Abstract
Understanding the dynamics of movement of bacteria within the environment and between species is crucial to unraveling the epidemiology of bacterial diseases and to developing biosecurity measures to prevent dissemination. Many arthropods, some beneficial and some detrimental, inhabit poultry houses. The lesser mealworm, Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae), is a pest commonly found in poultry litter that can harbor pathogens involved in both human and animal health issues. Current farm management practices perpetuate persistent infestations contributing to the dispersal of beetles and pathogens. To study the dissemination of bacteria by this beetle, we require the ability to differentiate internal from external sources of bacteria carried by the beetle. In this study, we tested previously described methods to externally disinfect beetles and found disinfectant efficacies between 40 and 98%. The irregular surface of the insect posed a challenge to cleansing procedures because the surface offered many recesses able to sequester bacteria. Complete bacterial disinfection was achieved with a serial treatment of ethanol and hydrogen peroxide or hydrogen peroxide/peracetic acid.
Keywords: lesser mealworm, external disinfection, Alphitobius diaperinus, bacteria, poultry litter beetle
Lesser mealworms, Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae), at all life stages, inhabit manure and feed in commercial poultry operations and are one of the most abundant insect species recovered from broiler chicken and turkey litter samples (Pfeiffer and Axtell 1980, Stafford et al. 1988, Axtell and Arends 1990, Rueda and Axtell 1997). Lesser mealworms are omnivorous scavengers that feed on manure, spilled chicken feed, cracked eggs, chicken carcasses, house fly maggots, and detritus (Pfeiffer and Axtell 1980, Axtell and Arends 1990, Rueda and Axtell 1997). In turn, these beetles are often fodder for chickens, wild birds, and opportunistic rodents. In addition, the beetles are inadvertently dispersed to neighboring residences by the spreading of beetle-containing manure on nearby fields (Armitage 1986). They are a prime candidate for participating in the transmission of bacteria among fauna inhabiting the poultry house environment and have previously been implicated in the transmission of several disease agents, including bacterial pathogens (De las Casas et al. 1968, 1973, 1976; Despins et al. 1994; McAllister et al. 1994, 1995, 1996; Watson et al. 2000).
Studies exploring the transfer of bacteria by litter beetles have been reported, but there is limited assessment of how a transferred bacterium is harbored by a beetle. This task requires the ability to differentiate bacteria carried externally or internally. Although several previous studies described protocols to disinfect the surface of beetles, no data were presented validating the efficacy of the techniques (De las Casas et al. 1968, 1972; Harein and De las Casas 1968; Harein et al. 1970, 1972; McAllister et al. 1994, 1995, 1996; Hald et al. 1998; Gray et al. 1999).
Beetle morphology can reduce the effectiveness of surface sterilization by preventing adequate access to bacteria. The insect integument and presumably the fecal material adhering to the exoskeleton provide refuge to bacterial organisms. Flexible joints, wings, and spiracles occurring between hard plates offer anchorage to microbes, whereas structures such as the elytra and the cuticle serve as protective covers (Chapman 1982a). In addition, ectodermal invaginations, such as sutures or sulci, may act to shield bacterial organisms from access and displacement during disinfectant procedures (Chapman 1982b). This study assesses the efficacy of previously described and newly developed methods to disinfect the external surface of beetles. We have devised a specific method resulting in complete surface bacterial disinfection for use in future studies to explore the environmental transfer of bacteria by this beetle.
Materials and Methods
Beetles
The Southern Plains Agricultural Research Center (SPARC) starter colony of A. diaperinus was a generous gift from a colony originally isolated from a poultry farm located in Wake County, North Carolina, and maintained by Dr. D. W. Watson (North Carolina State University, Raleigh, NC). The SPARC colony was initiated and has remained in production since 2004. Beetles were reared in 1,000-ml wheat bran (Morrison Milling Co., Denton, TX) in plastic containers (15 by 15 by 30 cm) with screen tops and held at 30°C under a photoperiod of 8:16 (L:D) h. Additionally within each cage, a 6-cm2 sponge moistened with deionized water (dH2O) and a 0.5-cm-thick slice of a medium-sized apple were replenished twice per week, and 30 ml of fishmeal (Omega Protein, Inc., Hammond, LA) was added to the wheat bran once per week.
