Key Points
Children frequently had multiple respiratory viruses detected. Although common, children with multiple viruses more frequently had cough and rhinorrhea. Children with influenza and respiratory syncytial virus were hospitalized most frequently. Routine screening and cohorting are recommended only for those with common respiratory pathogens.
Keywords: child, codetection, respiratory infection, viral infection
Abstract
Background
Children with acute respiratory tract infection (ARTI) frequently exhibit virus-virus codetection, yet the clinical significance of ARTI remains contentious. Using data from a prospective cohort of children with influenza-like illness, we examined the virology of ARTI and determined the clinical impact of virus-virus codetection.
Methods
Children aged 6 to 59 months who presented to a tertiary pediatric hospital between influenza seasons 2008 and 2012 with fever and acute respiratory symptoms were enrolled, and nasal samples were collected. Respiratory viruses were identified by culture and polymerase chain reaction. We compared demographics, presenting symptoms, and clinical outcomes of children with a single-virus infection and those in whom 2 or more viruses were detected (virus-virus codetection). We used logistic regression models and estimated marginal means to calculate the adjusted odds ratios and probabilities of symptom presentation, prescription of antibiotics, and hospitalization.
Results
Of 2356 children, a virus was detected in 1630 (69.2%) of them; rhinovirus (40.8%), influenza (29.5%), and respiratory syncytial virus (26.4%) were detected most commonly. Two or more viruses were detected in 25% of these children. After we adjusted for demographic factors, children with virus-virus codetection had greater odds of presenting with cough (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.2–3.1) and rhinorrhea (aOR, 1.8; 95% CI, 1.1–2.9) than those with a single-virus infection, although both symptoms were common. Children with influenza and respiratory syncytial virus combined had the highest probability of hospitalization (55%; 95% CI, 35%–73%), which was significantly greater than for those with influenza infection alone (22%; 95% CI, 16%–29%).
Conclusions
Overall, virus-virus codetection has limited impact on clinical severity among children with influenza-like illness. However, infection with specific pathogen pairs might be associated with more severe outcomes. Routine diagnostics to identify specific viruses should be restricted to common pathogens.
INTRODUCTION
Acute respiratory tract infections (ARTIs) in children place a significant burden on families and the community. Commonly recognized viral pathogens that cause ARTI include influenza viruses, respiratory syncytial viruses (RSVs), parainfluenza viruses, human rhinoviruses, adenoviruses, and coronaviruses [1, 2]. Advances in laboratory diagnostic techniques have resulted in the discovery of new viruses, including human metapneumovirus (hMPV) and polyomaviruses [3, 4], yet a number of these pathogens have an uncertain pathogenicity [5, 6].
Codetection can be defined as the detection of 2 or more pathogens in a single sample. With the improved sensitivity, availability, and affordability of modern diagnostics, virus-virus codetections are increasingly being found. The incidence of virus-virus codetection has been reported to be between 15% and 45%, depending on age, location, and testing methods [7–9]. The clinical significance of codetection in patients with ARTI remains contentious; the literature has described negligible to deleterious effects [9, 10].
With this study, we describe the virology of ARTI in children aged 6 months to 4 years who presented to a tertiary pediatric hospital in Australia with influenza-like illness during influenza season. This study also enabled us to specifically examine the impact of virus-virus codetection on clinical symptoms and outcomes.
MATERIALS AND METHODS
Study Setting and Patients
Western Australia (WA) spans 2.5 million km2 and has a population of approximately 2.5 million people, 7% of whom are younger than 5 years [11]. Princess Margaret Hospital for Children (PMH) is the only tertiary pediatric hospital in the state and is located in metropolitan Perth, where approximately 80% of the population resides [12].
Commencing in 2008, the Western Australia Influenza Vaccine Effectiveness (WAIVE) study was an observational cohort study established to determine the effectiveness of inactivated influenza vaccine. Patient recruitment was conducted at PMH (and at selected general practices in metropolitan WA in 2008–2009). Because of the small numbers recruited and differences in presentations, data from children who presented to the general practices were removed from our analyses.
