Abstract
Background
Invasive pneumococcal disease (IPD) continues to be a significant burden in children despite the implementation of two generations of conjugate vaccines. Serotype replacement by nonvaccine serotypes is reported in multiple areas around the world. This study is a continuation of previous studies and describes the incidence, serotype distribution, and antibiotic resistance pattern of Streptococcus pneumoniae serotypes causing IPD at Children's Medical Center Dallas after introduction of 13-valent pneumococcal conjugate vaccine (PCV13).
Methods
Streptococcus pneumoniae isolates from normally sterile sites were collected from January 1, 1999 to June 30, 2014. Demographic and clinical information was extracted for analysis. Incidence of IPD was calculated using inpatient and emergency center admissions to Children's Medical Center of Dallas as the denominator. Isolates were serotyped and penicillin/cefotaxime susceptibilities were determined. Selected nontypeable isolates were further characterized by multilocus sequence typing. A χ2 test and the Cochran–Armitage Trend Test for trend analysis were used to evaluate change in serotype and antibiotic susceptibility patterns over time.
Results
Comparison of the different study periods showed a significant reduction in the incidence of IPD in PCV13 era compared with prevaccine era and PCV7 era (P < .05). Children younger than 24 months showed the largest reduction of disease incidence. More than 40% of patients with IPD had a documented comorbidity. Cases of pneumonia continued to decrease in the PCV13 era (P < .002). The most common non-PCV13 serotypes after vaccine introduction were as follows: 23B, 6C, 23A, 9N/L, and 12. Penicillin resistance by meningitis breakpoint decreased significantly in the PCV13 era.
Conclusions
After introduction of PCV13 in Dallas, incidence of IPD caused by strains contained in the vaccine and penicillin resistance continued to decrease. Serotype replacement phenomena and persistence of PCV7 serotypes were documented. Patients with comorbidities represented a large percentage of patients with IPD. Concerns for geographic variation in serotype replacement phenomena arise from the present study.
Keywords: Streptococcus pneumoniae, IPD, pneumococcal conjugate vaccine, serotype distribution
Streptococcus pneumoniae is known to cause invasive and noninvasive disease in the pediatric population worldwide [1]. Invasive pneumococcal disease (IPD) continues to be a significant burden on children after the implementation of two generations of conjugate vaccines: the 7-valent pneumococcal conjugate vaccine (PCV7) and the 13-valent pneumococcal conjugate vaccine (PCV13) [2, 3]. These vaccines were designed to induce serotype-specific antibody production against the most prevalent circulating serotypes. 7-Valent pneumococcal conjugate vaccine (Wyeth Pharmaceuticals, Philadelphia, Pennsylvania), introduced in 2000, included serotypes 4, 6B, 9 V, 14, 18C, 19F, and 23F. At the time of introduction, PCV 7 was estimated to cover 82% of IPD in pediatric patients younger than 5 years of age [4]. 13-Valent pneumococcal conjugate vaccine, introduced in 2010 (Prevnar-13; Pfizer), added 6 additional serotypes, 1, 3, 5, 6A, 7F, and 19A, which were responsible for 44%–64% of the residual cases of IPD in the pediatric population [4].
After PCV7 was included in the US immunization schedule, a rapid decrease in the incidence of pneumococcal disease was observed in children of all ages. At the same time, different research groups reported serotype replacement by nonvaccine serotypes in their communities [5–8]. The mechanisms accounting for a particular nonvaccine serotype to increase after vaccine introduction are not completely understood [4]. After PCV7, serotype 19A was the most frequently encountered serotype causing invasive disease, and it has also been associated with a higher occurrence of antibiotic resistance compared with other nonvaccine serotypes [5, 9].
Decline in the incidence of IPD by PCV13-related serotypes is a reasonable expectation based on epidemiologic data after PCV7 introduction. Multiple studies from different areas in the United States and in European countries have documented early effectiveness of PCV13 in the reduction of nasopharyngeal carriage and incidence of IPD caused by vaccine serotypes [3, 10–15].
Our previous reports about the epidemiology and evolution of IPD in Children's Medical Center Dallas (CMCD) after PCV7 introduction documented a sustained IPD incidence reduction [1, 9]. The objective of the present study was to assess the incidence, serotype distribution, antibiotic resistance pattern, comorbidities, and viral coinfection of S pneumoniae serotypes causing IPD at CMCD after introduction of both PCV7 and PCV13 vaccines.