Disinfecting Agents
Hydrogen peroxide (H2O2) (Sigma-Aldrich, St. Louis, MO), 95% ethanol (EtOH) (EMD Chemicals, Gibbstown, NJ), Tween 80 (Amresco, Solon, OH), and sodium hypochlorite (NaOCl) (Sigma-Aldrich) were diluted to working concentrations with sterile dH2O. The 7.35% H2O2/0.23% peracetic acid was a commercially available formulation, SporGon (Decon Labs, Inc., Bryn Mawr, PA).
These agents were used individually or in combination to formulate and evaluate 11 disinfection protocols designed for comparison to previously described techniques or to test new combinations for increased efficacy. The protocols were grouped into EtOH (three), NaOCl (four), and H2O2 (four)-based protocols as described below. All protocols were evaluated on individual beetles held and disinfected in 1.5-ml tubes covered with a sterile barrier film (Parafilm; Sigma-Aldrich).
For each protocol, the tube in which the beetle was immersed was covered by the barrier film, inverted three times, and sonicated for 2 min at 40 kHz (model 8851–34, Cole Parmer, Vernon Hills, IL) before the next step. The beetle was transferred into a new sterile tube for each successive immersion or rinse. Evaporation was performed by transferring the beetle into a sterile tube, which was placed into a sterile, biosafety hood to allow the EtOH to evaporate. Sonication was used to assist in dislodging of the bacteria and to ensure uniformity of the agitation procedure for comparison of protocols.
EtOH-Based Protocols. (A) Each beetle was immersed in 95% EtOH and then rinsed in sterile dH2O for 30 s followed by sonication for 2 min; the rinse was repeated. (B) Each beetle was immersed in 70% EtOH and the EtOH evaporated for 5 min. (C) Each beetle was immersed in 95% EtOH, and the EtOH evaporated for 5 min.
NaOCl-Based Protocols. (D) Each beetle was immersed in 2% NaOCl/10% Tween 80 and then rinsed in sterile dH2O for 30 s followed by sonication for 2 min; the rinse was repeated. (E) Each beetle was immersed in 2% NaOCl/10% Tween 80, allowed to soak an additional 8 min, and then immersed in 70% EtOH. The beetle was then rinsed in sterile dH2O for 30 s followed by sonication for 2 min; the rinse was repeated twice. (F) Each beetle was immersed in 2% NaOCl/10% Tween 80, allowed to soak an additional 8 min, and then immersed in 95% EtOH and the EtOH evaporated for 5 min. (G) Each beetle was immersed in 95% EtOH, followed by immersion in 2% NaOCl:10% Tween 80, and allowed to soak an additional 8 min. The beetle was then immersed in 70% EtOH and the EtOH evaporated for 5 min.
H2O2-Based Protocols. (H) Each beetle was immersed in 20% H2O2, followed by immersion in sterile dH2O. (I) Each beetle was immersed in 20% H2O2, followed by immersion in 95% EtOH and the EtOH evaporated for 5 min. (J) Each beetle was immersed in 95% EtOH and the EtOH evaporated for 5 min, followed by immersion in 20% H2O2. (K) Each beetle was immersed in 95% EtOH and the EtOH evaporated for 5 min, followed by immersion in 7.35% H2O2/0.23% peracetic acid.