Patient recruitment coincided with the annual influenza seasons. The start and end of the influenza seasons were defined by the Infectious Diseases Surveillance Unit at PathWest Laboratory Medicine WA by using a combination of indicators, including the weekly proportion of positive laboratory influenza test results. As a guide, 2 consecutive weeks with more than 10% positive influenza test results often coincides with the beginning of influenza season in WA. Additional details on study design are described elsewhere [13].
All children 6 to 59 months of age who presented to PMH with a history of fever (according to parental report) or with a measured temperature of greater than 37.5°C at presentation and with at least 1 acute respiratory symptom within the previous 96 hours were eligible for enrollment. All children transited through the PMH emergency department. A proportion of these children were subsequently admitted to the hospital, and the remainder of them were discharged home from the emergency department. Children with a known immunodeficiency disorder, with current or recent immunosuppressive treatment, or who received immunoglobulin in the previous 3 months were excluded from the study.
Patient demographics, medical history, and presenting symptoms were collected through a parental questionnaire. Comorbidities recorded included prematurity, asthma, and chronic cardiac, neurological, and respiratory conditions. Influenza vaccination status was determined by parental report and confirmed through the Australian Childhood Immunisation Register or by contacting immunization providers. Vaccination status for other vaccines was not collected. A follow-up questionnaire regarding illness outcomes, including details of hospital admission(s), use of antibiotics, and time to recovery, was given to families to complete within 7 to 10 days after enrollment. A retrospective review of medical records was undertaken when hospitalization data were recorded incorrectly or missing. No follow-up was conducted for antibiotic use if data were missing.
Respiratory Virus Detection
Samples were collected from the children at enrollment with midturbinate nasal swabs (Copan Diagnostics, Inc., Murrieta, California). If a nasopharyngeal aspirate had already been collected by hospital staff as part of clinical care, that sample was used in lieu of a nasal swab. Viral culture (Madin-Darby canine kidney cells, diploid lung fibroblasts) and multiplex tandem polymerase chain reaction (PCR) were used to detect all viruses except picornaviruses and hMPV [14, 15]. Picornaviruses were detected by using nested PCR [16] targeting the 5′ untranslated region of the picornavirus genome, and sequencing was used to assist with the identification of rhinoviruses and enteroviruses. hMPV was tested by using an immunofluorescent assay (SimulFluor hMPV immunofluorescent assay [Millipore, Temecula, California]) and PCR. All patients were subjected to the same panel of tests, and testing methods were consistent throughout the study period with the exception of testing for hMPV, which was based on clinical need. Although both immunofluorescence and PCR assays were used throughout the study period, PCR testing was more common in later years.
For all viruses (except hMPV), positive viral detection was defined as detection by viral culture and/or PCR. Positive detection of hMPV was defined as detection by immunofluorescence and/or PCR. All influenza types/subtypes (i.e., influenza A/H1N1, A/H3N2, and B) were grouped for analysis. Similarly, subgroups of parainfluenza viruses (i.e., parainfluenza types 1–4) were grouped together for analysis. Infection was defined as the detection of 1 or more viruses (i.e., rhinovirus, influenza, RSV, parainfluenza, adenovirus, coronavirus, and/or hMPV). Codetection was defined as detection of 2 or more viruses in a single diagnostic sample.
Definitions and Statistical Analysis
Prematurity was defined as less than 37 weeks of gestation at birth. Out-of-home care was defined as attendance at a playgroup, mothers’ group, day care center, kindergarten, or preschool. Hospital length of stay refers to the duration from admission to discharge. Symptoms investigated included cough, rhinorrhea, wheeze, dyspnea, rash, diarrhea, and vomiting, and the outcomes investigated were antibiotic prescription and hospital admission.
