivo ROCV infection, we used a C57BL/6 mouse model, which we have previously demonstrated as being more susceptible to ROCV infection than BALB/c mice [12]. Our results showed that the CNS regions (the spinal cord and brain) are preferential targets of ROCV, further confirming the neurotropic phenotype of ROCV [2, 3, 13, 27]. Interestingly, the highest viral loads were found in the cortex and hippocampus of the CNS, in agreement with histopathologic analysis performed during autopsies of humans infected with ROCV, which showed damage of the same regions in the CNS [27]. Thus, these data could explain the high mortality rate and irreversible damage observed in ROCV-infected individuals [3, 27]. However, it is still unclear how the virus is able to invade the CNS. Although our recent study has shown that the prM-E proteins of ROCV are responsible for neuroinvasion of ROCV and mortality in C57BL/6 mice [21], the exact residues in these proteins and the mechanisms responsible for neuropathogenesis of ROCV infection are still unknown. It is worth noting that ROCV was readily detected in olfactory bulbs 3 days after infection, suggesting that this may be a possible route for ROCV invasion into the CNS.
Given that ROCV can be detected in both blood specimens and CNS specimens, we proceeded to characterize the hematological profiles. Our data demonstrated that blood leukocyte counts were reduced during the course of infection, which is in agreement with observations in ROCV-infected patients [3]. Humans infected with WNV and JEV also showed a decreased number of leukocytes and lymphocytes [28–30]. In contrast to levels in blood, CD45+ cells, which represent total leukocytes [31, 32], were detected at high levels in brains from ROCV-infected mice, suggesting that ROCV infection results in significant infiltration of immune cells into the brain. Interestingly, further detailed analysis showed an increase in the percentage of monocytes and CD11bhighCD45high macrophages in blood specimens and brains from ROCV-infected mice, respectively. The trafficking of inflammatory monocytes has been linked to the expression of CCR2 and production of CCL2 [18], which was also observed previously with WNV infection [15, 25]. Here, we detected an elevated CCL2 level in serum specimens 1 day after infection, while no significant increase in the CCL2 level was detected in the brain during this period. This implies that the high level of CCL2 in the blood may correspond to monocytosis. It is well known that increased CCL2 levels in the blood promote the egress of monocytes from the bone marrow to interact with the corresponding CCR2 receptor expressed on the surface of monocytes [25, 33–35].
Not surprisingly, an increased percentage of CD8+ T cells was detected in brains from ROCV-infected C57BL/6 mice. This is consistent with other ROCV studies involving BALB/c mice [13, 14], as well as with findings reported for WNV [25, 26], suggesting a role of CD8+ T cells during ROCV infection. Somewhat surprisingly, we observed no neutrophil infiltration in brains from ROCV-infected C57BL/6 mice. However, neutrophil infiltration into the brain was reported for ROCV-infected BALB/c mice [13]. This suggests that different genetic backgrounds may influence neutrophil infiltration into the brain in response to ROCV infection, resulting in a different neuropathogenesis during ROCV infection. Similarly, an absence of neutrophils was observed in brains during autopsies of the majority of humans during the ROCV outbreak [27]. Interestingly, the role of infiltrated neutrophils in the CNS is well described in viral-induced encephalitis [36, 37], as it was found to determine permissiveness to WNV infection, thereby acting as a Trojan horse for delivering the virus to the CNS [38]. It is likely that ROCV may exploit other cell types to invade the CNS, but further investigations are required to test this hypothesis.
We also found a significant reduction in the percentage of microglia cells (CD11b+CD45−) in the brain. We believe that depletion of the microglia cells in ROCV-infected mice could be attributed to deterioration of the neuronal tissues as a consequence of proinflammatory cytokines (TNF-α and CCL-2) produced by glial cells and infiltrated leukocytes. Thus, our findings support observations described by Barros et al, who showed that ROCV infection induced neuronal degeneration and apoptosis induced by inflammatory cytokines from glial and macrophage cells [13]. Interestingly, our mouse model showed no difference in IL-1β levels between ROCV-infected and uninfected mice in any tissue, while increased levels of IL-1β were observed in BALB/c mice [13]. It is worth noting that WNV and JEV infections induce significant levels of IL-1β in the CNS [39, 40]. Hence, our data suggest that different innate immunity processes may be induced by these closely related viruses.