Experimental Design
Three replications of each experimental wash protocol were conducted using 30 beetles per protocol per replication. To collect a sample of the resident bacteria present on the outer surface of the beetles before external disinfection (PRE-wash), each beetle was immersed in 1 ml of tryptic soy broth (TSB; Difco, Sparks, MD) at room temperature. The beetle was removed and immediately subjected to one of the experimental disinfection protocols (see above). An aliquot of 0.1 ml of PRE-wash TSB was serially diluted, spread on blood agar plates (Becton Dickinson, Sparks, MD), and incubated overnight at 37°C. Bacterial load, expressed as colony-forming units (CFU), was determined after an 18–24-h incubation. After each disinfection protocol was completed, surviving residual bacteria were sampled (POST-wash) by again immersing the beetle in 1 ml of TSB at room temperature. The beetle was removed, and an aliquot of 0.1 ml of POST-wash TSB was serially diluted, spread on blood agar plates, and incubated overnight at 37°C. CFU were determined after an 18–24-h incubation. To ensure detection of bacterial contamination below the plating threshold of 10 CFU, the remaining 0.9 ml of PRE-wash and POST-wash TSB was enriched by incubation overnight at 37°C (ENRICH). After incubation, an aliquot of 0.1 ml of TSB was spread on a blood agar plate and incubated overnight at 37°C. The presence or absence of bacteria was recorded after 18–24-h incubation at 37°C. CFU data were analyzed by logistic regression and enrichment data were analyzed by exact logistic regression in PROC LOGISTIC (SAS Institute, Cary, NC; Agresti 2002). Representative colonies of bacteria present were collected from PRE- and POST-wash blood plates for identification by growth on selective media or by ribotyping.
For PRE-TSB control, 60 beetles were taken from the same cage and split into two groups of 30 beetles. From one group, beetles were placed into individual tubes containing TSB. These served as PRE-wash samples for this control study to ensure that the tested beetles were contaminated with bacteria. Beetles from the second group, without prior immersion in TSB, were subjected to protocol K as described above.
Bacterial Isolation and Preliminary Identification Culture Methods
Individual bacteria from mixed cultures were grown in TSB and then subcultured onto blood agar plates for selection of isolated colonies. Individual isolates were identified by growing them on a bank of selective and differential media and then comparing the results from the unknown isolate to data for known species (Atlas 1997). To identify aerobic bacteria, 10-μl aliquots were streaked onto Brilliant Green agar (BGA; Becton Dickinson), MacConkey (Becton Dickinson), m Enterococcus (ME; Becton Dickinson), Rogosa (Becton Dickinson), CHROMagar E. coli, and CHROMagar Orientation (CHROMagar, Paris, France) plates, and then incubated at 37°C for 24 h. Each initial colony selection was streaked onto fresh media to ensure cultural purity. A bacterial lawn of each pure isolate culture was prepared on blood agar for definitive identification using automated ribotyping.
Ribotype Characterization
Bacterial isolates from blood agar plates were incubated at 37°C for 24 h to allow a bacterial lawn to form. Samples were collected while in log phase growth. Bacteria were suspended in a neutral pH buffer (Qualicon, Inc., Wilmington, DE), heated at 90°C for 10 min, combined with two additional lytic enzymes (Qualicon, Inc.), and analyzed according to manufacturer's instructions using the restriction enzymes EcoRI or PvuII. The RiboPrinter microbial characterization system (Qualicon, Inc.) characterizes the 5, 16, and 23S rRNA and flanking regions of a bacterial sample by using specified restriction enzymes. The resulting rRNA pattern (RiboPrint pattern) was automatically characterized and identified by comparing the pattern to reference patterns in the bacterial database provided by Qualicon, Inc. (containing >6000 isolates) and a custom, food animal-specific database (containing >400 isolates) developed by C.S. Identification requires that the ribopattern be an 85% or greater match to an existing static ribopattern. When ribotype identification match was <85%, isolate identification was confirmed using, API Staph, API 20E, API 20NE, API 20 Strep, ID 32 STAPH (Analytical Profile Index, bioMerieux, Inc., Durham, NC) manual identification test strips.