METHODS
Identification of Invasive Pneumococcal Disease
Streptococcus pneumoniae isolates causing IPD from January 1999 to June 2014 were collected from the CMCD microbiology laboratory by the same method described in previous report [9]. Invasive pneumococcal disease cases were defined as the isolation of S pneumoniae from a normally sterile site (eg, blood, pleural fluid, cerebrospinal fluid, join fluid). The evidence of chest radiograph infiltrates and isolation of the bacteria from at least 1 of the following sites confirmed the diagnosis of pneumococcal pneumonia: blood, pleural fluid, or empyema. Because some cases of pneumonia can present with bacteremia, clinical syndromes were divided into isolated pneumonia, bacteremic pneumonia, and isolated bacteremia. Medical records of patients with culture-confirmed IPD at CMCD were reviewed for demographic and clinical data extraction. If a subject had recurrent IPD or multiple serotypes isolated, it was included in the analysis only once. All patient information was obtained in accordance with the Health Information Protection and Portability Act guidelines.
Study Time Definition
Three study periods were defined according to the time of introduction of PCV7 and PCV13 vaccines in Dallas: pre-PCV era (January 1999 to December 2000), PCV7 era (January 2001 to December 2010), and PCV13 era (January 2011 to June 2014).
Specimen Processing/Serotyping/Antimicrobial Susceptibility Testing
Pneumococcal isolates were serotyped by the capsular swelling method using commercially available rabbit antipneumococcal antisera (Statens Serum Institute, Copenhagen, Denmark). The pneumococcal strains that did not react with all pooled antisera were classified as nontypeable. Selected nontypeable isolates were analyzed by multilocus sequence typing (MLST) from 2000 to 2010, and then they were serotyped by using the antipneumococcal antisera based on the serotypes in the MLST database.
Antimicrobial susceptibility information was obtained from the CMCD microbiology laboratory using the standard breakpoints established by the Clinical and Laboratory Standards Institute. In January 2008, the Clinical and Laboratory Standards Institute published new nonmeningitis breakpoints for parenteral penicillin therapy [16]. Penicillin and cefotaxime breakpoints used in this study are the 2008 breakpoints by isolate location and meningitis breakpoint. From 1999 to 2002, susceptibilities to penicillin and cefotaxime were performed by the epsilometric test. Starting in 2003, antibiotic susceptibilities were determined by MICroSTREP microtiter method (Dade MicroScan Inc., West Sacramento, California), a microbroth dilution method using Mueller-Hinton media with 3% lysed horse blood.
Genetic Analysis of Nontypeable Isolates
Genetic identification of some nontypeable serotypes was determined by MLST. Multilocus sequence typing analysis was carried out according to previously published methods [17]. Internal fragments of the 7 housekeeping genes, aroE, gdh, gki, recP, spi, xpt, and ddl, were amplified by polymerase chain reaction (PCR) from chromosomal DNA obtained from these S pneumoniae isolates. The amplified PCR gene products were purified using the QIAGEN QIAquick PCR purification kit (QIAGEN Inc., Valencia, California), and then sequenced in both the 5’ and 3’ directions using the ABI Big Dye Terminator 3.1 Sequencer (Applied Biosystems Inc, Foster City, California), and analyzed on ABI capillary instruments by the University of Texas Southwestern Medical School's McDermott Center Sequencing Core Facility.
Allele and sequence type assignments were made using the MLST Web site (http://spneumoniae.mlst.net). All alleles not already present in the pneumococcal MLST database were verified by resequencing the gene fragment on both strands.
Respiratory Viruses Detection
Polymerase chain reaction-based methods were used for identification of viruses. The Multi-Code Respiratory Virus Panel (Eragen Biosciences, Inc., Madison, Wisconsin) test was used from 2009 until February 2010 (influenza A, influenza B, respiratory syncytial virus [RSV], parainfluenza virus, adenovirus, human metapneumovirus, and rhinovirus). Then, the panel was changed to the xTAG Respiratory Viral Panel (Luminex Molecular Diagnostics, Austin, Texas) for detection of the following 8 viruses and subtypes: influenza A, influenza A subtype H1, influenza A subtype H3, influenza B, RSV, human metapneumovirus, rhinovirus, and adenovirus. After September 2012, the xTAG RVP was replaced by the FilmArray Respiratory Panel (Idaho Technologies, Salt Lake City, Utah), and the test was able to identify viruses (adenovirus, coronavirus HKU1, coronavirus NL63, coronavirus 229E, coronavirus OC43, human metapneumovirus, rhinovirus/enterovirus, influenza A, influenza A/H1, influenza A/H1–2009, influenza A/H3, influenza B, parainfluenza 1, parainfluenza 2, parainfluenza 3, parainfluenza 4, RSV) and several atypical bacteria (Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae).