Because we found that CCL2 levels were significantly increased during ROCV infection, we further investigated the role of this mediator in Ccr2−/− mice. We found that the increased disease severity and mortality in Ccr2−/− mice was associated with a reduction in the number of infiltrated macrophages and that this did not correlate with the observed viral load in the brain. An associated impairment in macrophage migration into the brain from the bloodstream in Ccr2−/− mice supports the notion that migration of macrophages into an infected brain is dependent on CCR2. It is possible that infiltrated macrophages in the brain may contribute to an antiviral effect by producing inflammatory cytokines such as TNF-α. This has previously been shown to increase disease severity and mortality in JEV and TBEV infections, using a Tnfa−/− mouse model, without influencing the viral load in the brain [41, 42]. In addition, it is also possible that other CCR2-expressing cells, such as CD8+ T lymphocytes, that we showed to have infiltrated the brains of ROCV-infected WT mice may play an active role in antiviral activity during ROCV infection. Together, this may explain the protective role of infiltrated macrophages in combination with other immune cells, such as T lymphocytes, against ROCV in the CNS without directly influencing the viral load.
Additionally, Ccr2−/− mice have been reported to exacerbate the outcome of disease promoted by other virus infections [26, 43–45]. Interestingly, CCL7, which also binds CCR2, has been reported to be involved in efficient recruitment of CD8+ T cells into the CNS that are required for effective viral clearance and survival [25]. Further studies evaluating the adoptive transfer of CCR2-sufficient cells into ROCV-infected Ccr2−/− mice should yield important insight regarding how macrophages/T cells influence ROCV antiviral responses.
In conclusion, we showed that ROCV preferentially replicates in the CNS, particularly in the cortex and hippocampus. Additionally, ROCV promotes increased numbers of monocytes in the periphery and infiltrated macrophages in the CNS. The lack of CCL2 receptor resulted in decreased infiltration of macrophages into the CNS and increased disease severity and mortality in ROCV-induced encephalitis, suggesting a protective role of infiltrated macrophages in the pathogenesis of ROCV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Sao Fundação de Amparo à Pesquisa do Estado de São Paulo (grants 2012/11169-3, 2014/15548-4, and 2014/02438-6; scholarship 2012/08778-8 to A. A. A.); the Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (scholarship 97/16 to A. A. A.); and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (scholarship 306471/2017-5 to V. H. A.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Acknowledgments. We thank Dr Willy W. Suen, for analysis of histopathologic data; Morgan E. Freney, for proofreading the manuscript; Soraya Jabur Badra, Sueli Aparecida Pereira Abramovicius, and Antonio Claudinho da Silva, for technical assistance; and the staff of the Virology Research Center of Ribeirao Preto Medical School, University of Sao Paolo, for use of its biosafety level 3 facilities.