Results
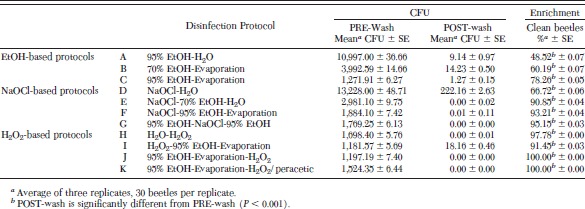
A comparison of external disinfection protocols demonstrated large variation in treatment efficacy (Table 1). External disinfection using 95% EtOH followed by a dH2O rinse (protocol A), disinfected less than one-half of the beetles. However, allowing external surface evaporation after treatment with EtOH instead of immediately performing a dH2O rinse, significantly improved disinfection by 61% (protocol C versus A; P < 0.0001). Using 2% NaOCl/10% Tween 80 followed by a dH2O rinse (protocol D) disinfected slightly more than one-half of the beetles. Adding a 70% EtOH treatment to the protocol significantly improved disinfection by 36% (protocol E verses D; P < 0.0003). Removing the dH2O rinse completely and adding a higher strength 95% EtOH treatment, which was subsequently allowed to evaporate, also significantly improved disinfection by 40% (protocol F versus D; P < 0.002). Prior treatment of 95% EtOH with NaOCl/Tween 80 improved disinfection by 19% (protocol C versus F; P < 0.03). However, the addition of a second 95% EtOH treatment before the NaOCl/Tween 80 and 95% EtOH treatments did not significantly increase efficacy (protocol G verses F). A dH2O rinse to wet the beetle followed by 20% H2O2 (protocol H) disinfected almost all of the beetles. The substitution of a 95% EtOH, which was subsequently allowed to evaporate, before treatment with 20% H2O2 or 7.35% H2O2/0.23% peracetic acid, disinfected 100% of the beetles (protocols J and K). However statistically nonsignificant the increase between protocols H versus J or K (2%), it gave us the outcome we desired, which was consistently reproducible complete disinfection of culturable, aerobic bacteria.
Table 1.
Comparison of the efficacy of lesser mealworm surface disinfection protocols
The measuring of resident bacteria before executing the disinfection protocol required a pretreatment of beetles by immersion in TSB. As this would not be a step normally included in the external disinfection protocol, we demonstrated that this initial immersion in TSB did not affect the efficacy of the disinfection protocol by testing protocol K (7.35% H2O2/0.23% peracetic acid) without PRE-wash immersion in TSB. A comparison between beetles exposed to prior TSB immersion and those disinfected without prior immersion showed no significant difference (P < 1.0) in wash efficacy. This demonstrated that the PRE-wash TSB immersion had no affect on the outcome of the disinfectant protocol.
The resident bacterial load of each beetle was measured individually before exposure to a disinfection protocol (Fig. 1). A frequency distribution of the log10 CFU of the results indicated that bacterial load was variable on individual beetles. Bacterial load ranged from 0 CFU on 20 beetles to 2.5 × 106 CFU on one beetle, averaging 7.6 × 103± 9.0 × 104 CFU, with a median of 1.2 × 103 CFU.
Fig. 1.
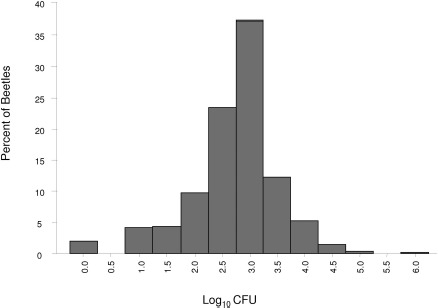
Frequency distribution of the bacterial load carried by individual beetles before disinfection procedures. A log10 transformation of the PRE-wash CFU data from 990 individual beetles was used to create the graph.
