Abstract
The aim of this study was to determine the potential modulatory effects of diets supplemented with spray-dried animal plasma (SDAP) or immunoglobulin concentrates (IC) on the immune response of rats challenged with Staphylococcus aureus enterotoxin B (SEB). Lewis rats were fed diets containing 80 g of SDAP/kg diet, 22.7 g of IC/kg diet, or milk proteins (Control diet) from postnatal d 21 (weaning) for 14 d. On d 30 and 33, rats were given SEB (0.5 mg/kg body weight; i.p.). Organized gut-associated lymphoid tissue (GALT) populations, intestinal secretion, mucosal and serum immunoglobulin concentrations, and neutrophil infiltration were studied. On d 35, blood was collected under anesthesia and samples of intestinal mucosa, Peyer's patches, mesenteric lymph nodes (MLN), and spleen were taken. SEB increased the water content of feces, which was prevented by diets containing either SDAP (P < 0.002) or IC (P < 0.001), indicating that plasma protein–supplemented diets can reverse the SEB-induced secretory response. In Peyer's patches, the diet containing SDAP partially prevented the SEB-induced increase in T lymphocytes (P < 0.1) and reduced the percentage of activated T helper cells (P < 0.05). In MLN, activated T lymphocytes were increased by SEB but they were not affected by diet. No effects of SEB or dietary supplementation on mucosal IgA and serum IgA and IgG were observed. The effects of SDAP supplementation on the lymphocyte populations of GALT in rats challenged with SEB support the view that SDAP can modulate the immune response and suggest that plasma protein supplementation can prevent GALT from possible activation by luminal bacterial superantigens.
Keywords: spray-dried plasma, immunoglobulin concentrates, intestinal inflammation, rat, Staphylococcus aureus enterotoxin B
Spray-dried animal plasma (SDAP)4 and serum proteins are complex mixtures of active proteins and other biologically important compounds that are used as additives in diets and supplements in farm animal production. SDAP promotes intestinal growth (1) and improves food intake and weight gain in postweaning pigs (2). These effects are more pronounced under conditions with a high number of pathogens than in environments with reduced potential pathogen exposure (3). This observation was confirmed by studies performed in animals challenged with pathogenic E. coli (4). This type of dietary supplement has been used as an alternative to antibiotics in the diet of young calves (5) and weanling pigs (6). Serum immunoglobulin concentrates (IC) improve viral gastroenteritis in children (7) and have been used in the treatment of diarrhea in AIDS patients infected with Cryptosporidium parvum (8). In both studies, significant reductions in stool frequency and weight loss were observed.
Mechanisms by which SDAP counteracts pathogen activity may include a reduction in pathogen adhesion. It was shown that several glycoproteins obtained from plasma can inhibit adhesion of E. coli to the small intestine (9). Moreover, immunoglobulins present in SDAP may bind to potential antigens in the lumen of the small intestine and prevent their attachment to mucosa and growth (10). Recent studies showed that SDAP reduces inflammatory cytokine expression in tissues exposed to lipopolysaccharide (LPS) (11) or E. coli (10). These results indicate that the mechanism of action of dietary SDAP and immunoglobulins on growth performance involves the immune system because the degree of immune cell activation may limit the availability of energy for growth (12) and may alter the structure and integrity of the intestinal mucosa (13). This hypothesis is consistent with the observation that SDAP can reduce tumor necrosis factor (TNF)-α expression in liver and spleen of farm animals exposed to thermal environment stress and challenged with LPS (14).
The objective of this study was to test the effects of SDAP and IC on cell populations of gut associated lymphoid tissue (GALT) and spleen, in rats challenged with enterotoxin B of Staphylococcus aureus (SEB). Staphylococcal exotoxins are potent activators of the immune system that can activate a high percentage of T cells by cross-linking major histocompatibility complex (MHC) class II molecules with a moiety on the variable portion of the β chain of the T-cell receptor (TCR) (15), hence called “superantigens.” They cause a variety of diseases in animals and humans ranging from food poisoning to shock. Injection of SEB in mice and rats is often used as a model to study the immunological mechanisms of a superantigen-activated shock, in conjunction with other promoting agents (16) or by administration of a repeated dose of the same agent. We also investigated the effects of dietary SDAP and IC on neutrophil infiltration and mucosal water permeability, in rats challenged with SEB.
MATERIALS AND METHODS
Animals and diets.
Male Wistar Lewis rats obtained from Harlan Ibérica were used throughout. Rats were kept under stable temperature and humidity conditions, with a 12-h light:dark cycle. At d 21 after birth, rats were weaned, distributed randomly into the various experimental groups and fed experimental diets until d 35. Groups studied were as follows: 1) healthy rats fed a control diet (Healthy); 2) rats fed a control diet and given SEB; 3) rats given SEB and fed a diet supplemented with spray-dried animal plasma (SEB-SDAP); and 4) rats given SEB and fed a diet supplemented with an immunoglobulin concentrate (SEB-IC). All protocols used in this study were approved by the ethical committees for animal experimentation of the Universitat de Barcelona and of the Regional Government (Departament d'Agricultura, Ramaderia i Pesca, Generalitat de Catalunya). Rats were killed between 0900 and 1130 h by cervical dislocation under anesthesia.