Statistical Analysis
For purposes of our analysis serotypes not included in either PCV vaccine group were classified into 2 groups: nonvaccine serotype group and nontypeable group. Nonvaccine serotype group represented all serotypes not contained in PCV7 or PCV13. Nontypeable group included the bacterial isolates that were not typeable by either capsular swelling method or MLST. Descriptive analyses were performed using medians, ranges, frequency distributions, and percentages. Incidence of IPD was calculated for each year using the sum of all hospitalized and emergency center patients, identified by unique medical record number, as the denominator. The Z-test was used to compare these rates at 0.05 significance level. A χ2 test and the Cochran–Armitage Trend test for trend analysis were used to test the change in serotype and antibiotic susceptibility patterns over time. The data were stratified into vaccine serotype and nonvaccine serotype invasive disease. Antimicrobial susceptibility patterns were determined for all strains. The association between serotypes and the types of illnesses was determined by calculating odds ratios and 95% confidence intervals. SAS 9.3 software (SAS Institute Inc., Cary, North Carolina) was used for most of the statistical analyses.
Consent
This study was approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center, Dallas and CMCD. Because this study did not require contact with patients or families, and because the identities of the patients were not revealed, informed consent was waived in accordance with the Institutional Review Board regulations.
RESULTS
Demographic Data
We identified 770 (52.3% male, 47.7% female) IPD cases at CMCD from January 1999 to June 2014 (Table 1). There were no cases of recurrent IPD during the study. Only 1 subject had invasive disease caused by 2 different serotypes: 14 and 19A. This patient was a 7-year-old female patient with Antley–Bixler syndrome and chronic lung disease who presented with bacteremia in 2014. Because both serotypes correspond to PCV13, they were included in the analysis as a single vaccine serotype. The median age of patients was 22 months (range, 0 months–18 years). The percentage of IPD cases in children younger than 24 months decreased consistently during the study time period (P = .008). In our study, 11% of all patients were 3 months of age or younger. The racial distribution showed the following: 260 (33.8%) children were Hispanic, 248 (32.2%) were White, 214 (27.8%) were Black, and 48 (6.2%) were classified as Other (Table 1).
Table 1.
Demographics and Clinical Features of the Study Population by Study Period
| Variable | Pre-PCV Era 1999–2000 | PCV7 Era 2001–2010 | PCV13 Era 2011– 2014* |
Total |
|---|---|---|---|---|
| (n) | (150) | (502) | (118) | (770) |
| Age (months) (n [%]) | ||||
| <24 Months | 98 [65.3] | 265 [52.8] | 46 [38.9] | 403 [52.3] |
| Gender (n [%]) | ||||
| Male | 65 [43.3] | 279 [55.6] | 59 [50] | 403 [52.3] |
| Race (n [%]) | ||||
| Black | 60 [40] | 125 [24.9] | 29 [24.6] | 214 [27.8] |
| Hispanic | 37 [24.7] | 174 [34.6] | 49 [41.5] | 260 [33.8] |
| White | 49 [32.6] | 170 [33.8] | 29 [25.6] | 248 [32.2] |
| Other | 4 [2.7] | 33 [6.7] | 11 [9.3] | 48 [6.2] |
| Diagnosis (n [%]) |
||||
| Isolated bacteremia | 74 [49.3] | 200 [39.8] | 65 [55.1] | 339 [44] |
| Pneumonia | 38 [25.3] | 159 [31.7] | 20 [17] | 217 [28.2] |
| Meningitis | 22 [14.7] | 83 [16.5] | 20 [17] | 125 [16.2] |
| Osteomuscular | 9 [6] | 16 [3.2] | 6 [5] | 31 [4] |
| Sinus/mastoid/ear | 3 [2] | 32 [6.6] | 5 [4.2] | 40 [5.2] |
| Peritonitis | 3 [2] | 10 [1.9] | 2 [1.7] | 15 [2] |
| Cardiovascular | 1 [0.7] | 2 [0.3] | 0 [0] | 3 [0.4] |
| Comorbidity (n [%]) | ||||
| Yes | 49 [32.7] | 212 [42.2] | 68 [57.6] | 329 [42.7] |
| Mortality (n [%]) | 6 [4] | 23 [4.6] | 6 [5.1] | 35 [4.5] |
| Vaccine serotype (n [%]) | ||||
| PCV7 | 71 [47.4] | 84 [16.7] | 29 [24.4] | 184 [23.9] |
| PCV13 | 17 [11.3] | 239 [47.6] | 47 [39.4] | 303 [39.3] |
| Nonvaccine serotype | 15 [10] | 148 [29.5] | 41 [34.5] | 204 [26.5] |
| Nontypeable | 0 [0] | 17 [3.4] | 2 [1.7] | 19 [2.4] |
| Nonavailable | 47 [31.3] | 14 [2.8] | 0 [0] | 61 [7.9] |
Abbreviations: IPD, invasive pneumococcal disease; PCV, pneumococcal conjugate vaccine.