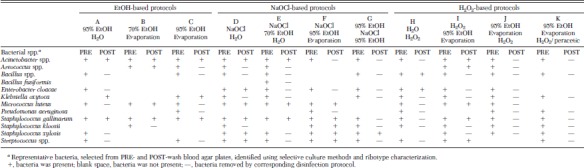
The species of bacteria carried by the beetles was relatively consistent. Although beetles were reared in separate cages, they used feed and bran from identical sources and were placed into a common cage as adults before use in experiments; thus, similar contamination profiles were found. The beetles were contaminated with 12 bacterial species, representing nine genera: Acinetobacter spp. (Brisou and Prévot; 97% API match), Aerococcus spp. (Williams; 100% API match), Bacillus spp. (Meyer and Gottheil; 97% ribotype match), Bacillus fusiformis (Cohn; 91% ribotype match), Enterobacter cloacae (Jordan; 94% ribotype match), Klebsiella oxytoca (Flügge; 94% ribotype match), Micrococcus luteus (Schtoeter; 91% ribotype match), Pseudomonas aeruginosa (Schroeter; 85% ribotype match), Staphylococcus gallinarum (Devrise; 94% ribotype match), Streptococcus kloosii (Schleifer; 92% ribotype match), Streptococcus xylosis (Schleifer and Kloos; 91% ribotype match), and Streptococcus spp. (Rosenbach; 92% API match). The bacteria showed varying sensitivities to the disinfection protocols (Table 2). Acinetobacter spp. and S. gallinarium seemed particularly resistant to EtOH- and NaOCl-based washes. M. luteus was also resistant to 70% EtOH and NaOCl washes, but it did not survive a 95% EtOH wash. Bacillus spp. and Enterobacter cloacae resisted sequential washing in dH2O and H2O2, but they were removed by sequential washing in 95% EtOH and H2O2. Although we did not identify the taxa, we noted that sequential washing in 95% EtOH and H2O2 also disinfected fungi present on the PRE-wash plates (data not shown).
Table 2.
Comparison of surface disinfection protocols by removal of specific bacterial species
Discussion
Litter beetles, especially the lesser mealworm, have become serious pests within the poultry brooder and laying industry. Because of their mobility, feeding habits, and prey potential, these beetles are implicated as mechanical vectors for diseases (e.g., Mareks disease, avian influenza, bacterial diseases, fowl pox, coccidiosis, and New Castle disease). The insect offers surfaces to support bacterial, fungal, and viral organisms (De las Casas et al. 1968, 1972, 1973; Harien et al. 1970; McAllister et al. 1994, 1995). These beetles have high reproductive rates and are difficult to control. Ultimately, they are portrayed as a reservoir source contributing to the persistence and transmission of pathogens among individual birds within a poultry facility (Harien et al. 1970, 1972; Brown et al. 1992; Hald et al. 1998).
Previous studies have attempted to surface sterilize beetles to establish the carriage of pathogens internally (De las Casas et al. 1968, 1972; Harein and De las Casas 1968; Harein et al. 1970, 1972; McAllister et al. 1994, 1995, 1996; Hald et al. 1998; Gray et al. 1999). However, validation of these sterilization methods was not presented. In this study, we tested the efficacy of disinfection protocols to remove culturable, aerobic bacteria from the external surface of A. diaperinus. The protocols were based on the use of three primary chemicals (EtOH, NaOCl, Tween 80, and H2O2), and the level of external disinfection was found to range from 48 to 100%. Sonication was used to assure uniformity of the agitation procedure for comparison of protocols. However, it should be noted that sonication has been reported to assist in the destruction of bacterial cells and thus could enhance the efficiency of the disinfection techniques used (Dehghani 2005).
The amount of bacteria carried by each individual beetle before disinfection varied greatly. The bacterial load is of course subject to many factors, such as moisture, litter and feed present, but it was interesting that in the same environment we could culture no bacterial from the external surface of several beetles. Perhaps the bacteria were sequestered in locations impervious to extrication. De las Casas et al. (1972) also observed this phenomena, finding that some beetles carried several thousand colonies, whereas others from the same cage carried few or no bacteria. Given the individual variation in bacterial load, the use of a large sample size is prudent for transmission studies.
Hald et al. (1998) investigated bacterial carriage by hairy fungus beetle, Typhaea stercorea L., by bulk disinfection in 70% EtOH followed by evaporation. In our study, only 60% of the A. diaperinus were surface disinfected by this method (protocol B). The reservoir competence of lesser mealworms for Salmonella and for E. coli was investigated by disinfection with dH2O followed by 70% EtOH and a second dH2O rinse (McAllister et al. 1994, 1996). Conclusions indicated that adult and larval A. diaperinus maintained a viable population of internal (homogenate) and external bacteria for a period of time sufficient to infect successive flocks of broiler chicks placed in the house. However, no data were presented demonstrating the effectiveness of the external disinfection procedure before homogenation. Our study indicates that this in an incomplete sterilization procedure. Gray et al. (1999) investigated the histerid beetle Carcinops pumilio (Erichson), measuring both surface and internal (homogenate) contamination after rinsing for 30 s in 95% EtOH followed by dH2O. These investigators concluded that bacteria were carried both externally and internally. However, no supporting data were presented to substantiate the disinfection procedure. In our study, disinfection with 95% EtOH followed by dH2O (protocol A) was only 48.52% effective. It is possible that if the surface disinfection procedures were insufficient, then bacteria harbored externally would be attributed to internal sources upon culturing of the insect homogenate.