Pelleted diets were formulated to meet NRC requirements (17) for laboratory animals (Table 1). SDAP is a feed ingredient obtained after separation of RBC by centrifugation of hygienically collected bovine blood from healthy animals (18). The IC was obtained by purification of the immunoglobulin fraction of bovine plasma (19). Both ingredients were spray-dried to obtain a stable powder product containing active immunoglobulins (175 g IgG/kg in SDAP and 617 g IgG/kg in IC). The maintenance of the native structure was checked by immunoelectrophoresis and Western blotting and the activity by an ELISA specifically recognizing E. coli (20). Diets supplemented with SDAP or IC were formulated to have a similar IgG concentration (14 g/kg of diet). Rats were monitored for daily food intake and body weight.
TABLE 1.
Composition of the experimental diets
| Control diet | SDAP diet1 | IC diet1 | |
|---|---|---|---|
| g/kg | |||
| Ingredient | |||
| Spray-dried animal plasma (SDAP) | — | 80 | — |
| Ig fraction concentrate (IC) | — | — | 22.7 |
| Corn starch | 149.3 | 262.1 | 187.1 |
| Dried skim milk | 530.7 | 337.2 | 470 |
| Sugar | 150 | 150 | 150 |
| Soybean oil | 70 | 70 | 70 |
| Cellulose | 50 | 50 | 50 |
| AIN-93 VX2 | 10 | 10 | 10 |
| AIN-93 GM2 | 35 | 35 | 35 |
| DL-methionine | 2.5 | 3.2 | 2.7 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| Nutrient composition | |||
| Crude protein | 182.6 | 180.1 | 181.8 |
| Fat | 75.2 | 73.7 | 74.8 |
| Fiber | 1.6 | 1.0 | 1.4 |
| Metabolizable energy, kJ/g | 14.6 | 14.9 | 14.7 |
APC Incorporated, Ankeny, IA.
Dyets Incorporated, Bethlehem, PA.
Induction of intestinal inflammation.
Intestinal inflammation was induced by the i.p. administration of SEB (Toxin Technologies) dissolved in PBS. Preliminary experiments indicated that 2 doses of SEB administered with a 3-d interval resulted in higher myeloperoxidase (MPO) activity and water feces content than single doses of SEB. For this reason we used a protocol based on the administration of 2 SEB doses (0.5 mg SEB/kg body weight), the first on d 30 (d 9 after weaning) and the second on d 33. Healthy rats received PBS. Rats were killed on d 35 (i.e., after a 14-d dietary treatment).
Water in feces.
At d 33, 34, and 35, spontaneously defecated stools were immediately collected and weighed. Feces were dried at 60°C for 3 d; the water content was measured and expressed as a percentage of the total wet weight.
Determination of MPO activity.
MPO activity was measured as an indicator of neutrophil infiltration in mucosal samples from the jejunum. The jejunum was excised, flushed with PBS, and opened lengthwise following the mesentery line. The mucosa was scraped using glass slides, weighed, and immediately frozen at −80°C. For analysis, thawed samples were suspended in PBS, homogenized (Polytron, Kinematica), and centrifuged (1300 × g, 20 min); the supernatant was used for mucosal IgA determination. Mucosal MPO was determined following the method described by Fedorak et al. (21) using o-dianisidine (0.6 g/L, Sigma) and 0.0016% (v/v) H2O2 (Sigma) solutions. Absorbance was measured every 15 s for 75 s at 460 nm in a UV-160A spectrometer (Shimadzu). One unit of MPO activity (UMPO) is defined as the amount of enzyme that degrades 1 μmol of H2O2/min at 25°C.
Immunoglobulin determination.
Serum and mucosal IgA were measured by sandwich ELISA using mouse anti-rat IgA (MARA-1, Labgen; 1.25 mg/L) as capture antibody and rat IgA (IR22, Labgen) as standard. Serum IgG was also measured by ELISA, using mouse anti-rat IgG (STAR 71, Serotec; 0.25 mg/L) as capture antibody and purified rat IgG (Labgen) as standard. In both assays, horseradish peroxidase-conjugated mouse anti-rat (RT-39 and RL-6, Sigma; 1:2000 dilution) was used as a secondary antibody. Color intensity was measured in a Microplate Reader (Multiscan, Labsystems).
Preparation of lymphocytes samples.
Mesenteric nodes were excised and lymphocyte suspension was obtained by passage through stainless steel sieves in RPMI medium (Innogenetics) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS; Innogenetics). Lymphocytes were then pelleted by centrifugation (600 × g, 10 min), resuspended in 2 mL of PBS containing 2% (v/v) FCS and 0.5 g/L sodium azide (PBS-FCS-Az). The lymphocyte pellet was then resuspended in 0.5 mL PBS-FCS-Az and cell counting and viability determination was done using acridine orange and ethidium bromide. In all cases, cell viability was >80%. The cell suspension was stored at 4°C until processing for immunostaining and flow cytometry.
To obtain spleen lymphocytes samples, the same protocol was followed; the only difference was an application of a Nycoprep gradient (Axis-Shield PoC AS) before the counting and viability determination.