Data cleaning and analyses were performed in Microsoft Excel, EpiBasic [17], and SPSS version 23 (SPSS, Inc., Chicago, Illinois). Categorical variables were compared by using Pearson's χ2 tests. Logistic regression models were used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) to compare those with single infection to those with virus-virus codetection. Dependent variables were symptoms (e.g., presence of cough or rhinorrhea) and outcome variables (e.g., hospitalization or use of antibiotics).
We calculated adjusted ORs (aORs) by including the following covariates in the logistic regression models: age, sex, Aboriginal status, prematurity, presence of comorbidities, out-of-home care, and household smoking. Age was included as a categorical variable in the models (6–11 months, 12–23 months, 2 years, 3 years, and 4 years [reference group]). Covariates were selected on the basis of known epidemiological or clinical risk factors for codetection. Data from all patients were included in the adjusted models unless they had data on 1 or more covariates missing. To investigate the impact of specific pathogen pairs, analyses were repeated for the most common pathogen pairs. Estimated marginal means of logistic regression models were used to calculate probabilities with 95% CIs for antibiotic prescribing and hospitalization for those infected with common pathogen pairs.
Ethical Approvals
This study was approved by the PMH Human Research Ethics Committee (approval 1673/EP), the Western Australian Aboriginal Health Ethics Committee (approval 212 06/08), and the University of Western Australia Research Ethics Committee (approval RA/4/1/6456).
RESULTS
Of 2715 patients recruited from 2008 to 2012, data for 2356 patients were available for analysis. Reasons for exclusion included incorrect or unknown age (n = 154 [42.9% of all excluded patients]), recruitment from general practice in 2008–2009 (n = 131 [36.5%]), incomplete pathogen testing (n = 29 [8.1%]), unknown vaccination history (n = 7 [1.9%]), incomplete data (n = 12 [3.3%]), multiple enrollments for the same episode of illness (n = 3 [0.8%]), and withdrawal from the study (n = 23 [6.4%]).
Of the 2356 patients enrolled, the majority of them (n = 1848 [78.4%]) were enrolled when they presented to the PMH emergency department. Of these patients, 6.3% (n = 117) were subsequently admitted to the hospital. The median age was 22.0 months (interquartile range, 14.0–35.0), 54.9% were male, and 5.7% were of Aboriginal or Torres Strait Islander decent. Children born preterm accounted for 13.5% (n = 319) of the patients. Children with comorbidities accounted for 15.1% (n = 355) of this cohort. Of those who had 1 or more comorbidity, asthma (n = 218 [61.4%]) and other chronic respiratory conditions (n = 54 [15.2%]) were the most common.
Of 2356 patients, questions relating to outcomes (e.g., antibiotics use) were completed for 52.8% (n = 1244). Although parents were requested to complete these questions 7 to 10 days after enrollment, the mean time to completion was 19.3 days (range, 0–149 days; median, 10 days). Data on antibiotic prescription after enrollment were available for 51.0% (n = 1201) of the patients, 483 (40.2%) of whom were prescribed antibiotics. Combining data from the questionnaires and a review of the hospital records resulted in near-complete data on hospitalization (99.4% [n = 2341]); 610 (26.1%) were hospitalized. Of those who were admitted to the hospital, the median length of stay was 2 days (interquartile range, 1–3 days).
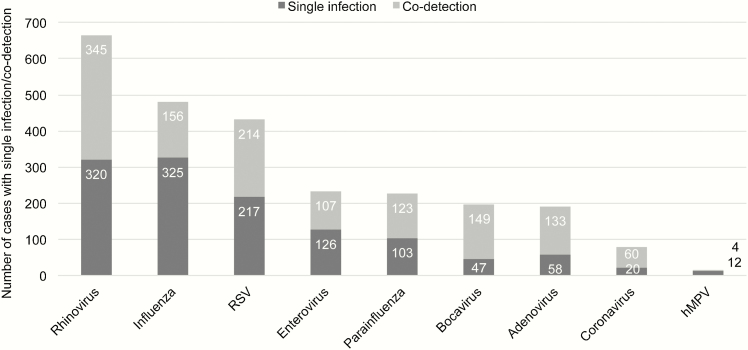
Overall, 1630 (69.2%) patients tested positive for a virus. In those with at least 1 virus detected, the most common were rhinovirus (n = 665 [40.8%]), influenza (n = 481 [29.5%]), and RSV (n = 431 [26.4%]) (Figure 1). Of those with a virus detected, 24.8% (n = 404) had at least 1 other virus codetected, and of them, 350 (86.6%) had 2 viruses detected, 52 (12.9%) had 3 viruses detected, and the remainder had 4 or more viruses detected.
