Abstract
Conjugated linoleic acid (CLA) is produced from linoleic acid (LA) by bacteria in the rumen of herbivores. CLA enters the human diet mainly via milk fat and fatty beef; it acts as an effective anticarcinogen and exhibits other important physiological effects. The objective of the current study was to investigate the capability of a LA-conjugating bacterial community isolated from a human volunteer and associated with germ-free rats to supply the host with CLA. Gnotobiotic rats were fed a diet enriched with esterified LA in the form of sunflower-seed oil. The control group was fed the same diet and remained germ-free. Bacterial cell counts, in vitro LA-conjugation activities, and CLA concentration in feces and in the contents of various intestinal segments were determined. After 10 wk, various tissues were analyzed for CLA concentrations. LA-conjugation activity was found only in feces, cecum and colon content samples from associated rats, but CLA accumulation in various body tissues did not differ significantly between the two groups. The ratio of CLA to LA in feces and in cecal and colonic contents did not differ between groups, indicating that the microorganisms in the cecum and the colon do not synthesize substantial amounts of CLA in vivo and therefore, do not contribute to the CLA supplementation of the host.
Keywords: anticarcinogen, conjugated linoleic acid, gnotobiotic rats, human diet, intestinal microorganisms
In the rumen of herbivores, linoleic acid (LA)2 (9c,12c-octadecadienoic acid) is a substrate of a bacterial activity that isomerizes the isolated double bonds to a conjugated system (Kepler et al. 1966). In this reaction, a mixture of isomers of conjugated linoleic acid (CLA) is produced that differs with respect to the position of the double bonds (Δ9Δ11 or Δ10Δ12) and the cis-trans stereochemistry (Ha et al. 1987). As a result of this activity, milk fat and body fat of ruminants are enriched with 8.9–87 μmol CLA/g fat, depending on the feeding regimen. These fats are the main source of CLA in the human diet. However, small amounts of CLA are also found in the body fat of monogastric animals as well as in plant oils (Chin et al. 1992, Ha et al. 1989, Jiang et al. 1996, Kelly et al. 1998, Lin et al. 1995, Precht and Molkentin 1997).
Following the report by Ha et al. (1987) on the anticarcinogenic activity of CLA isolated from grilled beef, CLA has received growing attention in biomedical research due to its important physiological effects at low dosage. Several investigations established that CLA is an effective anticarcinogen, inhibiting skin, mammary and forestomach neoplasia in humans and rodents (Ha et al. 1990, Ip 1997, Ip et al. 1991 and 1995, Liu and Belury 1997, Liu and Belury 1998, Parodi 1997, Rose 1997, Thompson et al. 1997) and decreasing the metastatic activity of human prostate cancer (Cesano et al. 1998). Furthermore, an antiatherogenic effect of CLA in hypercholesterolemic hamsters (Nicolosi et al. 1997) and rabbits (Lee et al. 1994), a decrease of body fat in mice (Park et al. 1997), and various other effects (Hayek et al. 1999, Houseknecht et al. 1998, Li and Watkins 1998, Sugano et al. 1998, Xiao et al. 1998) were demonstrated. The toxicity of CLA seems to be very low (Scimeca 1998). The mechanisms of action of CLA are not well understood, but a simple antioxidative effect can be excluded (van den Berg et al. 1995). However, as recently shown, CLA activates the γ-class of peroxisome proliferator-activated receptors (Houseknecht et al. 1998), nuclear hormone receptors controlling the expression of genes involved in lipid homeostasis.
In spite of the important physiologic effects of CLA, very little is known about the production of CLA by bacteria. One ruminal organism capable of CLA production is Butyrivibrio fibrisolvens (Kepler et al. 1966). Aerobic bacteria isolated from the vaginal tract, the bronchial system, and dairy starter cultures conjugate LA (Fairbank et al. 1988, Jack et al. 1994, Jiang et al. 1998). The increase in CLA in the adipose tissue of animals in response to feeding free LA was explained by the activity of intestinal bacteria (Chin et al. 1994). However, the experimental system was unrealistic because free LA does not occur in substantial amounts in human or animal diets but rather, in a form esterified to glycerol. For this reason, the germ-free and associated rats in our study were fed a diet enriched in esterified LA. Although Chin et al. (1994) found no CLA accumulation in rat tissue when a corn oil-fortified diet was fed, they did not prove the LA-conjugating activity of the intestinal flora. The experiments of Chin et al. (1994) with germ-free rats were not convincing because germ-free rats and conventional control rats had been fed different diets. Although the first studies of bacterial CLA production from triacylglycerol in humans were discouraging (Herbel et al. 1998), we decided to conduct an animal study with gnotobiotic rats because preliminary in vitro experiments in our laboratory demonstrated the capability of human intestinal bacteria to transform LA to CLA. The aim of the study was to test whether this observed in vitro activity was relevant to the in vivo situation. Here we present for the first time the association of germ-free rats fed a diet enriched with esterified LA with a bacterial community of human origin with proven capability of LA-conjugation. The in vitro and in vivo LA-conjugating activities and the amount of CLA in the body tissues compared to a germ-free control group were investigated.
MATERIALS AND METHODS
Animals and diet.
Germ-free rats of the inbred strain AVN-Ipcv-Wistar-Rehbrücke were used for the experiments. They were obtained from the germ-free breeding colony of the department. The germ-free status of the breed was confirmed by a modified method of Kunstyr (1992) twice a month by inoculation of the medium described below with fresh fecal samples. The cultures were macroscopically and microscopically examined for the absence of bacteria and fungi after incubation for 2 wk at 37°C and room temperature. Feces were microscopically examined after gram-staining. The serological methods of the FELASA health monitoring recommendations were applied to verify the absence of rat-specific viruses, bacteria, and protozoa (Kraft et al. 1994). No antigens of coronavirus of the rat, reovirus typ 3, Theiler's murine encephalomyelitisvirus, pneumoniavirus of the mouse, Sendai virus, Kilham rat virus, Toolan H-1 virus, Clostridium piliforme, Mycoplasma pulmonis, M. arthritidis, Pasteurella pneumotropica, and Toxoplasma gondii could be detected by specific antibodies. The protocol for the animal experiment was approved by the Ministry of Nutrition, Agriculture, and Forestry, Brandenburg, Germany.