To obtain lymphocyte suspension from Peyer's patches (PP), the tissue was incubated in RPMI medium supplemented with 10% (v/v) FCS and 1 mmol/L of dithiothreitol (Sigma) for 5 min at 37°C. Lymphocyte suspension was obtained through stainless steel mesh and then centrifuged (600 × g, 10 min), resuspended in 44% (v/v) Percoll (Amersham Biosciences), and placed over 67.5% (v/v) Percoll. After centrifugation (600 × g, 30 min), the interface between the 44 and 67.5% layers was removed, and the cells were washed in PBS-FCS-Az and centrifuged (600 × g, 10 min).
Lymphocyte labeling by immunofluorescence.
Lymphocyte subsets were determined after double staining with a panel of anti-rat monoclonal antibodies and analyzed by flow cytometry. The phenotype of lymphocyte subsets were stained with anti-CD45RA (OX33 unconjugated, Pharmingen) to label B lymphocytes, anti-CD3 [G4.18, fluorescein isothiocyanate (FITC)-conjugated, Pharmingen] to stain T lymphocytes, anti-CD4 (W3/25, FITC-conjugated, Labgen) to detect T helper lymphocytes, anti-CD8 (OX-8, FITC-conjugated, Labgen) to label T suppressor/cytotoxic, anti-TCRαβ (R73, unconjugated, Pharmingen) and anti-TCRγδ [V65, phycoerythrin (PE)-conjugated, Pharmingen] to recognize the cells expressing the corresponding TCR, anti-CD25 (NDS61, unconjugated, Labgen) to stain activated T lymphocytes, and anti-NKR-P1A (10/78, unconjugated, Pharmingen) to detect natural killer (NK) cells.
Cells (3 × 105) were incubated with primary nonlabeled mouse monoclonal antibodies for 20 min at 4°C and subsequently washed in PBS-FCS-Az. Cells were incubated for 20 min with PE-conjugated goat anti-mouse IgG antibody (Sigma) diluted in PBS-FCS-Az containing 2% (v/v) rat serum to avoid cross-reactions. After a second wash, cells were incubated with normal mouse immunoglobulins (15 min at 4°C) and then labeled with FITC-conjugated antibodies (20 min at 4°C). Finally, cells were washed in PBS, fixed with 10 g/L paraformaldehyde, and stored at 4°C in the dark until analysis. For each rat, a negative control staining was included (human CD7). All antibodies were used at saturating concentration. Samples were counted in a flow cytometer (Epics Elite, Coulter).
Blood determinations.
Total red and white blood cell counts, hematocrit, hemoglobin, and leukocyte differential counts were determined automatically by means of a Coulter Counter JT hemocytometer.
Statistical analyses.
Means of replicate experiments were compared. Results are given as means ± SEM, n = 7–10. To analyze the effect of the enterotoxin administration, SEB rats were compared with the Healthy rats by an ANOVA using SPSS-10.0 software (SPSS). To study the effect of dietary supplementation on the intestinal inflammation model, rats fed the supplemented diets (SEB-SDAP, SEB-IC) were compared with rats fed the unsupplemented diet (SEB) by another ANOVA followed by Scheffé's post-hoc test. Differences were considered significant at P < 0.05.
RESULTS
Food intake and body weight.
Food intake, which was monitored from weaning (d 21) up to the end of the experimental period (d 35), did not differ among the groups (Fig. 1A). The estimated intake of dietary active IgG during the 14-d experimental period was ∼1.8 g IgG/rat. The mean body weight of rats at weaning was 45 g and the growth rate was similar in all groups, with no effect of SEB or dietary supplementation (Fig. 1B).
FIGURE 1.
Food intake (A) and body weight (B) of Lewis rats fed a control diet (Healthy) and rats challenged with SEB (SEB) and supplemented with SDAP (SEB-SDAP) and with IC (SEB-IC) for 14 d. Results are expressed as means ± SEM, n = 7–10. In most cases, bars were smaller than symbols.
Water content in feces.
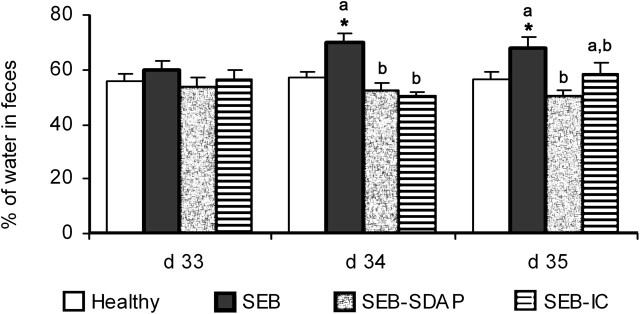
At d 33 (i.e., just before the second SEB dose) there were no differences in the water content in feces between groups (Fig. 2). However, at d 34, there was an increase in the SEB group with respect to Healthy rats (P < 0.05). This increase was prevented by dietary supplements in both the SEB-SDAP (P < 0.002) and SEB-IC (P < 0.001) groups. At d 35, the water content of feces from rats fed the diet containing SDAP was still significantly lower than the SEB group (P < 0.05).
FIGURE 2.