In studies investigating recovery of bacteria from granary weevils, Sitophilus granarius (L.), and A. diaperinus adults, pupae, and larvae, surface disinfection using 2% NaOCl/Tween 80-based protocols were performed (De las Casas et al. 1968, 1972; Harein and De las Casas 1968). The authors noted that neither treatment disinfected completely; therefore, the effectiveness of these techniques was determined by subsequent individual exposure of each beetle to nutrient broth and removal of contaminated specimens from the study. We determined that washing in 2% NaOCl/Tween 80 followed by dH2O was only 66.72% effective (protocol D). Similarly, De las Casas et al. (1968) determined that ≈50% of the pupae subjected to these disinfection procedures were still contaminated. In our study, the simple addition of an EtOH rinse step after disinfection by NaOCl/Tween 80 increased effectiveness to an average of 92.33% (protocol E and F).
Lesser mealworms have been surface sterilized against infectious bursal disease virus with 10% H2O2 followed by rinsing in dH2O (McAllister et al. 1995). In our study, disinfection with 10% H2O2 was <100% effective against bacteria (data not shown). An increase to 20% H2O2 (protocol H) was found to be 97.8% effective against bacteria. The addition of a 95% EtOH treatment before a 20% H2O2 wash resulted in consistent, complete surface bacterial disinfection.
H2O2 is often used as an antimicrobial or bleaching agent. However, it is an unstable compound that quickly neutralizes itself by reverting to oxygen and water. Stability depends upon many factors, but solutions of H2O2 which are kept in dark, inert containers that are completely free of contamination are relatively stable. Commercial solutions, however, usually contain minute amounts of impurities, which can cause decomposition; therefore, stabilizers are sometimes added (Goor 1989, Hess 1995, CHEMINFO 2005). Concentrated H2O2 solutions can also react exothermically with solvents. Although H2O2 proved to be the best cleansing agent, its instability in pure form and exothermic reactivity at high concentrations made it problematic to use; therefore, we investigated a commercially available substitute. The substitute consisted of a less concentrated formulation of 7.35% H2O2 with the addition of 0.23% peracetic acid, a stabilizer, a surfactant and a corrosion inhibitor. Our results demonstrate that this formulation was as effective as 20% H2O2 treatment.
The thin epicuticle secreted on the outside of the cuticle serves to minimize loss of insect body water, contributing to their success in a terrestrial environment (Bursell and Clements 1967). The epicuticle consists of several layers, including a superficial wax or lipid layer of long-chain hydrocarbons and esters of fatty acids and alcohols (Chapman 1982a, Lockey 1988). This deterrent to desiccation also presumably diminishes the effectiveness of surface disinfection by H2O2 or NaOCl treatment due to its hydrophobic nature. We found that prior treatment with EtOH enhanced the efficacy H2O2 and NaOCl treatments. Because lipids are soluble in organic solvents, it is likely that EtOH treatment either diminished the waxy layer or wetted the exterior sufficiently to allow a subsequent agent improved access to the beetle surface and invaginations, and the microorganisms residing there.
Surface disinfections that included EtOH or NaOCl were detrimental to the beetles and often resulted in their death. H2O2 treatment seemed less deleterious because the beetles usually survived the treatment. For our purposes and for many of the studies discussed, survival of the specimens was not required. However, for future studies where survival is desired, a modification in the protocols to a protocol less adverse to the survival of the beetles while still maintaining efficacy as a disinfectant will be necessary.