Twelve 6-wk-old germ-free male rats of 138 ± 13 g body weight were randomly divided into two groups. They were fed an irradiated pelleted diet (25 kGy) consisting of a commercial rat-breeding diet (Altromin fortified® type 1311; Altromin, Lage, Germany) with the following composition: crude protein (225 g/kg), crude fat (50 g/kg), crude fiber (45 g/kg), ash (65 g/kg), moisture (135 g/kg) and nitrogen-free extract (480 g/kg). The diet was supplemented with 60 g of sunflower-seed oil (Brölio, Hamm, Germany), 20 mg BHT as an antioxidant (Sigma, Deisenhofen, Germany), and 7.5 g of CaCO3 per kg diet, resulting in 110 g of fat and 12 g of calcium/kg diet. The diet was stored at room temperature. Rats were maintained in positive-pressure isolators (Metall & Plastik, Radolfzell, Germany) and housed individually in polycarbonate cages on irradiated wood chips at 22 ± 2°C, 55 ± 5% relative humidity on a 12-h light-dark cycle (0700–1900). They had free access to diet and autoclaved distilled water. Coprophagy was not prevented. Diet samples were taken once per week from the isolators and analyzed for total fat and CLA (see below). In one group, six rats were inoculated intragastrically on two successive days with a CLA-producing mixed bacterial culture by intragastric application. The control group received water by the same procedure and remained germ-free throughout the experiment.
Fresh fecal samples were taken directly from the anus of every rat twice per week and analyzed for bacterial cell counts and LA-conjugating activity (see below). Once per week, rats were weighed, the bedding material was removed and replaced by 0.125 m2 irradiated cellulose tissue. On the next morning, the feces were collected and stored frozen at -20°C and fresh bedding material was provided. After 70 experimental days, the body weight gain of the animals was nearly stagnant and they had developed sufficient adipose tissue. The rats were killed by decapitation. Blood was collected and incubated for 1 h at 37°C and subsequently overnight at 4°C for clotting. The blood samples were centrifuged at 3200 × g for 1 h at 4°C and the serum stored at -20°C. Cecal and colonic contents were removed under sterile conditions and immediately analyzed for cell counts and LA-conjugating activity. Tissues were removed and stored at -20°C.
Enrichment of CLA-producing bacteria.
For enrichment of CLA-producing bacteria, a human fecal sample, obtained from a healthy volunteer who had not undergone antibiotic treatment during the past year, was suspended under anoxic conditions in the dilution buffer (see below) and diluted 1000-fold. Two-hundred microliters of the suspension were inoculated into 5 mL of culture medium in rubber-stoppered anoxic cultivation tubes containing the following compounds (per L): 9 g of tryptic peptone from meat, 1 g of proteose peptone, 3 g of meat extract, 4 g of yeast extract, 3 g of NaCl, 2 g of Na2HPO4, 6 g of glucose, 0.5 mL of Tween 80, 0.1 g of MgSO4 × 7 H2O, 5 mg of FeSO4 × 7 H2O, 3.4 mg of MnSO4 × 2 H2O, 0.25 g l-cysteine × HCl, 0.25 g l-cystine, 10 mg of hemin and 1 mg of resazurin. The gas phase consisted of 80 vol% of N2 and 20 vol% of CO2, and the pH was adjusted to 6.8–7.0. After autoclaving, 4 mmol/L of filter-sterilized (0.2 μm sterile filter; Sartorius, Göttingen, Germany) LA were added (Sigma). Cultures were shaken at 37°C on a rotary shaker (140 revolutions/min). After incubation for at least 90 h, samples were subjected to CLA analysis (see below). Cultures producing CLA were subcultured several times.
Determination of living and total bacterial cell counts.
Samples of feces, cecum and colon content were processed anoxically under a gas atmosphere of 95 vol% of N2 and 5 vol% of H2 within 15 min after collection. Approximately 200 mg of sample was thoroughly mixed with 2 mL of dilution buffer (4.5 g of KH2PO4, 6 g of Na2HPO4 × 2 H2O, 1 mg of resazurin, 0.6 g of cysteine × HCl per L distilled water, pH 6.8) in preweighed glass tubes. The tubes were weighed again and centrifuged for 10 s at 1.3 × g to remove large particles. The supernatant was diluted anoxically (50-fold for germ-free animals and 104- to 106-fold for associated animals) and an aliquot of 0.1 mL plated inside an anoxic chamber (Coy Laboratory Products, Grass Lake, MI) onto agar plates. The agar (15 g/L) was composed of the medium described above without LA. After inoculation the plates were incubated under 80 vol% of N2 and 20 vol% of CO2 at 37°C for 2 d. The colonies were subsequently enumerated.
Total bacterial cell counts were determined by examining dilutions of feces and cecum and colon contents in a counting chamber. Dry mass of samples was determined by drying preweighed samples of approximately 0.4 g for 16 h at 80°C.
Determination of LA-conjugating activity and quantification of LA and conjugated LA.