Changes in the water content in feces (expressed as % of wet weight) in rats challenged with a double dose of SEB (50 μg i.p. at d 30 and 33) and fed supplements of plasma (SEB-SDAP) and immunoglobulin concentrate (SEB-IC). Results are expressed as means ± SEM, n = 7–10. *Different from Healthy rats, P < 0.05. Means for rats challenged with SEB without a common letter differ, P < 0.05.
MPO activity.
Groups given SEB had a mucosal MPO activity (3.0 ± 0.41 UMPO/g mucosa) that was significantly higher than in Healthy rats (2.0 ± 0.13 UMPO/g mucosa; P < 0.05). Enzymatic activity of MPO in the groups SEB-SDAP (3.06 ± 0.37 UMPO/g mucosa) and SEB-IC (2.88 ± 0.35 UMPO/g mucosa) did not differ from that observed in the SEB group.
Blood variables.
Hematological values were not modified by SEB treatment or dietary supplementation (data not shown). In Healthy rats, mucosal IgA (17.9 ± 1.15 μg secreted IgA/g), serum IgA (12.8 ± 0.85 mg/L), and serum IgG (975 ± 79 mg IgG/L) were not altered by either SEB treatment or dietary supplementation (data not shown).
Effects of SEB and diets on lymphocyte populations.
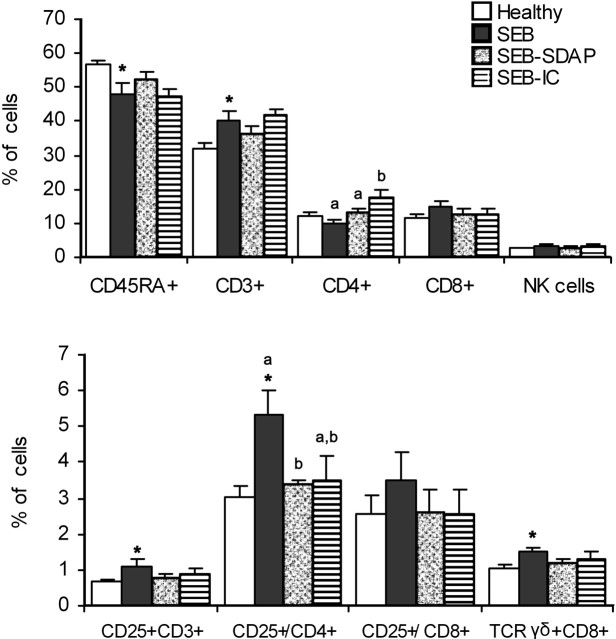
In Peyer's patches (Fig. 3), the SEB group had a higher percentage of T lymphocytes (P < 0.05) and a significantly lower proportion of B lymphocytes compared with Healthy rats (P < 0.05). SDAP and IC supplementation did not significantly modify the percentage of T and B cells in the SEB group. CD4+ T lymphocytes (T helper cells) were 12% of total lymphocytes, and SEB administration did not modify this percentage. For the supplemented groups, the percentage of this population was significantly increased in the group fed the IC-containing diet (P < 0.05), whereas there were no effects in rats fed SDAP. SEB induced a 15% increase (P = 0.093) in CD8+ lymphocytes (i.e., the suppressor/cytotoxic T population) compared with the Healthy group. No effects of diets were observed in the CD8+ population.
FIGURE 3.
Lymphocyte populations in Peyer's patches from Healthy rats and rats challenged with SEB and fed diets supplemented with SDAP or IC. CD45RA+ (as B lymphocytes), CD3+ (as T lymphocytes), CD4+ (as helper T lymphocytes), CD8+ (as suppressor/cytotoxic T lymphocytes), NK (as natural killer cells), CD25+CD3+ (as total activated T lymphocytes), CD25+/CD4+ (as the fraction of activated T helper lymphocytes), CD25+/CD8+ (as the fraction of activated suppressor/cytotoxic T lymphocytes), and γδ-T lymphocytes as percentages with respect to the total number of lymphocytes. Results are expressed as means ± SEM, n = 8–10. *Different from Healthy rats, P < 0.05. Means for rats challenged with SEB without a common letter differ, P < 0.05.
The activated T lymphocyte percentage in Peyer's patches was significantly increased in the SEB group compared with Healthy rats (Fig. 3). In groups fed SDAP or IC supplements, no differences in activated T lymphocytes were observed with respect to the SEB group. SEB administration increased activated CD4+ cells from 3 to 5.4% (P < 0.01), and this increase was prevented by the SDAP-supplemented diet (3.4%; P < 0.05). The IC supplement also tended to prevent (P = 0.080) CD4+ activation (3.5%). Activated cells expressing the CD8+ phenotype did not differ between experimental groups.
In Peyer's patches, the percentage of γδ-T lymphocytes increased after SEB treatment (P < 0.05). Although diets supplemented with SDAP or IC did not modify values obtained in the SEB group, there was a reduction in this population, with a pattern similar to that observed in the populations of total activated and CD4+ activated T cells (Fig. 3B). The NK cell population also tended to increase after SEB treatment (3.44 vs. 2.68% in Healthy rats; P = 0.094), and no effect of diets was observed.