In summary, this study assessed the efficacy of previously described and newly developed methods to disinfect the external surface of A. diaperinus. Although many of the bacterial disinfecting procedures examined cleaned >90% of the beetles, we endeavored to find a protocol that disinfected 100% of the insects. When investigating bacterial transfer, complete exoskeleton disinfection is important to discern conclusively an internal source of contamination. Several previously reported studies used protocols that purportedly disinfected the surface of beetles; however, we could find no data validating the efficacy of the described techniques. Although it is possible that the insects harbor bacterial organisms internally, insufficiently sterilized exoskeletons can contaminate subsequent homogenized specimens. Therefore, it would be unclear whether the bacteria being measured were actually being harbored internally. We found that a combination of treatment with 95% EtOH, allowed to evaporate, followed by a 20% H2O2 or 7.35% H2O2/0.23% peracetic acid wash removed all culturable, aerobic bacteria from the external surface of the adult lesser mealworms.
Acknowledgements
We thank Jesus Esquivel and Sharon Mowery for assistance in establishing the beetle colony; Gretchen Jones, Kate Andrews, Jason Leger, and Melanie Sandera for technical assistance; and Sara Duke for assistance with statistical analysis.
Footnotes
Mention of trade names, companies, or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement of the products by the U.S. Department of Agriculture.
References Cited
- Agresti A. 2002. Categorical data analysis, 2nd ed. Wiley, Hoboken, NJ. [Google Scholar]
- Atlas R.M. 1997. Handbook of microbiological media, 2nd ed. CRC Press, Boca Raton, FL. [Google Scholar]
- Armitage D. M. 1986. Population changes of four species of insects in three deep pit poultry houses. Entomol. Mon. Mag. 122: 75–77. [Google Scholar]
- Axtell R. C., Arends J. J. 1990. Ecology and management of arthropod pests of poultry. Annu. Rev. Entomol. 35: 101–126. [DOI] [PubMed] [Google Scholar]
- Brown D. J., Olsen J. E., Bisgaard M. 1992. Salmonella enterica: infection, cross infection and persistence within the environment of a broiler parent stock unit in Denmark. Zbl. Bakt. 277: 129–138. [DOI] [PubMed] [Google Scholar]
- Bursell E., Clements A. N. 1967. The cuticular lipids of the larva of Tenebrio molitor L. (Coleoptera). J. Insect Physiol. 13: 1671–167. [Google Scholar]
- Chapman R. F. 1982a. The integument, pp. 501–528. InThe insects, structure and function, 3rd ed. Harvard University Press, Cambridge, MA. [Google Scholar]
- Chapman R. F. 1982b. The tracheal system and respiration in terrestrial insects, pp. 529–553. InTheinsects, structure and function, 3rd ed. Harvard University Press, Cambridge, MA. [Google Scholar]
- CHEMINFO. 2005. Canadian Centre for Occupational Health & Safety. Hamilton, Ontario, Canada: (www.intox.org). [Google Scholar]
- De las Casas E., Pomeroy B. S., Harein P. K. 1968. Infection and quantitative recovery of Salmonella typhimurium and Escherichia coli from within the lesser mealworm, Alphitobius diaperinus (Panzer). Poult. Sci. 47: 1871–1875. [DOI] [PubMed] [Google Scholar]
- De las Casas E., Harein P. K., Pomeroy B. S. 1972. Bacteria and fungi within the lesser mealworm collected from poultry brooder houses. Environ. Entomol. 1: 27–30. [DOI] [PubMed] [Google Scholar]
- De las Casas E., Harein P. K., Deshmukh D. R., Pomeroy B. S. 1973. The relationship between the lesser mealworm and avian viruses: I. Reovirus 24. Environ. Entomol. 2: 1043–1047. [Google Scholar]