*Until June 2014. In addition, in 2014, 2 different serotypes caused IPD in 1 patient.
Frequency of Disease
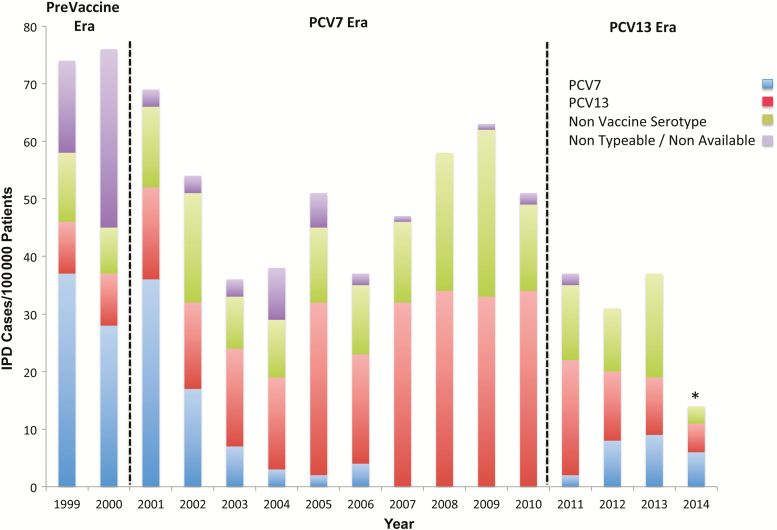
Analysis of the overall incidence of disease showed a significant decrease in IPD incidence rates (P < .0001) (Figure 1). When analysis was performed by study period, the difference in disease incidence between the PCV7 era and PCV13 era did not reach statistical significance (P < .11). Regarding the distribution of serotypes, PCV7 serotypes and nonvaccine serotypes continued to be an important cause of IPD in the PCV13 era (Figure 1).
Figure 1.
Annual incidence of invasive pneumococcal disease (IPD) and serotype distribution at Children's Medical Center Dallas, TX from 1999 to 2014. *June 2014. Abbreviation: PCV, pneumococcal conjugate vaccine.
Site of Infection
The principal illnesses observed during the study were as follows: isolated bacteremia 339 (44%), pneumonia 217 (28.2%), and meningitis 125 (16.2%). Analysis of IPD by site of infection during the 3 study periods did not show significant variation except for pneumonia and isolated bacteremia. Cases of pneumonia during PCV7 era were 31.7% compared with 17% during PCV13 era. (P < .002). The opposite effect was observed for cases of isolated bacteremia with an increase from 39.8% to 55.1% between PCV7 and PCV13 eras (P < .004).
Changes in Serotype Distribution
Pneumococcal isolates from 690 (89.6%) of the 770 patients included in the study were available for serotyping. Forty-seven (58.8%) of the missing samples were from 1999 and 2000 when the isolates were retrospectively collected from CMCD microbiology laboratory freezers. Twenty-nine serotypes were characterized by MLST between 2000 and 2010.
Analysis of each vaccine group by study time period showed that PCV7 serotypes decreased during PCV 7 era but continued to cause IPD in the PCV13 era. During the PCV13 era, 29 cases of IPD were caused by PCV7 isolates. Serotype 19F (38%) was the most common isolate in this group, followed by serotype 14 (31%), 6B (13.8%), 23 F (13.8%), and 9 V (3.4%). Patients with comorbidities represented 58.6% of these cases. Of the 41.4% of patients without known underlying medical conditions, 50% were patients younger than 6 months of age at the time of presentation.
Serotype 19A was the most common vaccine serotype isolated after PCV7 introduction. However, analysis of individual frequencies of vaccine serotypes in the PCV13 era showed that the most significant change noted in serotype distribution is the progressive reduction in the frequency of 19A isolates. The most common non-PCV13 serotypes identified were 23B (n = 13, 30.2%), 6C (n = 11, 25.5%), 23A (n = 10, 23.3%), 9N/9L (n = 3, 7%), and 12 (n = 2, 4.7%).