In the MLN, SEB treatment or the SDAP- and IC-containing diets did not modify the proportion of main lymphocyte populations. Percentages of T and B lymphocytes from spleen were not affected by SEB treatment or by the experimental diets (Table 2). Spleen CD4+ and CD8+ cells were not modified by SEB or by diets. The proportion of activated T lymphocytes in MLN was increased by SEB treatment (2.0 ± 0.25% compared with 1.4 ± 0.17% in Healthy rats; P < 0.05) but no effect of the experimental diets was observed (data not shown).
TABLE 2.
Percentages of CD45RA+, CD3+, CD4+ and CD8+ lymphocytes in mesenteric lymph nodes and spleen of Healthy rats 1
| CD45RA+ | CD3+ | CD4+ | CD8+ | |
|---|---|---|---|---|
| MLN | 11.1 ± 0.4 | 81.5 ± 0.8 | 55.8 ± 0.7 | 16.6 ± 0.4 |
| Spleen | 21.5 ± 1.0 | 57.3 ± 2.1 | 52.4 ± 2.2 | 8.1 ± 0.4 |
Values are means ± SEM, n = 7–10.
DISCUSSION
Few studies exist on the response of T cells of the GALT upon exposure to staphylococcal enterotoxin in the rat. The model employed in this study, based on the administration of 2 consecutive i.p. SEB doses, aimed to induce a mild inflammatory state with few repercussions on animal welfare, to avoid interference with the normal food intake pattern. The i.p. route is widely used in models of intestinal inflammation (15,22) because the oral route has the disadvantage that the amount of SEB reaching the mucosa is variable because it can be hydrolyzed by enteric enzymes, thus requiring coadministration of trypsin inhibitors (23). We chose rats at the weaning period because the mucosal immune system is still immature and therefore more susceptible to infection. In our model, food intake and growth rates were unaffected, indicating that basic intestinal and metabolic functions were not impaired. This is remarkable because the period chosen is a time at which rats (and mammals in general) move from liquid to solid food, thus becoming more susceptible to dietary and environmental aggressions (24).
In an intestinal inflammatory state there is mucosal neutrophil infiltration and hence increase mucosal MPO activity. Franco-Penteado et al. (25) observed an increase in MPO activity 4 h after subplantar injection of a single dose of SEB but this effect disappeared after 2 d. However, McKay et al. (26) did not find any increase in MPO activity 4 h or 2 d after i.p. administration of SEB in mouse jejunum. In our study, mucosal MPO was increased 2 d after the second SEB dose, indicating that the effects of the superantigen on neutrophil infiltration are long lasting. The increase in MPO activity was not prevented by the SDAP- or IC-supplemented diets.
Permeability of the intestinal epithelium is regulated by several stimuli, and enhanced paracellular permeability contributes to secretory diarrhea in humans and farm animals (27). Toxins such as SEB stimulate secretion of interferon (IFN)-γ and TNF-α from lymphocytes; these cytokines disassemble tight-junction protein complexes and enhance paracellular permeability of microvascular endothelial cells (28). In the intestine, both effects eventually lead to alteration of the mucosal barrier and induction of intestinal secretion and diarrhea. TNF-α can downregulate Na+/K+-ATPase in mice (29) and change Cl− and K+ transport toward secretion in the human colon (30). Furthermore, IFN-γ is involved in selective downregulation of intestinal Na+ absorption, in part by decreasing expression and activity of the apical membrane Na+ transporters NHE2 and NHE3 (31), which can also contribute to intestinal secretion.
Our study shows that SDAP and IC reduce an inflammatory marker such as diarrhea in SEB-treated rats, which may improve nutrient absorption and electrolyte homeostasis. Other studies performed with plasma supplements showed positive health effects; for example, oral supplementation of calves with bovine serum can ameliorate C. parvum-induced diarrhea (32) and decrease the severity of disease in young calves exposed to coronavirus (33).
SEB administration or diets had no effect on hematological variables, which did not differ from reference values (34), or on serum immunoglobulins, which were similar to other rat strains (35,36). The observation that administration of SEB or dietary supplementation did not modify these systemic variables indicates that SEB actions are restricted mainly to the intestine. Interestingly, IgA levels in intestinal mucosa were not modified by SEB administration. This indicates that local stimulation of the immune system by SEB does not involve B-cell activation but T-cell–dependent enteropathy (15,23).
The distribution of B and T cells and of T helper and T suppressor/cytotoxic populations in Peyer's patches of Healthy rats in our study is in agreement with studies performed in adult rats of several strains (37,38). In Peyer's patches, SEB increases the T lymphocyte population, supporting the view that SEB binds mainly to the TCR (15). When analyzing the activated lymphocytes within the 2 major subpopulations, CD4+ and CD8+, we observed that the main effect of SEB is circumscribed to the T helper cells, in agreement with results of McKay et al. (26) and Noël et al. (39). This increase in T helper activation was prevented by dietary supplementation with SDAP. The γδ T-lymphocyte values for the cytotoxic cells in Peyer's patches from Healthy rats were in the range of values found for CBA/J mice (40). This population, together with the NK cells, increased when rats were treated with the double SEB dose, indicating that SEB also enhances cytotoxic cell populations. This confirms previous observations in mice (41) and is consistent with observations in humans [γδ-T cells, (42); NK cells, (43)]. Dietary supplementation with SDAP brings the percentages of these cytotoxic populations close to the values of Healthy rats. The different effects of SDAP and IC can be due to the different biological activity of their protein composition (SDAP contains all plasma components, whereas IC contains only the Ig fraction). Therefore, although immunoglobulins present in plasma may be the main agents responsible for SDAP activity, other functional proteins, such as transferrin, growth factors, and enzymes, may also contribute to the effects of SDAP.