Two hundred microliters of the anoxic suspensions prepared from feces or from cecum or colon contents (see above) were transferred into two rubber-stoppered anoxic cultivation tubes filled with 5 mL of the liquid medium described above under 80 vol% of N2 and 20 vol% of CO2. Filter-sterilized LA was added to a final concentration of 4 mmol/L, and the cultures were shaken at 37°C. Media without inoculum served as controls. Since the media were turbid due to the addition of LA, microbial growth was monitored by light microscopy. CLA production was measured as follows: The cultures were vigorously shaken, then 1-mL samples were subjected to lipid extraction with 5 mL of chloroform/methanol (2:1). After centrifugation at 1600 × g for 15 min at 4°C, a 3-mL aliquot of the chloroform layer was dried in a rotoevaporator at 4°C (Jouan, Saint-Herblain, France). The samples were dissolved in 0.5 mL of HPLC-grade acetonitrile and analyzed for LA and CLA using an HPLC system (Gynkotek, Munich, Germany) equipped with a nucleosil C18 reversed-phase column (250 × 4.6 mm, 5 μm particle size; Bischoff Chromatography, Leonberg, Germany) and a precolumn (type K1, 16 × 4.6 mm; Bischoff Chromatography). Samples of 10 μL were injected by an automatic injection system (GINA, Gynkotek). An isocratic mobile phase of 16.15 mol/L acetonitrile with 16.4 mmol/L acetic acid was pumped at a flow rate of 2 mL/min at 25°C over the column for 12 min per sample. Samples exceeding the calibration range were diluted 10- to 50-fold with acetonitrile prior to injection. The retention times of LA and CLA were between 6.9 and 7.3 min. LA and CLA were identified by diode-array detection (234 nm for CLA and 205 nm for LA). Calibration was performed by injecting purified CLA and LA (Sigma).
Determination of CLA in tissues, diet, serum, and feces, and cecum and colon contents.
Tissue and diet samples (about 0.2 g), serum (1 mL), feces, cecum and colon contents (∼2.0 g each) were exactly weighed and analyzed in duplicate. Feces, and cecum- and colon-content samples were ground with a mortar to a fine powder after freezing with liquid nitrogen and addition of purified sand. Pellets of the rat diet were ground at room temperature without any additions. Serum samples were vigorously shaken. Petroleum ether (10 mL) was added and the tubes shaken for 16 h at room temperature to extract the lipids. Samples were filtered into preweighed tubes (Teflon-sealed, screw-capped glass test tubes) and dried in a rotoevaporator at 4°C and 190 × g (Jouan, Saint-Herblain, France). Tubes were weighed again to determine the amount of total fat extracted. Triacylglycerol in serum was quantified enzymatically with glycerol phosphate oxidase with a test kit (No 336; Sigma). To liberate the fatty acids, the fat extracted from tissues, serum and diet samples was hydrolyzed by addition of 2 mL 1 mol/L of NaOH in methanol and incubation for 15 min in a boiling water bath. One milliliter 5.5 mol/L H2SO4 was added to neutralize the NaOH. Free fatty acids were extracted twice with 5 mL hexane. Hexane layers were pooled and evaporated to dryness (see above). Fatty acids were dissolved in 1 mL of acetonitrile and subjected to quantification of LA and CLA by HPLC (see above). In feces and cecum and colon contents, only free CLA and LA were determined.
To evaluate the CLA quantification method, coconut oil and white adipose tissue from beef, lamb and pork were obtained from a local market and subjected to LA and CLA analyses. The values for CLA concentration were 0.15 ± 0.02 (coconut oil), 26.0 ± 7.5 (beef), 13.6 ± 4.99 (lamb) and 1.85 ± 0.14 (pork) μmol/g fat, respectively. These values are in accordance with literature data (Ha et al. 1990), demonstrating the accuracy of the method used. As a further control, both LA and CLA were added in defined quantities to coconut oil and subjected to the extraction and analysis process. The values obtained were in accordance with the added amounts and demonstrated a reliable recovery of CLA and LA.
Statistical analysis.
Results are expressed as mean ± sd. Body weight gain, CLA and LA concentrations, and relative concentrations of fat and dry mass were compared for the associated and germ-free group with the two-sided Student's t-test for unpaired data. Activities of LA conjugation were compared for the associated and germ-free group with the two-sided Welch-test. P-values ≤ 0.02 were considered to be significant.
RESULTS
Dietary composition and growth of rats.
The LA and CLA concentrations of the diet remained stable under the storage conditions as indicated by the analysis of diet samples (n = 10) taken during the experiment. The total fat concentration of the diet was 94 ± 8.7 g/kg, containing 6.24 ± 0.36 μmol of CLA/g fat.
The body weights of the rats increased from an initial 131.7 ± 6.9 g to 401.7 ± 6.9 g at the end of the study for the germ-free rats and from an initial 144.0 ± 18.8 g to 405.0 ± 18.9 g at the end of the study for the associated rats. The body-weight gain did not differ significantly between groups. Hence, colonization of the one group of rats with the mixed bacterial culture did not affect the body weight.
Bacterial mixed culture, bacterial cell counts in feces and cecum and colon content.
The mixed bacterial culture enriched from a human fecal sample exhibited CLA-producing activity (up to 1.4 mmol of CLA/L) in vitro when incubated in the presence of 4 mmol of free LA/L. Only free CLA, but no esterified CLA was detected when culture samples were analyzed in parallel for free and esterified CLA. The concentration of free CLA was 0.857 ± 0.026 mmol CLA/L (n = 6). When the same samples were saponified with the method described to analyze esterified CLA in adipose tissue, a value of 0.789 ± 0.07 mmol CLA/L was obtained. This control shows that the bacteria do not incorporate CLA into their lipids. CLA production was strictly dependent on the growth phase of the cultures and occurred only in the stationary phase (data not shown). Isolation of a single strain capable of CLA production was not successful. None of the isolates from the mixed culture produced CLA any longer. Highly diluted (up to 1010-fold) liquid cultures showed growth, but no CLA production. Based on colony morphology, at least four different microorganisms could be distinguished in the mixed culture. The coccoid and short rod-shaped organisms stained gram-positive or gram-negative. Two of them were strict anaerobes.
Total bacterial cell counts expressed as log cells/(g dry mass) were 11.4 ± 0.17 for feces, 11.7 ± 0.14 for cecum contents and 11.3 ± 0.139 for colon contents, respectively. Living bacterial cell counts expressed as log cells/(g dry mass) were 11.1 ± 0.45 for feces, 11.4 ± 0.351 for cecum contents and 10.6 ± 0.65 for colon contents, respectively. These data indicated that the intestine of the rats had been successfully colonized by the applied bacteria. The bacterial cell counts in the feces were stable throughout the experiment. There were no significant differences in cell counts of cecal and colonic contents, indicating that the bacteria did not prefer one habitat more than the other. The control group was kept germ-free throughout the experiment.