Antibiotic Susceptibility of Pneumococcal Isolates
The resistance patterns of isolates to penicillin and cefotaxime by study time period are shown in Table 2. Two different breakpoints were included: isolate location breakpoint and meningitis breakpoint. Penicillin resistance interpreted by meningitis breakpoint has significantly decreased from the pre-PCV era (53.9%) to the PCV13 era (35.3%) (P < .015). These results contrast with the pattern of resistance described by isolate location breakpoint. Resistance to penicillin according to that breakpoint increased significantly from pre-PCV era to PCV13 era from 0% to 8.2% (P < .008). Cefotaxime susceptibility did not show a significant change between pre-PCV era and PCV13 era for either breakpoint.
Table 2.
Antibiotic Susceptibility to Penicillin and Cefotaxime by Study Time Period
| Penicillin | n | Susceptibility (%) | ||
|---|---|---|---|---|
| S | I | R | ||
| Pre-PCV Era | 143 | |||
| Isolate location breakpoint | 96.7 | 3.3 | 0 | |
| Meningitis breakpoint | 46.1 | 53.9 | ||
| PCV7 Era | 483 | |||
| Isolate location breakpoint | 90.8 | 8.6 | 0.6 | |
| Meningitis breakpoint | 51.1 | 48.9 | ||
| PCV13 Era | 116 | |||
| Isolate location breakpoint | 88.2 | 3.4 | 8.2 | |
| Meningitis breakpoint | 64.7 | 35.3 | ||
| Cefotaxime | ||||
| Pre-PCV Era | 140 | |||
| Isolate location breakpoint | 91.3 | 8 | 0.7 | |
| Meningitis breakpoint | 72.1 | 18.6 | 9.3 | |
| PCV7 Era | 479 | |||
| Isolate location breakpoint | 91 | 6.8 | 2 | |
| Meningitis breakpoint | 80.4 | 10.2 | 9.4 | |
| PCV13 Era | 116 | |||
| Isolate location breakpoint | 98.3 | 1.7 | 0 | |
| Meningitis breakpoint | 90.5 | 7.8 | 1.7 | |
Abbreviation: PCV, pneumococcal conjugate vaccine.
Comorbidities
Three hundred twenty (41.6%) study patients had a documented comorbidity. The most common group of comorbidities included the following: malignancy (17.5%), history of prematurity (12.5%), genetic disorders (10.9%), renal disease (9.1%), and asthma (7.5%). When information was analyzed by study period, no statistically significant variations were noted within the above-mentioned groups. The number of patients with sickle cell anemia who developed IPD during the study decreased from 7 (14.3%) in the pre-PCV era to 3 (4.5%) in the PCV13 era (P < .09). The majority of the patients who had comorbidity developed infection caused by a serotype included in PCV13 (36.6%) compared with PCV7 era (24%). It is interesting to note that the phenomenon of serotype replacement is more evident in the subgroup of patients with comorbidities, with nonvaccine serotypes causing 33.1% of the cases of IPD compared with 26.5% in the rest of the cohort (P < .05).
Viral Coinfection
Widespread use of viral respiratory panel was introduced in our hospital in 2009. Of the 56.1% of patients tested in 2009 and 2010 for viral coinfection, 46.8% had a positive result. For the PCV13 era, 43.7% had viral testing, with 60% of those results returning positive. The overall percentage of patients with IPD diagnosis and a viral coinfection was 21%. The most common respiratory viruses isolated were rhinovirus/enterovirus (26.2%), RSV (23%), and rhinovirus (18%).
DISCUSSION
Our study provides an epidemiologic description of IPD in Dallas, Texas, using continuous epidemiologic surveillance in infants and children hospitalized at CMCD before, during, and after the implementation of both PCV vaccines. Data on the immunogenicity of PCV13 vaccine were obtained from several noninferiority studies using PCV7 for comparison [18]. It has been described in the literature that even a single dose of PCV7 is able to elicit antibody response [18]. This response is presumed to be higher once PCV13 is given because of immunologic priming. Study of the kinetics on immune responses to PCV vaccines have shown comparable antibody persistence for the 7 common serotypes in cases of combined immunization. In addition, children with previous PCV13 immunization had higher antibody responses for the 6 additional serotypes compared with patients vaccinated with PCV7 alone [19].