Figure 1.
Frequency of pathogen detection and codetection. Detections of enterovirus and bocavirus were excluded from subsequent analyses. Abbreviations: RSV, respiratory syncytial viruses; hMPV, human metapneumovirus.
A greater proportion of children with multiple viruses detected were younger than 2 years than those with a single-virus infection (65.4% vs 51.2%, respectively; p < .001; Table 1). Those with codetection also had greater odds of presenting with cough and rhinorrhea than those with a single-virus infection, although both symptoms were common in both groups (Table 2). This effect remained after adjusting for other covariates. It should be noted that, although less common, diarrhea was observed more frequently in children with viral codetection. There were no significant differences between patients with a single-virus infection and those with virus-virus codetection in the odds of being prescribed antibiotics (aOR, 1.1; 95% CI, 0.8–1.5) or being hospitalized (aOR, 1.1; 95% CI, 0.8–1.4) (Table 2).
Table 1.
Cohort Characteristics According to Infection Statusa
| Characteristic | Frequency (n = 2356) | |||||
|---|---|---|---|---|---|---|
| No Pathogen (n = 726) | Single-Virus Infection (n = 1226) | Virus-Virus Codetection (n = 404) | ||||
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | |
| Aged <2 y | 382 (52.62) | 48.91–56.30 | 628 (51.22) | 48.38–54.06 | 264 (65.35) | 60.48–69.98 |
| Male sex | 397 (54.68) | 50.98–58.35 | 668 (54.49) | 51.65–57.30 | 228 (56.44) | 51.44–61.33 |
| Aboriginal or Torres Strait Islander descent | 33 (4.55) | 3.15–6.32 | 71 (5.79) | 4.55–7.25 | 31 (7.67) | 5.27–10.71 |
| Preterm birth | 102 (14.05) | 11.60–16.79 | 158 (12.89) | 11.06–14.89 | 59 (14.60) | 11.31–18.43 |
| ≥1 comorbidity | 117 (16.12) | 13.51–19.00 | 183 (14.93) | 12.98–17.05 | 55 (13.61) | 10.42–17.35 |
| >4 h in out-of-home care | 442 (60.88) | 57.22–64.45 | 825 (67.29) | 64.59–69.91 | 299 (74.01) | 69.44–78.22 |
| Smoking in household | 154 (21.21) | 18.29–24.37 | 283 (23.08) | 20.75–25.55 | 107 (26.49) | 22.24–31.07 |
| Influenza vaccine on year of admission | 188 (25.90) | 22.74–29.24 | 303 (24.71) | 22.32–27.23 | 100 (24.75) | 20.62–29.26 |
Abbreviation: CI, confidence interval.
aExact 95% CIs are presented. Denominators include cases with missing data. Detections of enterovirus or bocavirus were ignored in counts of single-virus and virus-virus codetection.
Table 2.