Activity of LA conjugation.
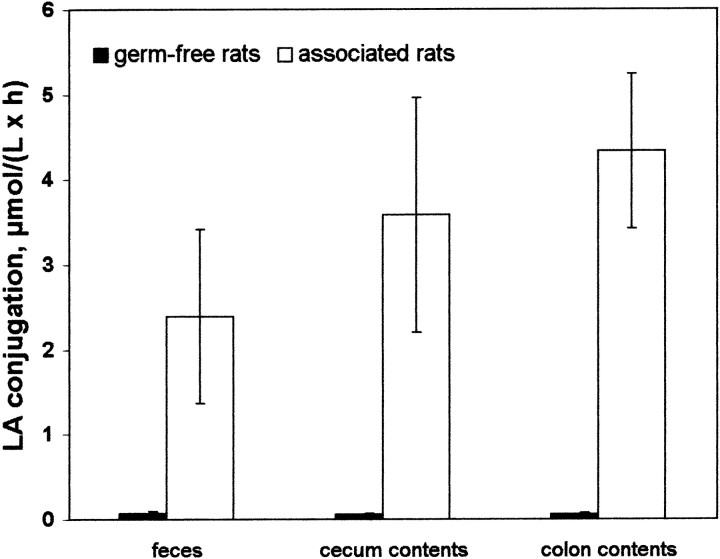
Samples obtained from germ-free rats exhibited no activity of LA-conjugation, whereas rats associated with the bacterial mixed culture had high activities in feces and in cecum or colon contents (Fig. 1). The LA-conjugating activities in the feces were constant throughout the experiment (data not shown). This led us to conclude that the LA-conjugating microorganisms had successfully colonized the intestinal tract of the rats. These results indicate that the LA-conjugation activity in the intestine was due to the microorganisms and that germ-free rats do not synthesize CLA in the intestine.
FIGURE 1.
Linoleic acid (LA) conjugation in feces and in cecum and colon contents of germ-free (n = 6) and associated (n = 6) rats fed a sunflower-seed oil enriched diet for 70 d. Associated rats were inoculated with a LA-conjugating bacterial mixed culture isolated from human feces. Throughout the experiment, fecal samples (n = 156 for germ-free and n = 144 for associated rats) and, at the end of the experiment, cecum and colon contents samples were analyzed in duplicate for in vitro LA conjugation. Activities found in samples from germ-free rats were not different from those in uninoculated controls. Values are expressed as mean ± sd. Germ-free and associated rats differed for each variable, P = 0.001.
Fat and dry mass concentration in feces and in cecum- and colon-contents.
The fat concentration of feces was significantly higher in associated than in germ-free rats (Fig. 2A). This may be due to the bacterial biomass in the feces of associated rats. The lipids of the bacterial cell membranes may contribute to the higher fat concentration (Carey et al. 1983). There were no significant differences in the fat concentration in cecum and colon contents of germ-free and associated rats, but this may be due to the small number of cecum and colon samples as compared to the high number of fecal samples. That the fat concentration of feces was higher than that of cecum or colon contents is probably due to the higher dry-mass concentration of feces.
FIGURE 2.
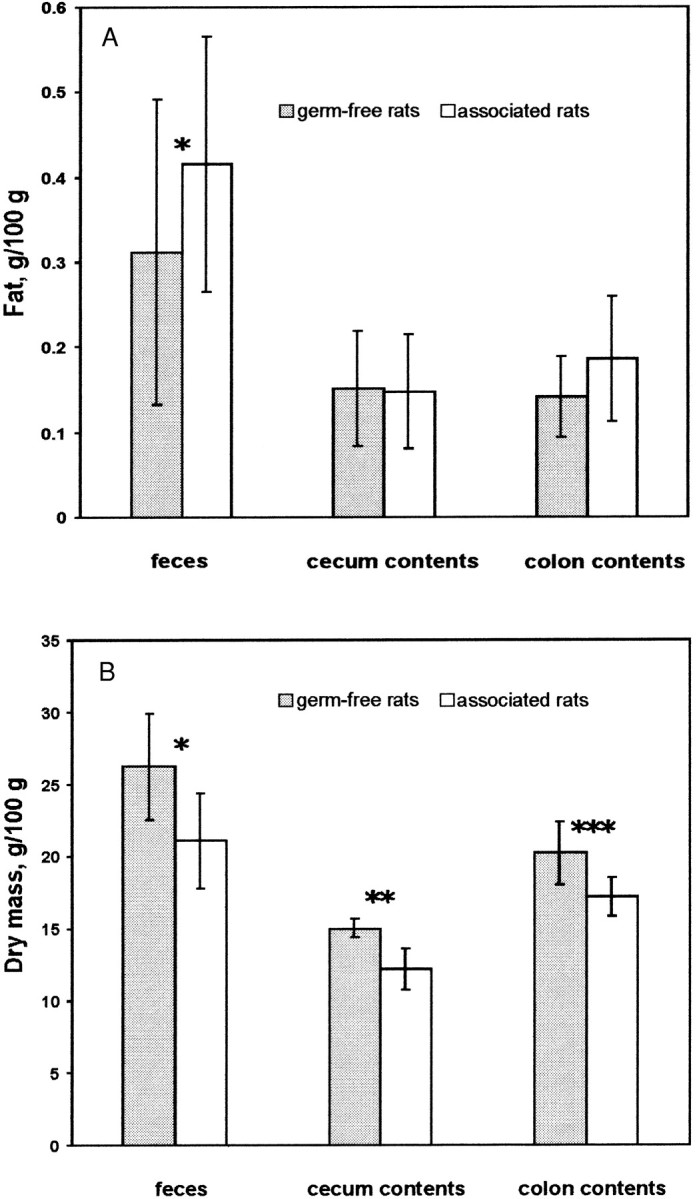
Proportions of fat (A) and dry mass (B) in feces, cecum and colon contents of germ-free (n = 6) and associated (n = 6) rats fed a diet enriched with sunflower-seed oil for 70 d. Associated rats were inoculated with an LA-conjugating mixed bacterial culture isolated from human feces. Throughout the experiment, fecal samples were analyzed (fat concentration analyses: n = 90 for germ-free and n = 95 for associated rats; dry mass analyses: n = 107 for both germ-free and associated rats). At the end of the experiment, cecum and colon contents samples were analyzed for dry mass and in duplicate for fat concentration. Values are expressed as mean ± sd * = significantly different from germ-free control (P = 0.001) ** = significantly different from germ-free control (P = 0.01) *** = significantly different from germ-free control (P = 0.02).