It is interesting to note that in vitro cross-reactivity between S pneumoniae serotypes does not necessarily indicate clinical protection [13]. For example, antibodies produced by PCV7 immunization against 19F have produced limited cross-protective immunity against serotype 19A [18]. Functional analysis of antibody production regarding PCV13 showed that this vaccine could provide enhanced protection against serotypes included in the vaccine and certain serotypes such as 6C when compared with PCV7 [18]. Our data provide information about the persistence of PCV7 serotypes in the PCV13 era. The most frequent PCV7 serotypes in our population were 19 F (38%) and 14 (31%). These findings contrast with recently reported IPD surveillance data [3, 7, 11, 20, 21]. However, our data are similar to findings of Loughlin et al [14] regarding PCV serotypes causing colonization. They describe that PCV7 serotypes persisted at low prevalence in their population in the following order of frequency: 19F, 23F, 18C, 14, and 6B.
Comparison of trends on IPD after the introduction of each vaccine must be done cautiously. Vaccine uptake for PCV7 and PCV13 was different because PCV 7 was a new vaccine at introduction, and it took several years to reach vaccine uptake rates above 80% [4]. In addition, soon after PCV7 was licensed, several publications raised attention to the phenomenon of serotype replacement [5–7, 13]. There are data suggesting that the distribution of S pneumoniae serotypes in the community can evolve independently of the vaccine. This points to the importance of natural variations in serotype distribution unrelated to the vaccine and the ability of certain serotypes to fill the new ecologic niche. Natural trends in pneumococcal serotype colonization and the effect of antibiotic use should also be considered [22]. Observational studies have provided most of the available data regarding the serotype replacement. It is important to consider possible methodology issues such as ecologic and sampling bias that could affect the surveillance systems used by those studies. At the same time, changes in laboratory methods used in clinical practice or epidemiologic reporting tools can potentially influence observed changes in IPD incidence [22].
After PCV7 introduction, the nonvaccine serotypes that were the most commonly isolated in pneumococcal pneumonia and empyema in children in France were as follows: 1, 3, 7F, and 19A [11]. Serotype 19A has been the most common replacement serotype reported in the literature since PCV7 was introduced [6, 18]. Likewise, serotype 19A was the most common vaccine serotype isolated in the PCV7 era in our study. The PCV13 era already shows progressive reduction in the frequency of this isolate. Zuccotti et al [13] reported 6C as the most frequent serotype causing IPD in Italy after PCV13 introduction. Serotype 6C has also been reported as a cause of IPD in the United States [8]. In our study, frequency of 6C isolates reached 25.5% of nonvaccine serotypes in the PCV13 era. Epidemiologic data from France suggests a strong impact of PCV13 on pediatric community-acquired pneumonia cases [11]. Lindstrand et al [10] showed that vaccination led to significant decrease of pneumonia cases for children 2 years of age and younger. Our data support these findings with the highest impact of both PCV seen in the overall incidence reduction of pneumonia cases and IPD in that age group. Investigators in Houston also reported reduction in PCV13 serotypes causing chronic sinusitis after the introduction of PCV13 [12].
Before introduction of PCV vaccine, there was evidence of geographic variation in the incidence of vaccine serotypes causing IPD in the United States [23]. After vaccine introduction, the magnitude of the reduction of vaccine-related IPD due to serotypes has been consistent across sites. The increase in incidence of IPD caused by nonvaccine serotypes has been described to be more variable [23]. The most common non-PCV13 serotypes after vaccine introduction in our population were 23B, 6C, 23A, 9N/L and 12. Kaplan et al reported that their most common non-PCV13 serotypes causing IPD in 2010 and 2011 were 33F, 22F, 12, 15B, 15C, 23A, and 11 [3]. de St Maurice et al reported similar data to Kaplan's group regarding PCV13 era surveillance in the Tennessee area. The most common serotypes causing IPD in their population were 22F, 33, 12F, and 38 [24]. These data suggest variation in the serotype replacement phenomena according to geographic location within the United States [3]. It is of interest to note that data from the National Immunization Survey from the Centers for Disease Control and Prevention show the following average estimated vaccination coverage for PCV vaccine during PCV13 era: Dallas (79.3%), Houston (85.7%), Texas (83.3%), and Tennessee (84.9%) [25].
Another important aspect of PCV surveillance includes the analysis of antimicrobial resistance of the strains causing IPD. The present study findings using the meningitis breakpoint show a significant reduction of penicillin resistance between the pre-PCV era and the PCV13 era (from 53.9% to 35.3%). This information is consistent with the data reported by Iroh Tam et al in 2014 showing an overall decrease in antibiotic resistance among bacterial isolates in the Boston area [21]. On the other hand, Olarte et al [12] found that penicillin and ceftriaxone minimum inhibitory concentrations were similar between the pre- and post-PCV eras.