References
- 1. Karabatsos N. Supplement to international catalogue of arboviruses including certain other viruses of vertebrates. Am J Trop Med Hyg 1978; 27:372–440. [DOI] [PubMed] [Google Scholar]
- 2. de Souza Lopes O, Coimbra TL, de Abreu Sacchetta L, Calisher CH. Emergence of a new arbovirus disease in Brazil. I. Isolation and characterization of the etiologic agent, Rocio virus. Am J Epidemiol 1978; 107:444–9. [DOI] [PubMed] [Google Scholar]
- 3. Tiriba AC, Miziara AM, Lorenço R, da Costa RB, Costa CS, Pinto GH. [Primary human epidemic encephalitis induced by Arbovirus found at the sea shore south of the State of São Paulo. Clinical study in an emergency hospital]. AMB Rev Assoc Med Bras 1976; 22:415–20. [PubMed] [Google Scholar]
- 4. de Souza Lopes O, de Abreu Sacchetta L, Coimbra TL, Pinto GH, Glasser CM. Emergence of a new arbovirus disease in Brazil. II. Epidemiologic studies on 1975 epidemic. Am J Epidemiol 1978; 108:394–401. [DOI] [PubMed] [Google Scholar]
- 5. Setoh YX, Amarilla AA, Peng NY, et al. Full genome sequence of Rocio virus reveal substantial variations from the prototype Rocio virus SPH 34675 sequence. Arch Virol 2018; 163:255–8. [DOI] [PubMed] [Google Scholar]
- 6. Medeiros DB, Nunes MR, Vasconcelos PF, Chang GJ, Kuno G. Complete genome characterization of Rocio virus (Flavivirus: Flaviviridae), a Brazilian flavivirus isolated from a fatal case of encephalitis during an epidemic in Sao Paulo state. J Gen Virol 2007; 88:2237–46. [DOI] [PubMed] [Google Scholar]
- 7. Straatmann A, Santos-Torres S, Vasconcelos PF, da Rosa AP, Rodrigues SG, Tavares-Neto J. Serological evidence of the circulation of the Rocio arbovirus (Flaviviridae) in Bahia. Rev Soc Bras Med Trop 1997; 30:511–5. [DOI] [PubMed] [Google Scholar]
- 8. Ferreira IB, Pereira LE, Rocco IM, Marti AT, Souza L, Iversson LB. Surveillance of arbovirus infections in the atlantic forest region, State of São Paulo, Brazil: I. detection of hemagglutination-inhibition antibodies in wild birds between 1978 and 1990. Revista do Instituto de Medicina Tropical de São Paulo 1994; 36:265–74. [DOI] [PubMed] [Google Scholar]
- 9. Pauvolid-Corrêa A, Campos Z, Juliano R, Velez J, Nogueira RM, Komar N. Serological evidence of widespread circulation of West Nile virus and other flaviviruses in equines of the Pantanal, Brazil. PLoS Negl Trop Dis 2014; 8:e2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silva JR, Romeiro MF, Souza WM, et al. A Saint Louis encephalitis and Rocio virus serosurvey in Brazilian horses. Rev Soc Bras Med Trop 2014; 47:414–7. [DOI] [PubMed] [Google Scholar]
- 11. Casseb AR, Cruz AV, Jesus IS, et al. Seroprevalence of flaviviruses antibodies in water buffaloes (Bubalus bubalis) in Brazilian Amazon. J Venom Anim Toxins Incl Trop Dis 2014; 20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amarilla AA, Fumagalli MJ, Figueiredo ML, et al. Ilheus and Saint Louis encephalitis viruses elicit cross-protection against a lethal Rocio virus challenge in mice. PLoS One 2018; 13:e0199071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Barros VE, Saggioro FP, Neder L, et al. An experimental model of meningoencephalomyelitis by Rocio flavivirus in BALB/c mice: inflammatory response, cytokine production, and histopathology. Am J Trop Med Hyg 2011; 85:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franca RF, Costa RS, Silva JR, et al. IL-33 signaling is essential to attenuate viral-induced encephalitis development by downregulating iNOS expression in the central nervous system. J Neuroinflammation 2016; 13:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Getts DR, Terry RL, Getts MT, et al. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med 2008; 205:2319–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Templeton SP, Kim TS, O’Malley K, Perlman S. Maturation and localization of macrophages and microglia during infection with a neurotropic murine coronavirus. Brain Pathol 2008; 18:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol 2008; 181:6417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Terry RL, Getts DR, Deffrasnes C, van Vreden C, Campbell IL, King NJ. Inflammatory monocytes and the pathogenesis of viral encephalitis. J Neuroinflammation 2012; 9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011; 11:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 2008; 26:421–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amarilla AA, Setoh YX, Periasamy P, et al. Chimeric viruses between Rocio and West Nile: the role for Rocio prM-E proteins in virulence and inhibition of interferon-α/β signaling. Sci Rep 2017; 7:44642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller SD, Karpus WJ, Davidson TS. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol 2010; Chapter 15:Unit 15.1. [DOI] [PubMed] [Google Scholar]