Frequency and Logistic Regression Models of Symptoms and Outcomes According to Infection Type
| Symptom or Outcome | Frequency (% [95% CI]) | Logistic Regression Models, Virus-Virus Codetection | ||
|---|---|---|---|---|
| Single-Virus Infection (n = 1226) | Virus-Virus Codetection (n = 404) | OR (95% CI) | aOR (95% CI)a | |
| Symptoms | ||||
| Cough | 88.66 (86.75–90.38) | 93.32 (90.43–95.55) | 1.95 (1.24–3.06) | 1.94 (1.21–3.13) |
| Rhinorrhea | 88.09 (86.15–89.85) | 93.32 (90.43–95.55) | 2.07 (1.32–3.23) | 1.79 (1.12–2.85) |
| Wheezing | 43.56 (40.76–46.39) | 49.01 (44.03–54.00) | 1.26 (1.01–1.58) | 1.20 (0.94–1.52) |
| Dyspnea | 45.84 (43.02–48.68) | 50.74 (45.75–55.72) | 1.23 (0.98–1.55) | 1.15 (0.91–1.47) |
| Rash | 17.86 (15.76–20.12) | 14.11 (10.86–17.89) | 0.75 (0.55–1.03) | 0.69 (0.49–0.95) |
| Diarrhea | 20.39 (18.17–22.76) | 27.23 (22.94–31.85) | 1.47 (1.13–1.90) | 1.33 (1.01–1.74) |
| Vomiting | 38.58 (35.85–41.37) | 42.82 (37.94–47.81) | 1.19 (0.94–1.50) | 1.16 (0.91–1.48) |
| Outcomes | ||||
| Antibiotics givenc | 19.98 (17.78–22.33) | 21.53 (17.62–25.87) | 1.19 (0.86–1.63) | 1.11 (0.79–1.54) |
| Admitted to hospital | 24.55 (22.16–27.06) | 26.24 (22.01–30.82) | 1.13 (0.87–1.46) | 1.09 (0.83–1.44) |
Denominators include those with missing data. The missing data (for single-virus infections and virus-virus codetections) for the children were cough (n = 4 and 3), rhinorrhea (n = 4 and 3), wheezing (n = 4 and 4), dyspnea (n = 5 and 4), rash (n = 30 and 9), diarrhea (n = 30 and 10), vomiting (n = 32 and 9), antibiotics given (n = 587 and 199), and admission to hospital (n = 6 and 2). Infections with either enterovirus or bocavirus were ignored in counts of single-virus infection and virus-virus codetection.
Abbreviations: CI, confidence interval; OR, odds ratio.
aModels presented are the odds of having a symptom/outcome in children with virus-virus codetection compared with children with single-virus infection.
bModels were adjusted for age, sex, Aboriginal status, preterm birth, presence of comorbidities, out-of-home care, and household smoking. All covariates listed were input as categorical variables.
cData were available for only 639 children with single-virus infection and 205 children with virus-virus codetection.
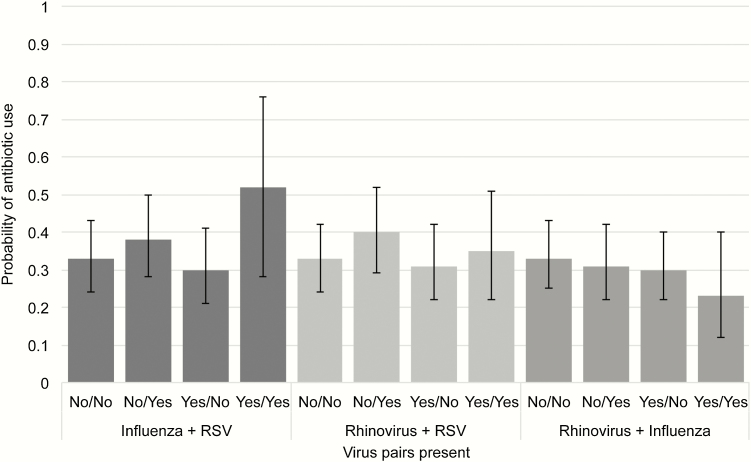
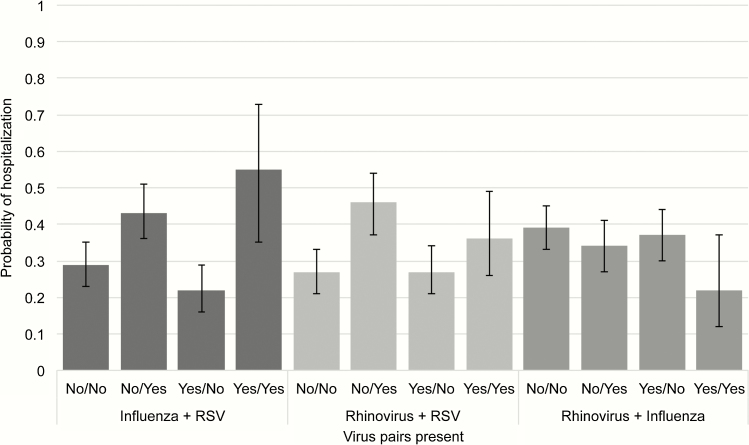
We then selected the 3 most common pathogens (rhinovirus, influenza, and RSV) and investigated associations of infection with specific pathogen pairs with antibiotic prescriptions and hospitalization. After adjusting for other covariates, patients with both influenza and RSV detected had a 52% probability (95% CI, 28%–76%) of being prescribed antibiotics, and there was a trend toward more frequent prescription than those with influenza or RSV infection alone (Figure 2). Similarly, the probability of being hospitalized was highest in those with influenza and RSV detected (probability, 55%; 95% CI, 35%–73%) and was significantly greater when compared with those with influenza infection alone (probability, 22%; 95% CI, 16%–29%) (Figure 3) and with a trend towards increased hospitalization observed compared with RSV infection alone (probability, 43%; 95% CI, 36%–51%).
Figure 2.
Probability (95% confidence intervals) of postenrollment antibiotic use according to pathogen pairs. Abbreviation: RSV, respiratory syncytial viruses.