Several studies reporting the effectiveness of PCV13 continue to document IPD cases in children with underlying medical conditions [5, 21]. Our data shows that the most common comorbidities listed included the following: malignancy, history of prematurity (chronic lung disease), and asthma. It is important to consider that although the difference of IPD incidence between study periods did not reach statistically significance, cases of IPD in the PCV13 era were more common in patients with comorbidities. We consider that phenomena as an indirect indicator of vaccine effectiveness. Likewise, cases of IPD in patients with sickle cell anemia trended down in our cohort. This is most likely a consequence of PCV vaccination enforced in this population.
Introduction of new viral detection technologies has prompted the analysis of viral coinfection of patients with IPD [26]. Our database showed that 21% of the patients had a positive viral study with rhinovirus, RSV, and rhinovirus/enterovirus being most common. Respiratory virus panels used during present study were unable to separate human rhinovirus and enterovirus. Interaction between rhinovirus and S pneumoniae has been described in human cell cultures. It is notable that rhinovirus seems to stimulate S pneumoniae adhesion to airway epithelial cells [27]. Clinical studies designed to analyze the clinical significance of the isolation of rhinovirus in patients with IPD are needed.
Our study has some limitations. Approximately 10% of pneumococcal isolates were not available for typing or were found to be nontypeable. Most of those samples belong to the pre-PCV era (1999–2000). We began prospective collection of samples from the CMCD microbiology laboratory late in 2000. In addition, MLST was not available starting 2011. However, only 2 isolates were classified as nontypeable during the PCV13 period. Our study was based on results from a single tertiary referral center, and hence, our findings may not be generalizable to other areas of the country. Children's Medical Center Dallas is the major referral center in the area, covering most of the pediatric population of the Dallas area. However, there are other medical centers, which were not included in the study, and the incidence of IPD could be overestimated or underestimated. Finally, although we could not associate vaccination status of individuals to development of IPD, we can point out that average PCV vaccination rates were slightly lower in the Dallas area compared with other areas of surveillance in the country. These findings could potentially contribute to some of the differences contained in this study in comparison with other reports.
CONCLUSIONS
Our findings show an overall reduction of IPD after introduction of PCV13 in the Dallas area. Penicillin resistance among bacterial isolates and occurrence of IPD caused by serotype 19A are decreasing as well. Interestingly, we documented persistence of PCV7 serotypes in the PCV13 era. The group with the most significant reduction of IPD corresponded to children younger than 24 months with the highest impact by clinical syndrome seen in the pneumonia group. Patients with comorbidities represented a significant percentage of patients with IPD. Additionally, concerns for geographic variation in serotype replacement phenomena arise from the present study. Continuing careful monitoring through national and international population-based IPD surveillance is necessary to determine the long-term impact of PCV13. Groups of emerging serotypes (including persistence of PCV7 serotypes), changes in antimicrobial susceptibility, and possible geographic differences in serotype distribution should be addressed in future studies.
Notes
Acknowledgments. We thank Shari Young for assistance in procuring Streptococcus pneumoniae isolates. We also thank Beverley Huet for her expertise in the statistical analysis.