The dry-mass percentages of fecal and cecum and colon contents samples from germ-free rats were significantly higher than those from associated rats (Fig. 2B). Usually, the feces of germ-free rats have a lower dry-mass concentration than those of conventional rats (Gordon and Bruckner 1984). This may be explained by the fact that the polysaccharides involved in water absorption are subject to degradation by the intestinal microorganisms in conventional rats but not in germ-free rats.
CLA concentration in feces and in cecum and colon contents.
In feces, cecum and colon contents samples, only free CLA and LA were determined, whereas in diet, serum, and tissue samples fat was saponified prior to fatty acid analysis. This was reasonable because the LA-conjugating bacterial enzyme system acts only on free LA (Kepler and Tove 1969). As a control, cecum content samples were analyzed in parallel for free and esterified CLA. Values for free CLA and for total (free plus esterified) CLA did not differ significantly either in the germ-free or in the associated rats (data not shown).
Both the LA and the CLA concentrations in feces and cecum and colon contents, expressed as μmol/g fat, were significantly higher in associated rats than in germ-free rats (data not shown). However, since the diet is the source for both LA and CLA in the intestine, the ratio of CLA to LA is a more relevant variable for reflecting the LA-conjugating bacterial activity in vivo than the CLA concentration itself. These ratios did not differ significantly between germ-free and associated rats (data not shown), indicating that the CLA in the intestine originated totally from the diet. Thus, the intestinal microorganisms do not synthesize substantial amounts of CLA from LA in vivo despite their ability to do so in vitro.
CLA concentration in various body tissues.
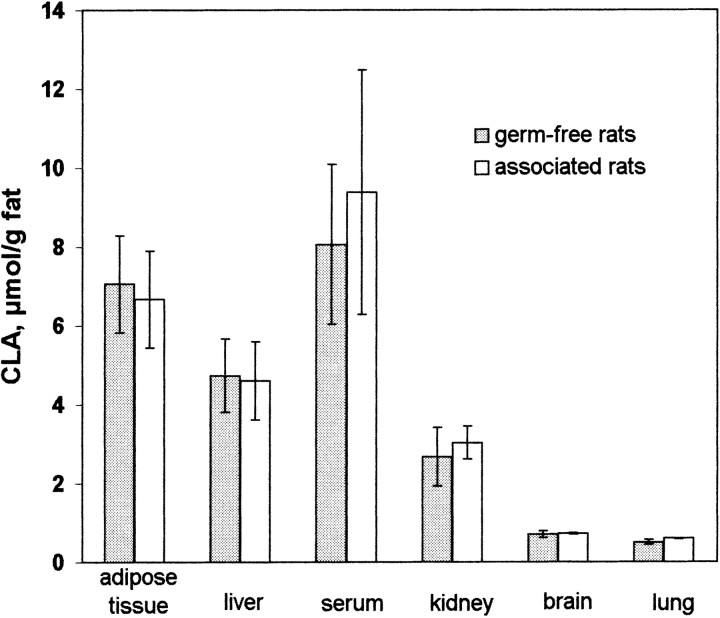
There were no significant differences in the CLA concentrations of various tissues between associated and germ-free rats, indicating that the CLA found in any tissue came from the diet and that the intestinal microorganisms do not contribute to the CLA supply of the host (Fig. 3).
FIGURE 3.
Conjugated linoleic acid (CLA) concentration of serum and various tissues from germ-free (n = 6) and associated (n = 6) rats fed a diet enriched with sunflower-seed oil for 70 d. Associated rats were inoculated with an LA-conjugating bacterial mixed culture isolated from human feces. For serum, fat was determined enzymatically as triacylglycerol. Fat concentrations of other tissues were determined gravimetrically after extraction. Samples were analyzed in duplicate. Values are expressed as mean ± sd.
DISCUSSION
The importance of the intestinal microorganisms to the supply of the host with the anticarcinogen CLA was investigated. It has already been shown (Chin et al. 1994) that free LA, but not corn oil, in the diet of conventional rats leads to an accumulation of CLA in the tissues, whereas the CLA concentration of tissues from germ-free rats is not affected by free LA in the diet. However, in that study (Chin et al. 1994), the germ-free rats were fed a diet different from that fed to the conventional rats. Furthermore, free LA usually is not added to human food but rather, eliminated during industrial processing because of its bad taste. Thus, it is more appropriate to use a diet rich in esterified LA to investigate the intestinal CLA synthesis in animals and humans. A recent study revealed that the daily intake of 16 g esterified LA by humans did not increase the plasma CLA concentrations (Herbel et al. 1998). Our study with germ-free and associated rats, fed a diet rich in esterified LA, confirms these results and also shows very clearly that even the presence of intestinal microorganisms capable of LA-conjugation under in vitro conditions does not necessarily lead to an increase in CLA in serum or tissues under in vivo conditions. This may be due to the inability of the microorganisms investigated to conjugate LA in vivo. This is supported by the fact that the CLA/LA ratio in feces and in cecum or colon contents was not significantly greater in the associated rats compared to the germ-free rats. In this context, it is interesting to note that the LA-conjugating enzyme system acts on free, but not on esterified LA (Kepler and Tove 1969). Moreover, the absence of free CLA in intestinal contents is not due to the incorporation of CLA into bacterial lipids, as was shown by controls. The extracellular location of the free CLA produced by bacteria was recently demonstrated by Jiang et al. (1998).