The phenotypic study in MLN shows that treatment with SEB does not affect the percentages of the main lymphocyte populations. However, the number of activated T lymphocytes is increased, an effect that is not modified by experimental diets. The effect of SEB on activated MLN lymphocytes was also reported after i.g. administration of the superantigen in mice (23). In the spleen, the cell populations were similar to those described for adult Lewis rats (44) and no effects of SEB were observed, supporting the view that SEB affects mainly the physiology of the intestinal epithelium (15). Because the systemic variables measured in the present study were not modified by SEB administration or diet supplementation, we conclude that the major effects of SEB and diets take place at the intestinal level, in agreement with previous observations (12,22).
The primary role of the immune response is the recognition, destruction, and elimination of antigens and pathogens. However, it is also clear that inappropriate immune reactions can result in tissue damage and pathology (26) that may lead to depression of growth. In this study, we showed that dietary SDAP and IC supplementation consistently reduced the percentage of several lymphocyte populations with specific functions in inflammatory states. The diet supplemented with SDAP prevented major activation of CD4+ cells produced by SEB, indicating that rats fed SDAP did not develop the same degree of activation of T helper cells. SDAP also prevented the increase in the γδ-T lymphocytes, which supports the hypothesis that a SDAP-containing diet can play a role in the modulation of the immune response by limiting the immune activation that would compromise the utilization of food energy (12). These systemic effects should be added to the well-known luminal effects of SDAP, preventing the activation of GALT by reducing the probability of bacterial agents and bacterial products reaching the intestinal mucosa (10).
The present study focused on the possible benefits of substituting conventional proteins with proteins prepared from animal plasma in preventing the symptoms of an experimental inflammation induced by the systemic administration of a superantigen. Our results show that SDAP (as well as IC, albeit to a lesser extent) protects GALT from possible activation provoked by S. aureus enterotoxin B. Extrapolation of the results obtained in the laboratory to the situation in farm animals is not straightforward because laboratory rats are not exposed to the same pathogen load that can affect farm animals. However, our results indicate a role for SDAP in limiting immune responses that could eventually compromise the utilization of energy from the diet.
Acknowledgments
We are grateful to M. Canela for expert assistance in statistical analysis. The valuable help of the staff of the Flow Cytometry Service of the Serveis Cientificotècnics of the Universitat de Barcelona is also acknowledged.
Abbreviations
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GALT
gut-associated lymphoid tissue
- IC
immunoglobulin concentrate
- INF
interferon
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- MLN
mesenteric lymph nodes
- MPO
myeloperoxidase
- NK
natural killer cells
- PE
phycoerythrin
- PP
Peyer's patches
- SDAP
spray-dried animal plasma
- SEB
staphylococcal enterotoxin B
- TCR
T-cell receptor
- TNF
tumor necrosis factor
- UMPO
units of myeloperoxidase
Footnotes
Supported by the programs PROFIT (FIT-060000–2000-188) and Eureka Euroagri (E!2452) and by grant 2001SGR0142 (Generalitat de Catalunya, Spain).
LITERATURE CITED
- 1. Torrallardona D., Conde M. R., Badiola I., Polo J. & Brufau J. (2003) Effect of fishmeal replacement with spray-dried animal plasma and colistin on intestinal structure, intestinal microbiology, and performance of weanling pigs challenged with Escherichia coli K99. J. Anim. Sci. 81:1220–1226. [DOI] [PubMed] [Google Scholar]
- 2. Hansen J. A., Nelssen J. L., Goodband R. D. & Weeden T. L. (1993) Evaluation of animal protein supplements in diets of early-weaned pigs. J. Anim. Sci. 71:1853–1862. [DOI] [PubMed] [Google Scholar]