Figure 3.
Probability (95% confidence intervals) of hospitalization according to pathogen pairs. Abbreviation: RSV, respiratory syncytial viruses.
DISCUSSION
This is one of the largest single-site prospective studies of children up to 4 years of age that specifically investigated the incidence of and clinical outcomes associated with virus-virus codetection. Our findings indicate that although differences in demographics, risk factors, and symptoms are identifiable, in general, virus-virus codetection is unlikely to be associated with more severe clinical illness among young children with influenza-like illness. Infection with specific pathogen pairs might be associated with an increased probability of hospitalization, as was observed with influenza and RSV. This finding has implications in pediatric healthcare facilities, where the isolation of all children with acute respiratory viral infection is difficult during periods of peak respiratory virus activity and cohorting of children is frequently required before the availability of diagnostic test results.
We detected small differences between the symptoms presented by patients with a single-virus infection and those presented by patients with virus-virus codetection. However, these symptoms were common and therefore likely to be of little clinical relevance. In contrast, the clinical outcomes chosen (i.e., antibiotic use and hospitalization) were more indicative of disease severity, but they are subject to clinical judgement and therefore can be less sensitive measures of disease severity. Accordingly, we observed no significant differences in the outcomes of children with single-virus infection and those with virus-virus codetection.
These results are consistent with data from previous systematic reviews, which found negligible differences between outcomes in children and adults with virus-virus codetection compared to peers with single-virus infection [18, 19]. However, the results of additional analyses of pathogen pairs suggest that some combinations of specific viral pathogens, such as influenza and RSV, are potentially more significant than others. This result corroborates data from our recently completed systematic review in which we specifically investigated clinical outcomes in children with codetection; we found no differences overall, but the results suggest that some pathogen-specific effects might be present [20]. Our data suggest that future research in this area should segregate analysis according to specific pathogen pairs when the numbers allow.
We chose to exclude bocavirus and enterovirus detections from the analyses because their pathogenicity in ARTI is still not well established. Bocavirus is often implicated in both symptomatic and asymptomatic codetection and is thought to have a prolonged period of shedding [6]; both of these features might confound any associations between codetection and clinical severity. In contrast, the results of studies on the role of enteroviruses in ARTI are suggestive of pathogenicity [21]; however, the numbers in those studies were small. For these reasons, detections of both viruses were excluded from the analyses presented here. Repeat analyses including these viruses did not change the overall findings (Supplementary Tables 1 and 2).