Sunflower-seed oil was chosen because of the high concentration of LA esterified to glycerol (657 g/kg; Stolper Bloch and Shils 1994). During triacylglycerol digestion, fatty acids in sn-1- and sn-3-positions of the glycerol are preferably hydrolyzed, whereas the fatty acid in the sn-2-position is preferentially absorbed as monoacylglycerol (Renaud 1995). Since 78% of the sn2-positions in oil from sunflower seeds are esterified with LA (personal communication: Dr. Klaus Ragotzky, Unilever, Hamburg, Germany), it can be calculated from the fatty-acid pattern in this oil that 407 g of LA/kg of oil are esterified to the sn-1- or sn-3-positions of the glycerol. This amount is hydrolyzed by the pancreatic lipases and preferably absorbed, but also does enter to some extent the cecum or colon. Calcium supplementation increases fecal fatty acid excretion slightly (Govers et al. 1996, Welberg et al. 1994). In the large intestine, fatty acids are present either in free form or precipitated as Ca2+-salts (Carey et al. 1983). Thus, the calcium concentration of the diet was increased from 9 to 12 g/kg in order to enhance the precipitation of LA with Ca2+ and to increase the proportion of LA reaching the cecum and the colon of the rats. Not surprisingly, we detected free LA and CLA in feces and in cecum and colon contents. Usually, fecal fatty acids in conventional rats are more saturated than in germ-free rats (Eyssen and Parmentier 1974). In our study, we were unable to confirm this observation for the associated rats (data not shown), indicating that the bacterial mixed culture did not reduce the double bonds in unsaturated fatty acids.
It is not known whether fatty acids can be actively absorbed in the large intestine. The absorption of fatty acids occurs preferentially in the small intestine (Carey et al. 1983). Absorption of long-chain fatty acids from the colon would be an important prerequisite for CLA formed in the colon to exert any systemic beneficial effects. However, CLA formed in the intestine may still have local effects on the colonic mucous membrane, particularly in humans. In rats, the situation may be different because of coprophagy (Soave and Brand 1991), which makes nutrients formed by the bacterial flora in the cecum or colon available. The fact that coprophagy was not prevented in this study supports the notion that no CLA was synthesized from LA in the large intestine when a diet rich in esterified LA was fed. Coprophagy might be the method of CLA absorption in conventional rats, in which the feeding of a free LA-enriched diet leads to an increase of CLA in the tissues (Chin et al. 1994).
The total concentrations of CLA in tissues of monogastric animals and humans vary considerably: 0.71–7.85 μmol/g of fat in rat tissue (Chin et al. 1994), 2.14 μmol/g of fat in pork (Chin et al. 1992) and 10.7–32.1 μmol/g of fat in human white adipose tissue (Ackman et al. 1981). These differences are due to differences in dietary CLA intake. The CLA concentrations found in this study (0.71–7.13 μmol/g of fat) are well within this range and are probably due to the relatively high amount of CLA (6.24 μmol/g of fat) in the diet offered to the rats. Casein, which is relatively high in CLA (Chin et al. 1994), was probably the major source of CLA in the diet. Vegetable oils are usually lower in CLA (0.36–2.49 μmol/g of fat) (Chin et al. 1992).
There are obviously fundamental differences between the microbial ecosystem of the rumen on the one hand and the cecum and colon on the other hand: In the rumen but not in the large intestine of monogastric animals, the addition of esterified LA-containing sunflower-seed oil stimulates the bacterial LA-conjugating activity and leads to an increase of CLA in the cow's milk fat (Kelly et al. 1998). The agriculture and food industries may take advantage of this fact and increase the CLA concentration of dairy products by supplementing the ruminants' feed with vegetable oils rich in esterified LA.
For humans, the sole source of CLA is the diet. CLA is formed by the rumen microorganisms and accumulates in milk fat and body fat of ruminants, which enter the human diet. The consumption of fat from ruminants is usually not recommended by nutritionists because of its high concentration of saturated fatty acids, although it is the best source of CLA. Recommendations should therefore be carefully reconsidered and the beneficial and undesirable features of milk fat and beef fat evaluated.
A bacterial mixed culture capable of in vitro CLA formation was used in our association experiments. Several attempts to isolate a pure culture were unsuccessful (data not shown). Although several aerobic or aerotolerant bacteria are able to conjugate LA to CLA (Fairbank et al. 1988, Jack et al. 1994), no such species has been isolated from the intestinal tract of monogastric animals or humans. Only Butyrivibrio fibrisolvens, the only known CLA-producing species inside the rumen, is found sometimes in the human intestine (Bryant 1986). However, whether B. fibrisolvens is also capable of CLA formation in the human intestine is unknown. The growth of B. fibrisolvens was completely inhibited by 4 mmol/L of LA added (data not shown), and cells exhibiting the characteristic morphology of this organism were not observed in our mixed culture. Therefore, the presence of B. fibrisolvens in our mixed culture seems very unlikely.
This investigation also demonstrates that certain metabolic capabilities of microorganisms easily observed in vitro do not necessarily occur in vivo. This appears to be particularly true for enzymatic activities that are not essential for survival of the microorganisms, such as the biotransformation of nonnutritive dietary compounds or the postulated production of certain vitamins.
Acknowledgments
We want to express our thanks to Susanne Dietrich, Ines Grüner and Renate Herzog for taking care of the animals and to Bärbel Scharfenberg for preparing media. We are indebted to Lynne Rogers-Blaut.