- 3. Coffey R. D. & Cromwell G. L. (1995) The impact of the environment and antimicrobial agents on the growth response of early-weaned pigs to spray-dried porcine plasma. J. Anim. Sci. 73:2532–2539. [DOI] [PubMed] [Google Scholar]
- 4. Van Dijk A. J., Enthoven P. M., Ven den Hoven S. G., Van Laarhoven M. M., Niewold T. A., Nabuurs M. J. & Beynen A. C. (2002) The effect of dietary spray-dried porcine plasma on clinical response in weaned piglets challenged with a pathogenic Escherichia coli. Vet. Microbiol. 84:207–218. [DOI] [PubMed] [Google Scholar]
- 5. Quigley J. D., 3rd & Drew M. D. (2000) Effects of oral antibiotics or bovine plasma on survival, health and growth in dairy calves challenged with Escherichia coli. Food Agric. Immunol. 12:311–318. [Google Scholar]
- 6. Torrallardona D., Conde R., Esteve-García E. & Brufau J. (2002) Use of dried plasma as an alternative to antimicrobial medication in weanling pigs. Anim. Feed Sci. Technol. 99:119–129. [Google Scholar]
- 7. Guarino A., Canani R. B, Russo S., Albano F., Canani M. B., Ruggeri F. M., Donelli G. & Rubino A. (1994) Oral immunoglobulins for treatment of acute rotaviral gastroenteritis. Pediatrics 93:12–16. [PubMed] [Google Scholar]
- 8. Greenberg P. D. & Cello J. P. (1996) Treatment of severe diarrhoea caused by Cryptosporidium parvum with oral bovine immunoglobulin concentrate in patients with AIDS. J. Acquir. Immune Defic. Syndr. 13:348–354. [DOI] [PubMed] [Google Scholar]
- 9. Sanchez R., Kanarek L., Koninkx J., Hendriks H., Lintermans P., Bertels A., Charlier G. & Van Driessche E. (1993) Inhibition of adhesion of enterotoxigenic Escherichia coli cells expressing F17 fimbriae to small intestinal mucus and brush-border membranes of young calves. Microb. Pathog. 15:207–219. [DOI] [PubMed] [Google Scholar]
- 10. Bosi P., Casini L., Finamore A., Cremokolini C., Merialdi G., Trevisi P., Nobili F. & Mengheri E. (2004) Spray-dried plasma improves growth and performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 82:1764–1772. [DOI] [PubMed] [Google Scholar]
- 11. Touchette K. J., Carroll J. A., Allee G. L., Matteri R. L., Dyer C. J., Beausang L. A. & Zannelli M. E. (2002) Effect of spray-dried plasma and lipopolysaccharide exposure on weaned pigs: I. Effects on the immune axis of weaned pigs. J. Anim. Sci. 80:494–501. [DOI] [PubMed] [Google Scholar]
- 12. Demas G. E., Chefer V., Talan M. I. & Nelson R. J. (1997) Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol. 273:R1631–R1637. [DOI] [PubMed] [Google Scholar]
- 13. Focchi C. (1997) Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am. J. Physiol. 273:G769–G775. [DOI] [PubMed] [Google Scholar]
- 14. Frank J. W., Carroll J. A., Allee G. L. & Zannelli M. E. (2003) The effects of thermal environment and spray-dried plasma on the acute-phase response of pigs challenged with lipopolysaccharide. J. Anim. Sci. 81:1166–1176. [DOI] [PubMed] [Google Scholar]
- 15. McKay D. M. (2001) Bacterial superantigens: provocateurs of gut dysfunction and inflammation?. Trends Immunol 22:497–501. [DOI] [PubMed] [Google Scholar]
- 16. Krakauer T. (1999) Immune response to staphylococcal superantigens. Immun. Res. 20:163–173. [DOI] [PubMed] [Google Scholar]
- 17. National Research Council (1995) Nutrient Requirements of Laboratory Animals 4th ed 1995National Academy Press; Washington, DC. [Google Scholar]
- 18. Coffey R. D. & Cromwell G. L. (2001) Use of spray-dried animal plasma in diets for weanling pigs. Pig News Info 22:39N–48N. [Google Scholar]
- 19. Lee Y.-Z., Sim J. S., Al-Mashikhi S. & Nakai S. (1988) Separation of immunoglobulins from bovine blood by polyphosphate precipitation and chromatography. J. Agric. Food Chem. 36:922–928. [Google Scholar]
- 20. Borg B. S., Campbell J. M., Russel L. E., Rodríguez C. & Ródenas J. (2002) Evaluation of the chemical and biological characteristics of spray-dried plasma protein collected from various locations around the world. Am. Assoc. Swine Vet.:97–100.