An important consideration when interpreting these findings is that active (and pathogenic) infection and viral shedding cannot be distinguished. Prolonged viral shedding for some respiratory viruses, particularly rhinovirus, has been well documented [22, 23]. Quantitative analysis might be of assistance in distinguishing these clinical states but has not yet become commonplace in the diagnostic laboratory for respiratory viruses.
One limitation of our study is that only children who presented to 1 hospital with influenza-like illness and fever were eligible for enrollment. As a consequence, it is possible that these children were at the more severe end of the disease spectrum, which might have biased our results. During the course of this study, there was a shift from using an antigen-based assay to using PCR for detecting hMPV, although both methods were used throughout the study period. We elected to include detections from both methods but acknowledge that differences in the performance of these methods would mean that potential cases of hMPV might have been missed in earlier samples. These changes, and clinical discretion in testing for hMPV, may explain the proportion of hMPV detections in this cohort, which was lower than that in other studies [24, 25].
Additional limitations of this study include missing outcomes data, particularly for antibiotic prescription. In addition, data on diagnosis at discharge were not collected, which might have helped to indicate the severity of symptoms. Moreover, despite enrolling nearly 2500 children, the number of patients infected with specific pathogens and pathogen pairs was relatively small.
Future studies using routinely collected, linked administrative data might assist in addressing both issues. Nonetheless, ours was one of the largest single-site studies to specifically investigate the effects of virus-virus codetection in young children by using a wide panel of tests for respiratory pathogens. Our results are similar to those reported elsewhere, which adds to the validity of the findings [26].
We conclude that the impact of virus-virus codetection on disease severity in children who present with influenza-like illness is likely to be limited to those infected with specific pathogen pairs. Therefore, routine screening for virus-virus codetection in this population should be restricted to those with common respiratory pathogens, and efforts to reduce cross infection should focus on these specific pathogens.
Supplementary Data
Supplementary materials are available at Journal of the Pediatric Infectious Diseases Society online.
Supplementary Material
Notes
Author contributions. C. C. B., P. C. R., P. V. E., and D. W. S. conducted the WAIVE study; P. C. R., D. W. S., and C. C. B. conceptualized this study; A. L. and S. T. conducted the laboratory work; Z. V. W. and N. T. C. conducted the preliminary data cleaning and analyses; F. J. L. conducted data cleaning and analyses with assistance from H. C. M., N. d. K., and C. C. B.; and F. J. L. and Z. V. W. jointly wrote the first draft of the manuscript. All authors critically revised and approved of the final version of the manuscript.
Acknowledgments. We acknowledge Peter Jacoby for his assistance with the logistic regression analyses and Gabriela Willis for her assistance with cross-checking the data, and we thank members of the Vaccine Trials Group, particularly Christine Robins, who managed the study. We also thank all the parents and children who participated in the WAIVE study.