Abbreviations
- CLA
conjugated linoleic acid
- LA
linoleic acid
LITERATURE CITED
- Ackman R. G., Eaton C. A., Sipos J. C. & Crewe N. F. (1981) Origin of cis-9, trans-11- and trans-9, trans-11-octadecadienoic acids in the depot fat of primates fed a diet rich in lard and corn oil and implications for the human diet. Can. Inst. Food Sci. Technol. J. 14:103–107. [Google Scholar]
- Bryant M. P. (1969) Butyrivibrio. Holt J. G., Sneath P. H. A., Mair N. S., Sharpe M. E. eds. Bergey's manual® of systematic bacteriology :1376–1379Williams & Wilkins; Baltimore. [Google Scholar]
- Carey M. C., Small D. M. & Bliss C. M. (1983) Lipid digestion and absorption. Ann. Rev. Physiol. 45:651–677. [DOI] [PubMed] [Google Scholar]
- Cesano A., Visonneau S., Scimeca J. A., Kritchevsky D. & Santoli D. (1998) Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer Res 18:1429–1434. [PubMed] [Google Scholar]
- Chin S. F., Liu W., Storkson J. M., Ha Y. L. & Pariza M. W. (1992) Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J. Food Compos. Anal. 5:185–197. [Google Scholar]
- Chin S. F., Storkson J. M., Liu W., Albright K. J. & Pariza M. W. (1994) Conjugated linoleic acid (9,11- and 10,12-octadecadienoic acid) is produced in conventional but not germ-free rats fed linoleic acid. J. Nutr. 124:694–701. [DOI] [PubMed] [Google Scholar]
- Eyssen H. & Parmentier G. (1974) Biohydrogenation of sterols and fatty acids by the intestinal microflora. Am. J. Clin. Nutr. 27:1329–1340. [DOI] [PubMed] [Google Scholar]
- Fairbank J., Ridgway L., Griffin J., Wickens D., Singer A. & Dormandy T. L. (1988) Octadeca-9–11-dienoic acid in diagnosis of cervical intraepithelial neoplasia. Lancet II: 329. [DOI] [PubMed]
- Gordon H. A. & Bruckner G. (1984) Anomalous lower bowel function and related phenomena in germ-free animals. Coates M. E., Gustafsson B. E. eds. Laboratory Animal Handbooks 9: The germ-free animal in biomedical research :193–213Laboratory Animals LTD; London. [Google Scholar]
- Govers M. J. A. P., Termont D. S. M. L., Lapré J. A., Kleibeuker J. H., Vonk R. J. & Van der Meer R. (1996) Calcium in milk products precipitates intestinal fatty acids and secondary bile acids and thus inhibits colonic cytotoxicity in humans. Cancer Res 56:3270–3275. [PubMed] [Google Scholar]
- Ha Y. L., Grimm N. K. & Pariza M. W. (1987) Anticarcinogens from fried ground beef: heat altered derivatives of linoleic acid. Carcinogenesis 8:1881–1887. [DOI] [PubMed] [Google Scholar]
- Ha Y. L., Grimm N. K. & Pariza M. W. (1989) Newly recognized anticarcinogenic fatty acids: Identification and quantification in natural and processed cheeses. J. Agric. Food Chem. 37:75–81. [Google Scholar]
- Ha Y. L., Storkson J. & Pariza M. W. (1990) Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res 50:1097–1101. [PubMed] [Google Scholar]
- Hayek M. G., Han S. N., Wu D., Watkins B. R., Meydani M., Dorsay J. L., Smith D. E. & Meydani S. N. (1999) Dietary conjugated linoleic acid influences the immune response of young and old C57BL/6NCrlBR mice. J. Nutr. 129:32–38. [DOI] [PubMed] [Google Scholar]
- Herbel B. K., McGuire M. K., McGuire M. A. & Shultz T. D. (1998) Safflower oil consumption does not increase plasma conjugated linoleic acid concentrations in humans. Am. J. Clin. Nutr. 67:332–337. [DOI] [PubMed] [Google Scholar]
- Houseknecht K. L., Vanden Heuvel J. P., Moya-Camarena S. Y., Portocarrero C. P., Peck L. W., Nickel K. P. & Belury M. A. (1998) Dietary conjugated linoleic acid normalizes impaired glucose tolerance in the Zucker diabetic fatty fa/fa rat. Biochem. Biophys. Res. Commun. 244:678–682. [DOI] [PubMed] [Google Scholar]
- Ip C. (1997) Review of the effects of trans fatty acids, oleic acid, n-3 polyunsaturated fatty acids, and conjugated linoleic acid on mammary carcinogenesis in animals. Am. J. Clin. Nutr. 66(suppl):1523S–1529S. [DOI] [PubMed] [Google Scholar]
- Ip C., Chin S. F., Scimeca J. A. & Pariza M. W. (1991) Mammary cancer prevention by conjugated dienoic derivative of linoleic acid. Cancer Res 51:6118–6124. [PubMed] [Google Scholar]
- Ip C., Scimeca J. A. & Thompson H. (1995) Effect of timing and duration of dietary conjugated linoleic acid on mammary cancer prevention. Nutr. Cancer 24:241–247. [DOI] [PubMed] [Google Scholar]
- Jack C. I. A., Ridgway E., Jackson M. J. & Hind C. R. K. (1994) Serum octa-9,11 dienoic acid—an assay of free radical activity or a result of bacterial production?. Clin. Chim. Acta 224:139–146. [DOI] [PubMed] [Google Scholar]
- Jiang J., Bjoerck L. & Fondén R. (1998) Production of conjugated linoleic acid by dairy starter cultures. J. Appl. Microbiol. 85:95–102. [DOI] [PubMed] [Google Scholar]
- Jiang J., Bjoerck L., Fondén R. & Emanuelson M. (1996) Occurrence of conjugated cis-9,trans-11-octadecadienoic acid in bovine milk: Effects of feed and dietary regimen. J. Dairy Sci. 79:438–445. [DOI] [PubMed] [Google Scholar]