- 23. Barros VE, dos Santos-Junior NN, Amarilla AA, et al. Differential replicative ability of clinical dengue virus isolates in an immunocompetent C57BL/6 mouse model. BMC Microbiol 2015; 15:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Posel C, Moller K, Boltze J, Wagner DC, Weise G. Isolation and flow cytometric analysis of immune cells from the ischemic mouse brain. J Vis Exp 2016; 53658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bardina SV, Michlmayr D, Hoffman KW, et al. Differential roles of chemokines CCL2 and CCL7 in monocytosis and leukocyte migration during West Nile virus infection. J Immunol 2015; 195:4306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim JK, Obara CJ, Rivollier A, Pletnev AG, Kelsall BL, Murphy PM. Chemokine receptor Ccr2 is critical for monocyte accumulation and survival in West Nile virus encephalitis. J Immunol 2011; 186:471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosemberg S. Neuropathology of S. Paulo south coast epidemic encephalitis (Rocio flavivurus). J Neurol Sci 1980; 45:1–12. [DOI] [PubMed] [Google Scholar]
- 28. Chaturvedi UC, Mathur A, Tandon P, Natu SM, Rajvanshi S, Tandon HO. Variable effect on peripheral blood leucocytes during JE virus infection of man. Clin Exp Immunol 1979; 38:492–8. [PMC free article] [PubMed] [Google Scholar]
- 29. Cunha BA, McDermott BP, Mohan SS. Prognostic importance of lymphopenia in West Nile encephalitis. Am J Med 2004; 117:710–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cunha BA, Minnaganti V, Johnson DH, Klein NC. Profound and prolonged lymphocytopenia with West Nile encephalitis. Clin Infect Dis 2000; 31:1116–7. [DOI] [PubMed] [Google Scholar]
- 31. Sedgwick JD, Schwender S, Imrich H, Dörries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A 1991; 88:7438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res 2005; 81:374–89. [DOI] [PubMed] [Google Scholar]
- 33. Mildner A, Mack M, Schmidt H, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 2009; 132:2487–500. [DOI] [PubMed] [Google Scholar]
- 34. El Khoury J, Luster AD. Mechanisms of microglia accumulation in Alzheimer’s disease: therapeutic implications. Trends Pharmacol Sci 2008; 29:626–32. [DOI] [PubMed] [Google Scholar]
- 35. D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci 2009; 29:2089–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michlmayr D, Bardina SV, Rodriguez CA, Pletnev AG, Lim JK. Dual function of Ccr5 during langat virus encephalitis: reduction in neutrophil-mediated central nervous system inflammation and increase in T cell-mediated viral clearance. J Immunol 2016; 196:4622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang P, Bai F, Zenewicz LA, et al. IL-22 signaling contributes to West Nile encephalitis pathogenesis. PLoS One 2012; 7:e44153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bai F, Kong KF, Dai J, et al. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis 2010; 202:1804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramos HJ, Lanteri MC, Blahnik G, et al. IL-1β signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog 2012; 8:e1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghoshal A, Das S, Ghosh S, et al. Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia 2007; 55:483–96. [DOI] [PubMed] [Google Scholar]
- 41. Hayasaka D, Shirai K, Aoki K, et al. TNF-α acts as an immunoregulator in the mouse brain by reducing the incidence of severe disease following Japanese encephalitis virus infection. PLoS One 2013; 8:e71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tun MM, Aoki K, Senba M, et al. Protective role of TNF-α, IL-10 and IL-2 in mice infected with the Oshima strain of tick-borne encephalitis virus. Sci Rep 2014; 4:5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Käufer C, Chhatbar C, Bröer S, et al. Chemokine receptors CCR2 and CX3CR1 regulate viral encephalitis-induced hippocampal damage but not seizures. Proc Natl Acad Sci U S A 2018; 115:E8929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Menasria R, Canivet C, Piret J, Gosselin J, Boivin G. Both cerebral and hematopoietic deficiencies in CCR2 result in uncontrolled herpes simplex virus infection of the central nervous system in mice. PLoS One 2016; 11:e0168034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Held KS, Chen BP, Kuziel WA, Rollins BJ, Lane TE. Differential roles of CCL2 and CCR2 in host defense to coronavirus infection. Virology 2004; 329:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.