Financial support. This work was supported by the Western Australia Department of Health, the University of Western Australia (University Postgraduate Award to F. J. L.), the National Health and Medical Research Council (grants APP1045668 [to H. C. M. and F. J. L.], APP1034254 [to H. C. M.], and APP1111596 [to C. C. B.]), and the Western Australian Department of Health/Raine Medical Research Foundation (clinician research fellowship to C. C. B.). No external funding was provided for other authors.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mahony JB. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev 2008; 21 : 716–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monto AS. Epidemiology of viral respiratory infections. Am J Med 2002; 112Suppl 6A : 4S–12. [DOI] [PubMed] [Google Scholar]
- 3. Debiaggi M, Canducci F, Ceresola ER, Clementi M. The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Virol J 2012; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bialasiewicz S, Whiley DM, Lambert SB et al. Presence of the newly discovered human polyomaviruses KI and WU in Australian patients with acute respiratory tract infection. J Clin Virol 2008; 41 : 63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linder JE, Kraft DC, Mohamed Y et al. Human rhinovirus C: age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol 2013; 131 : 69–77. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Söderlund-Venermo M. Human bocavirus—the first 5 years. Rev Med Virol 2012; 22 : 46–64. [DOI] [PubMed] [Google Scholar]
- 7. Aberle JH, Aberle SW, Pracher E, Hutter H-P, Kundi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-[gamma] response. Pediatr Infect Dis J 2005; 24 : 605–10. [DOI] [PubMed] [Google Scholar]
- 8. Peng D, Zhao D, Liu J et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J 2009; 6 : 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kouni S, Karakitsos P, Chranioti A, Theodoridou M, Chrousos G, Michos A. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin Microbiol Infect 2013; 19 : 772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses 2012; 6 : 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Codde J. Rates Calculator, version 9.5.5. Perth, Western Australia: Health Information Centre, Department of Health; 2013. [Google Scholar]
- 12. Australian Bureau of Statistics. Regional population growth, Australia, 2012–13. Available at: http://www.abs.gov.au/ausstats/abs@.nsf/Previousproducts/3218.0Feature Article22012-13?opendocument&tabname=Summary&prodno=3218.0&issue=2012-13&num=&view=. Accessed November 13, 2015. [Google Scholar]
- 13. Blyth CC, Jacoby P, Effler PV et al. Effectiveness of trivalent flu vaccine in healthy young children. Pediatrics 2014; 133 : e1218–25. [DOI] [PubMed] [Google Scholar]
- 14. Chidlow GR, Harnett G, Williams S, Levy A, Speers D, Smith DW. Duplex real-time reverse transcriptase PCR assays for rapid detection and identification of pandemic (H1N1) 2009 and seasonal influenza A/H1, A/H3, and B viruses. J Clin Microbiol 2010; 48 : 862–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chidlow GR, Harnett GB, Shellam GR, Smith DW. An economical tandem multiplex real-time PCR technique for the detection of a comprehensive range of respiratory pathogens. Viruses 2009; 1 : 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ireland DC, Kent J, Nicholson KG. Improved detection of rhinoviruses in nasal and throat swabs by seminested RT-PCR. J Med Virol 1993; 40 : 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juul S, Frydenberg M. EpiBasic version 2.0.Aarhus, Denmark: Aarhus University; 2011. [Google Scholar]
- 18. Asner SA, Science ME, Tran D, Smieja M, Merglen A, Mertz D. Clinical disease severity of respiratory viral co-infection versus single viral infection: a systematic review and meta-analysis. PLoS One 2014; 9 : e99392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goka EA, Vallely PJ, Mutton KJ, Klapper PE. Single and multiple respiratory virus infections and severity of respiratory disease: a systematic review. Paediatr Respir Rev 2014; 15 : 363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lim FJ, de Klerk N, Blyth CC, Fathima P, Moore HC. Systematic review and meta-analysis of respiratory viral coinfections in children. Respirology 2016; 21 : 648–55. [DOI] [PubMed] [Google Scholar]
- 21. Imamura T, Oshitani H. Global reemergence of enterovirus D68 as an important pathogen for acute respiratory infections. Rev Med Virol 2015; 25 : 102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loeffelholz MJ, Trujillo R, Pyles RB et al. Duration of rhinovirus shedding in the upper respiratory tract in the first year of life. Pediatrics 2014; 134 : 1144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol 2004; 72 : 695–9. [DOI] [PubMed] [Google Scholar]
- 24. Fathima S, Lee B, May-Hadford J, Mukhi S, Drews S. Use of an innovative web-based laboratory surveillance platform to analyze mixed infections between human metapneumovirus (hMPV) and other respiratory viruses circulating in Alberta (AB), Canada (2009–2012). Viruses 2012; 4 : 2754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ali SA, Williams JV, Chen Q et al. Human metapneumovirus in hospitalized children in Amman, Jordan. J Med Virol 2010; 82 : 1012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dierig A, Heron LG, Lambert SB et al. Epidemiology of respiratory viral infections in children enrolled in a study of influenza vaccine effectiveness. Influenza Other Respir Viruses 2014; 8 : 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.