- Kelly M. L., Berry J. R. & Dwyer D. A. et al. (1998) Dietary fatty acid sources affect conjugated linoleic acid concentrations in milk from lactating dairy cows. J. Nutr. 128:881–885. [DOI] [PubMed] [Google Scholar]
- Kepler C. R., Hirons K. P., McNeill J. J. & Tove S. B. (1966) Intermediates and products of the biohydrogenation of linoleic acid by Butyrivibrio fibrisolvens. J. Biol. Chem. 241:1350–1354. [PubMed] [Google Scholar]
- Kepler C. R. & Tove S. B. (1969) LinoleateΔ12-cis,Δ11-trans-isomerase. Colowick S. P., Kaplan N.O., Lowenstein J. M. eds. Methods in Enzymology. Lipids :105–109Academic Press XIV; New York, London. [Google Scholar]
- Kraft V., Deeny A. A., Blanchet H. M., Boot R., Hansen A. K., Hem A., van Herck H., Kunstyr I., Milite G., Needham J. R., Nicklas W., Perrot A., Rehbinder C., Richard Y. & De Vroey G. (1994) Recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit breeding colonies. Lab. Anim. 28:1–12.8158962 [Google Scholar]
- Kunstyr I. (1992) Investigation of germ-free animals. Kunstyr I. eds. Diagnostic Microbiology for Laboratory Animals :42–44Gustav Fischer Verlag Stuttgart, Jena, New York. [Google Scholar]
- Lee K. N., Kritchevsky D. & Pariza M. W. (1994) Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 108:19–25. [DOI] [PubMed] [Google Scholar]
- Li Y. & Watkins B. A. (1998) Conjugated linoleic acid alter bone fatty acid composition and reduce ex vivo prostaglandin E2 biosynthesis in rats fed n-6 or n-3 fatty acids. Lipids 33:417–425. [DOI] [PubMed] [Google Scholar]
- Lin H., Boylston T. D., Chang M. J., Luedecke L. O. & Shultz T. D. (1995) Survey of the conjugated linoleic acid contents of dairy products. J. Dairy Sci. 78:2358–2365. [DOI] [PubMed] [Google Scholar]
- Liu K.-L. & Belury M. A. (1997) Conjugated linoleic acid modulation of phorbol ester-induced events in murine keratinocytes. Lipids 32:725–730. [DOI] [PubMed] [Google Scholar]
- Liu K.-L. & Belury M. A. (1998) Conjugated linoleic acid reduces arachidonic acid content and PGE2 synthesis in murine keratinocytes. Cancer Lett 127:15–22. [DOI] [PubMed] [Google Scholar]
- Nicolosi R. J., Rogers E. J., Kritchevsky D., Scimeca J. A. & Huth P. J. (1997) Dietary conjugated linoleic acid reduces plasma lipoproteins and early aortic atherosclerosis in hypercholesterolemic hamsters. Artery 22:266–277. [PubMed] [Google Scholar]
- Park Y., Albright K. J., Liu W., Storkson J. M., Cook M. E. & Pariza M. W. (1997) Effect of conjugated linoleic acid on body composition in mice. Lipids 32:853–858. [DOI] [PubMed] [Google Scholar]
- Parodi P. W. (1997) Cows' milk fat components as potential anticarcinogenic agents. J. Nutr. 127:1055–1060. [DOI] [PubMed] [Google Scholar]
- Precht D. & Molkentin J. (1997) Effect of feeding on conjugated cisΔ9,transΔ11-octadecadienoic acid and other isomers of linoleic acid in bovine milk fats. Nahrung 41:330–335. [DOI] [PubMed] [Google Scholar]
- Renaud S. C., Ruf J. C. & Petithory D. (1995) The positional distribution of fatty acids in palm oil and lard influences their biologic effects in rats. J. Nutr. 125:229–237. [DOI] [PubMed] [Google Scholar]
- Rose D. P. (1997) Effects of dietary fatty acids on breast and prostate cancers: evidence from in vitro experiments and animal studies. Am. J. Clin. Nutr. 66:1513S–1522S. [DOI] [PubMed] [Google Scholar]
- Scimeca J. A. (1998) Toxicological evaluation of dietary conjugated linoleic acid in male Fischer 344 rats. Food Chem. Toxicol. 36:391–395. [DOI] [PubMed] [Google Scholar]
- Soave O. & Brand C. D. (1991) Coprophagy in animals: A review. Cornell Vet 81:357–364. [PubMed] [Google Scholar]
- Stolper Bloch A. & Shils M. E. (1994) Appendices. Shils M. E., Olson J. A., Shike M. eds. Modern nutrition in health and disease 8th ed :A-102.Williams & Wilkins; Baltimore. [Google Scholar]
- Sugano M., Tsujita A., Yamasaki M., Noguchi M. & Yamada K. (1998) Conjugated linoleic acid modulates tissue levels of chemical mediators and immunoglobulins in rats. Lipids 33:521–527. [DOI] [PubMed] [Google Scholar]
- Thompson H., Zhu Z., Banni S., Darcy K., Loftus T. & Ip C. (1997) Morphological and biochemical status of the mammary gland as influenced by conjugated linoleic acid: implication for a reduction in mammary cancer risk. Cancer Res 57:5067–5072. [PubMed] [Google Scholar]
- van den Berg J. M. J., Cook N. E. & Tribble D. L. (1995) Reinvestigation of the antioxidant properties of conjugated linoleic acid. Lipids 30:599–605. [DOI] [PubMed] [Google Scholar]
- Welberg J. W. M., Monkelbaan J. F. & de Vries E. GE, et al. (1994) Effects of supplemental dietary calcium on quantitative and qualitative fecal fat excretion in man. Ann. Nutr. Metab. 38:185–191. [DOI] [PubMed] [Google Scholar]
- Xiao Y. F., Wright S. N., Wang G. K., Morgan J. P. & Leaf A. (1998) Fatty acids suppress voltage-gated Na+ currents in HEK293t cells transfected with the alpha-subunit of the human cardiac Na+ channel. Proc. Natl. Acad. Sci. USA 95:2680–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]