Financial support. This work was funded by University of Texas Southwestern and Children's Medical Center Dallas.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Messina AF, Katz-Gaynor K, Barton T, et al. Impact of the pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr Infect Dis J 2007; 26:461–7. [DOI] [PubMed] [Google Scholar]
- 2. Bryant KA, Block SL, Baker SA, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics 2010; 125:866–75. [DOI] [PubMed] [Google Scholar]
- 3. Kaplan SL, Barson WJ, Lin PL, et al. Early trends for invasive pneumococcal infections in children after the introduction of the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2013; 32:203–7. [DOI] [PubMed] [Google Scholar]
- 4. Link-Gelles R, Taylor T, Moore MR; Active Bacterial Core Surveillance Team. Forecasting invasive pneumococcal disease trends after the introduction of 13-valent pneumococcal conjugate vaccine in the United States, 2010–2020. Vaccine 2013; 31:2572–7. [DOI] [PubMed] [Google Scholar]
- 5. Kaplan SL, Barson WJ, Lin PL, et al. Serotype 19A is the most common serotype causing invasive pneumococcal infections in children. Pediatrics 2010; 125:429–36. [DOI] [PubMed] [Google Scholar]
- 6. Yildirim I, Stevenson A, Hsu KK, Pelton SI. Evolving picture of invasive pneumococcal disease in Massachusetts's children: a comparison of disease in 2007–2009 with earlier periods. Pediatr Infect Dis J 2012; 31:1016–21. [DOI] [PubMed] [Google Scholar]
- 7. Feikin DR, Kagucia EW, Loo JD, et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med 2013; 10:e1001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Green MC, Mason EO, Kaplan SL, et al. Increase in prevalence of Streptococcus pneumoniae serotype 6C at eight children's hospitals in the United States from 1993 to 2009. J Clin Microbiol 2011; 49:2097–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Techasaensiri C, Messina AF, Katz K, et al. Epidemiology and evolution of invasive pneumococcal disease caused by multidrug resistant serotypes of 19A in the 8 years after implementation of pneumococcal conjugate vaccine immunization in Dallas, Texas. Pediatr Infect Dis J 2010; 29:294–300. [DOI] [PubMed] [Google Scholar]
- 10. Lindstrand A, Bennet R, Galanis I, et al. Sinusitis and pneumonia hospitalization after introduction of pneumococcal conjugate vaccine. Pediatrics 2014; 134:e1528–36. [DOI] [PubMed] [Google Scholar]
- 11. Angoulvant F, Levy C, Grimprel E, et al. Early impact of 13-valent pneumococcal conjugate vaccine on community-acquired pneumonia in children. Clin Infect Dis 2014; 58:918–24. [DOI] [PubMed] [Google Scholar]
- 12. Olarte L, Hulten KG, Lamberth L, et al. Impact of the 13-valent pneumococcal conjugate vaccine on chronic sinusitis associated with Streptococcus pneumoniae in children. Pediatr Infect Dis J 2014; 33:1033–6. [DOI] [PubMed] [Google Scholar]
- 13. Zuccotti G, Mameli C, Daprai L, et al. Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine 2014; 32:527–34. [DOI] [PubMed] [Google Scholar]
- 14. Loughlin AM, Hsu K, Silverio AL, et al. Direct and indirect effects of PCV13 on nasopharyngeal carriage of PCV13 unique pneumococcal serotypes in Massachusetts’ children. Pediatr Infect Dis J 2014; 33:504–10. [DOI] [PubMed] [Google Scholar]
- 15. Gounder PP, Bruce MG, Bruden DJ, et al. Effect of the 13-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae--Alaska, 2008–2012. J Infect Dis 2014; 209:1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. CLSI. Performance Standards for Antimicrobial Susceptibility Testing—18th Informational Supplement. CLSI document M100-S18 Wayne, PA: Clinical and Laboratory Standards Institute, 2008. [Google Scholar]
- 17. Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 1998; 144(pt 11):3049–60. [DOI] [PubMed] [Google Scholar]
- 18. Grant LR, O'Brien SE, Burbidge P, et al. Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS One 2013; 23;8:e74906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quinet B, Laudat F, Gurtman A, et al. Pneumococcal conjugate vaccine-elicited antibody persistence and immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in children previously vaccinated with 4 doses of either 7-valent or 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2014; 33:1065–76. [DOI] [PubMed] [Google Scholar]
- 20. Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis 2014; 59:1066–73. [DOI] [PubMed] [Google Scholar]
- 21. Iroh Tam PY, Madoff LC, Coombes B, Pelton SI. Invasive pneumococcal disease after implementation of 13-valent conjugate vaccine. Pediatrics 2014; 134:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosen JB, Thomas AR, Lexau CA, et al. Geographic variation in invasive pneumococcal disease following pneumococcal conjugate vaccine introduction in the United States. Clin Infect Dis 2011; 53:137–43. [DOI] [PubMed] [Google Scholar]
- 24. de St Maurice A, Grijalva CG, Fonnesbeck C, et al. Racial and regional differences in rates of invasive pneumococcal disease. Pediatrics 2015; 136:e1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Immunization Survey (NIS) CDC. Survey of immunization coverage among U.S. pre-school children ages 19 through 35 months. Available at: http://www.cdc.gov/vaccines/imz-managers/coverage/nis/child/index.html. Accessed 7 January 2016. [Google Scholar]
- 26. Vu HT, Yoshida LM, Suzuki M, et al. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr Infect Dis J 2011; 30:11–8. [DOI] [PubMed] [Google Scholar]
- 27. Ishizuka S, Yamaya M, Suzuki T, et al. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis 2003; 188:1928–39. [DOI] [PubMed] [Google Scholar]