- 21. Fedorak R. N., Haeberlin B., Empey L. R., Cui N., Nolen H., III, Jewell L. D. & Friend D. R. (1995) Colonic delivery of dexametasone from a prodrug accelerates healing of colitis in rats without adrenal suppression. Gastroenterology 108:1688–1699. [DOI] [PubMed] [Google Scholar]
- 22. Benjamin M. A., Lu J., Donnelly G., Dureja P. & McKay D. M. (1998) Changes in murine jejunal morphology evoked by the bacterial superantigen Staphylococcus aureus Enterotoxin B are mediated by CD4+ T cells. Infect. Immun. 66:2193–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spiekermann G. M. & Nagler-Anderson C. (1998) Oral administration of the bacterial superantigen staphylococcal enterotoxin B induces activation and cytokine production by T cells in murine gut-associated lymphoid tissue. J. Immunol. 161:5825–5831. [PubMed] [Google Scholar]
- 24. Pachá J. (2000) Development of intestinal transport function in mammals. Physiol. Rev. 80:1633–1667. [DOI] [PubMed] [Google Scholar]
- 25. Franco-Penteado C. F., Desouza I., Teixeira S. A., Ribeiro-DaSilva G., De Nucci G. & Antunes E. (2001) Role of nitric oxide on the increased vascular permeability and neutrophil accumulation induced by staphylococcal enterotoxin B into the mouse paw. Biochem. Pharmacol. 61:1305–1311. [DOI] [PubMed] [Google Scholar]
- 26. McKay D. M., Benjamin M. A. & Lu J. (1998) CD4+ T cells mediate superantigen-induced abnormalities in murine jejunal ion transport. Am. J. Physiol. 275:G29–G38. [DOI] [PubMed] [Google Scholar]
- 27. Toivola D. M., Krishnan S., Binder H. J., Singh S. K. & Bishr Omary M. (2004) Keratins modulate colonocyte electrolyte transport via protein mistargeting. J. Cell Biol. 164:911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nusrat A., Turner J. R. & Madara J. L. (2000) Molecular physiology and pathophysiology of tight junctions IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am. J. Physiol. 279:G851–G857. [DOI] [PubMed] [Google Scholar]
- 29. Musch M. W., Clarke L. L., Mamah D., Gawenis L. R., Zhang Z., Ellsworth W., Shalowitz D., Mittal N. & Efthimiou P., et al. (2002) T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J. Clin. Investig. 110:1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmitz H., Fromm M., Bode H., Scholz P., Riecken E. O. & Schulzke J. D. (1996) Tumor necrosis factor-α induces Cl− and K+ secretion in human distal colon driven by prostaglandin E2. Am. J. Physiol. 271:G669–G674. [DOI] [PubMed] [Google Scholar]
- 31. Rocha F., Musch M. W., Lishanskiy L., Bookstein C., Sugi K., Xie Y. & Chang E. B. (2001) IFNγ downregulates expression of Na+/H+ exchangers NHE2 and NHE3 in rat intestine and human Caco-2/BBe cells. Am. J. Physiol. 280:C1224–C1232. [DOI] [PubMed] [Google Scholar]
- 32. Hunt E., Fu Q., Armstrong M. U., Rennix D. K., Webster D. W., Galanko J. A., Chen W., Weaver E. M. & Argenzio R. A. et al. (2002) Oral bovine serum concentrate improves cryptosporidial enteritis in calves. Pediatr. Res. 51:370–376. [DOI] [PubMed] [Google Scholar]
- 33. Arthington J. D., Jaynes C. A., Tyler H. D., Kapil S. & Quigley J. D. (2002) The use of bovine serum protein as an oral support therapy following coronavirus challenge in calves. J. Dairy Sci. 85:1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ringler D. H. & Dabich L. (1979) Haematology and clinical biochemistry. Baker H. J., Lindsey J. R., Weisbroth S. H. eds. The Laboratory Rat I:105–122 Academic Press New York, NY. . [Google Scholar]
- 35. Elphick G. F., Greenwood B. N., Campisi J. & Fleshner M. (2003) Increased serum nIgM in voluntary physically active rats: a potential role for B-1 cells. J. Appl. Physiol. 94:660–667. [DOI] [PubMed] [Google Scholar]
- 36. Herías M. V., Hessle C., Telemo E., Midtvedt T., Hanson L. A. & Wold A. E. (1999) Immunomodulatory effects of Lactobacillus plantarum colonizing the intestine of gnotobiotic rats. Clin. Exp. Immunol. 116:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lyscom N. & Brueton M. J. (1982) Intraepithelial, lamina propria and Peyer's patch lymphocytes of the rat small intestine: isolation and characterization in terms of immunoglobulin markers and receptors for monoclonal antibodies. Immunology 45:775–783. [PMC free article] [PubMed] [Google Scholar]
- 38. Kroese F.G.M. (1998) Immunology of the rat. Pastoret P. P., Griebel P., Bazin H., Govaerts A. eds. Handbook of Vertebrate Immunology 1998Academic Press; San Diego, CA. [Google Scholar]
- 39. Nöel C., Florquin S., Goldman M. & Braun M. Y. (2001) Chronic exposure to superantigen induces regulatory CD4+ T cells with IL-10-mediated suppressive activity. Int. Immunol. 13:431–439. [DOI] [PubMed] [Google Scholar]
- 40. Gryglewski A., Szczepanik M., Majcher P., Popiela T. & Ptak W. (1997) Different patterns of γδ and αβ T cell redistribution in the mouse after a partial gastrectomy. J. Surg. Res. 73:137–142. [DOI] [PubMed] [Google Scholar]
- 41. Okamoto S., Kawabata S., Nakagawa I. & Hamada S. (2001) Administration of superantigens protects mice from lethal Listeria monocytogenes infection by enhancing cytotoxic T cells. Infect. Immun. 69:6633–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rust C.J.J. & Koning F. (1993) γδ T cell reactivity towards bacterial superantigens. Semin. Immunol. 5:41 (abs.). [DOI] [PubMed] [Google Scholar]
- 43. D'Orazzio J. A., Burke G. W. & Stein-Streilein J. (1995) Staphylococcal enterotoxin B activates purified NK cells to secrete IFN-γ but requires T lymphocytes to augment NK cytotoxicity. J. Immunol. 154:1014–1023. [PubMed] [Google Scholar]
- 44. Yaqoob P., Newsholme E. A. & Calder P. C. (1994) The effect of dietary lipid manipulation on rat lymphocyte subsets and proliferation. Immunology 82:603–610. [PMC free article] [PubMed] [Google Scholar]