- De las Casas E., Harein P. K., Deshmukh D. R., Pomeroy B. S. 1976. Relationship between the lesser mealworm, fowl pox and Newcastle disease virus in poultry. J. Econ. Entomol. 69: 775–779. [DOI] [PubMed] [Google Scholar]
- Dehghani M. H. 2005. Effectiveness of ultrasound on the destruction of E. coli. Am. J. Environ. Sci. 1: 187–189. [Google Scholar]
- Despins J. L., Axtell R. C., Rives D. V., Guy J. S., Ficken M. D. 1994. Transmission of enteric pathogens of turkeys by darkling beetle larva (Alphitobius diaperinus). J. Appl. Poult. Res. 3: 61–65. [Google Scholar]
- Goor G. 1989. Hydrogen peroxide, pp. 443–466. InGerhartz W., Yamamoto Y. S. [eds.], Ullmann’s encyclopedia of industrial chemistry, 5th ed., vol. A 13. VCH Verlagsgesellschaft, Weinheim, Deerfield Beach, FL. [Google Scholar]
- Gray J. P., Maddox C. W., Tobin P. C., Jennifer D. G., Pitts C. W. 1999. Reservoir competence of Carcinops pumilio for Salmonella enteritidis (Eubacteriales: Enterobacteriaceae). J. Med. Entomol. 36: 888–891. [DOI] [PubMed] [Google Scholar]
- Hald B., Olsen A., Madsen M. 1998. Typhaea stercorea (Coleoptera: Mycetophagidae), a carrier of Salmonella enterica serovar infantis in a Danish broiler house. J. Econ. Entomol. 91: 660–664. [DOI] [PubMed] [Google Scholar]
- Harein P. K., De las Casas E. 1968. Bacteria from granary weevils collected from laboratory colonies and field infestations. J. Econ. Entomol. 61: 1719–1720. [Google Scholar]
- Harein P. K., De las Casas E., Pomeroy B. S., York M. D. 1970. Salmonella spp. and serotypes of Escherichia coli isolated from the lesser mealworm collected in poultry brooder houses. J. Econ. Entomol. 63: 80–82. [DOI] [PubMed] [Google Scholar]
- Harein P. K., Delas Casas E., Larsen C. T., Pomeroy B. S. 1972. Microbial relationship between the lesser mealworm and its associated environment in a turkey brooder house. Environ. Entomol. 1: 189–194. [Google Scholar]
- Hess W. T. 1995. Hydrogen peroxide, pp. 961–995. InKroschwitz J. I., Howe-Grant M. [eds.], Kirk-Othmer encyclopedia of chemical technology, 4th ed., vol. 13. Wiley, New York. [Google Scholar]
- Lockey K. H. 1988. Review - lipids of the insect cuticle: origin, composition and function. Comp. Biochem. Physiol. 89B: 595–645. [Google Scholar]
- McAllister J. C., Steelman C. D., Skeeles J. K. 1994. Reservoir competence of the lesser mealworm (Coleoptera: Tenebrionidae) for Salmonella typhimurium (Eubacteriales: Enterobacteriaceae). J. Med. Entomol. 31: 369–372. [DOI] [PubMed] [Google Scholar]
- McAllister J. C., Steelman C. D., Newberry L., Skeeles J. K. 1995. Isolation of infectious bursal disease virus from the lesser mealworm, Alphitobius diaperinus (Panzer). Poult. Sci. 74: 45–49. [DOI] [PubMed] [Google Scholar]
- McAllister J. C., Steelman C. D., Skeeles J. K., Newberry L. A., Gbur E. E. 1996. eservoir competence of the Alphitobius diaperinus (Coleoptera: Tenebrionidae) for Escherichia coli (Eubacteriales: Enterobacteriaceae). J. Med. Entomol. 33: 983–9872. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D. G., Axtell R. C. 1980. Coleoptera of poultry manure in caged layer houses in North Carolina. Environ. Entomol. 9: 21–28. [Google Scholar]
- Rueda L. M., Axtell R. C. 1997. Arthropods in litter of poultry (broiler chicken and turkey) houses. J. Agric. Entomol. 14: 81–91. [Google Scholar]
- Stafford K. C., Collison C. H., Burg J. G., Cloud J. A. 1988. Distribution and monitoring lesser mealworms, hide beetles, and other fauna in high-rise, caged layer poultry houses. J. Agric. Entomol. 5: 89–101. [Google Scholar]
- Watson D. W., Guy J. S., Stringham S. M. 2000. Limited transmission of turkey coronavirus in young turkeys by adult Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Med. Entomol. 37: 480–483. [DOI] [PMC free article] [PubMed] [Google Scholar]


