Abstract
Background
Infection control measures have played a major role in limiting human/camel-to-human transmission of Middle East respiratory syndrome coronavirus (MERS-CoV); however, development of effective and safe human or camel vaccines is warranted.
Methods
We extended and optimized our previous recombinant adenovirus 5 (rAd5)–based vaccine platform characterized by in vivo amplified and CD40-mediated specific responses to generate MERS-CoV S1 subunit-based vaccine. We generated rAd5 constructs expressing CD40-targeted S1 fusion protein (rAd5-S1/F/CD40L), untargeted S1 (rAd5-S1), and Green Fluorescent Protein (rAd5-GFP), and evaluated their efficacy and safety in human dipeptidyl peptidase 4 transgenic (hDPP4 Tg+) mice.
Results
Immunization of hDPP4 Tg+ mice with a single dose of rAd5-S1/F/CD40L elicited as robust and significant specific immunoglobulin G and neutralizing antibodies as those induced with 2 doses of rAd5-S1. After MERS-CoV challenge, both vaccines conferred complete protection against morbidity and mortality, as evidenced by significantly undetectable/reduced pulmonary viral loads compared to the control group. However, rAd5-S1– but not rAd5-S1/F/CD40L–immunized mice exhibited marked pulmonary perivascular hemorrhage post–MERS-CoV challenge despite the observed protection.
Conclusions
Incorporation of CD40L into rAd5-based MERS-CoV S1 vaccine targeting molecule and molecular adjuvants not only enhances immunogenicity and efficacy but also prevents inadvertent pulmonary pathology after viral challenge, thereby offering a promising strategy to enhance safety and potency of vaccines.
Keywords: MERS-CoV, vaccine, immunopathology, CD40L, adenovirus
In this study, we describe a potent and safe recombinant adenovirus 5–based Middle East respiratory syndrome coronavirus (MERS-CoV) vaccine expressing MERS-CoV S1 as an antigen and incorporating CD40L as a targeting ligand and molecular adjuvant. The vaccine protected transgenic mice against lethal challenge without vaccine-associated immunopathology.
The Middle East respiratory syndrome coronavirus (MERS-CoV) is a novel zoonotic virus that was first isolated from a fatal human case in Saudi Arabia in 2012 [1]. The virus can cause severe acute and fatal respiratory symptoms associated with systemic infection and occasional multiorgan failure [1]. As of October 2018, MERS-CoV has caused >2250 laboratory-confirmed infections with an approximately 35% mortality rate in 27 countries, with Saudi Arabia being the most affected country [2]. Most global cases are linked to the Arabian Peninsula, where MERS-CoV continues to be endemic for >6 years with the potential to spread globally, as seen in the 2015 outbreak in South Korea [3].
The spike (S) glycoprotein of MERS-CoV (1353 aa) is a type I trimeric membrane protein expressed on the virus surface, and binds to dipeptidyl peptidase 4 (DPP4) on target cells [4]. It is comprised of an N-terminal globular S1 subunit (aa 1–751) containing the receptor-binding domain (RBD) and a membrane-proximal S2 subunit (aa 752–1353) consisting of a transmembrane domain and an intracellular cytoplasmic domain (CD) involved in viral fusion with target cells [4, 5]. Given its critical role in viral replication, the S protein has been the focus for MERS-CoV vaccine development similar to severe acute respiratory syndrome coronavirus (SARS-CoV), where it has been the main target for vaccines that led to robust induction of neutralizing antibody (nAb)–mediated protection in immunized animals [6–8].
Several vaccine candidates based on full-length or truncated S protein including vectored, DNA, and nanoparticle vaccines, as well as S or RBD protein–based subunit vaccines, have been developed and investigated in animal models [9–17]. Although some of these candidates can induce high levels of nAbs in vaccinated animals and have entered phase 1 clinical trials [15–17], most of these candidates are associated with low immunogenicity requiring multiple doses [18]. Importantly, vaccine research on other members of the coronaviruses including SARS-CoV [19–27] revealed several safety concerns associated with the use of S-based vaccines, including inflammatory and immunopathological effects such as pulmonary eosinophilic infiltration and antibody (Ab)–mediated disease enhancement following subsequent viral challenge of vaccinated animals. Recently, we also showed that whole inactivated MERS-CoV vaccine (WIV) is associated with significantly increased pulmonary eosinophilic infiltration accompanied by elevated levels of Th2 cytokines in vaccinated human DPP4 transgenic (hDPP4 Tg+) mice in response to virus challenge [28]. Given these previous observations, a MERS-CoV vaccine based on the full-length S protein could pose potential safety concerns similar to those associated with SARS-CoV vaccines. While the neutralizing epitope-rich S1 region could be considered as an alternative target for an effective and safe MERS vaccine, S1-based protection could clearly be enhanced through appropriate immunological modulation.
CD40 ligand (CD40L), a type II membrane protein, is a key co-stimulatory molecule and an essential regulator of the immune system. It is expressed transiently on activated CD4+ T cells mainly [29, 30]. CD40L and its receptor CD40, which is constitutively expressed on all antigen-presenting cells (APCs) [29–31], represent a crucial link between innate and adaptive immunity [31, 32]. The potential of CD40L as a molecular adjuvant has been investigated by several groups using multiple strategies [33–36]. We have previously developed a nonreplicating recombinant adenovirus 5 (rAd5) vectored prototype vaccine in which secreted viral proteins are targeted to CD40-expressing APCs using CD40L [37, 38]. Such studies provided a proof of concept for a vaccine platform characterized by enhanced durability, breadth, potency, and universal protection against influenza viruses in different mouse models. Here, we further extended and optimized our previous platform using rAd5 expressing a secreted and CD40-targeted fusion protein comprised of head globular ectodomains of both MERS-CoV S1 protein and murine CD40L (rAd5-S1/F/CD40L) using a trimerization motif to express the fusion protein as a trimer and a nonpolar linker to provide flexibility. The vaccine was then subjected to systematic analyses of protection and safety in the highly MERS-CoV permissive hDPP4 Tg+ mouse model.
MATERIALS AND METHODS
In Silico Design of the Immunogen and Generation of rAd5 Vaccines
The fusion gene was designed and codon-optimized to express a secreted and CD40-targeted consensus MERS-CoV S1 subunit (amino acids 1–747). In brief, all available MERS-CoV S sequences were downloaded from the GenBank database, and the dataset was filtered by removing sequences containing ambiguous amino acid codes (BJOUXZ). The final dataset was multiply aligned using CLUSTALW, the Shannon entropy for each position was determined, and the consensus S1 subunit sequence was obtained. The signal peptide from the MERS-CoV S protein was maintained at the N-terminus to secrete the fusion protein from rAd5-infected cells. The synthetic fusion gene was synthesized by linking the coding region of MERS-CoV S1 to a histidine tag coding sequence, followed by coding region for the fibritin trimerization motif [37] connected via a nonpolar amino acid linker to the ectodomain of CD40L (amino acids 117–260) coding region to express the fusion protein S1/F/CD40L. The fusion gene was then used to generate the proposed rAd5 construct (rAd5-S1/F/CD40L) in addition to rAd5 vaccines expressing secreted and consensus S1 protein alone (rAd5-S1) and a vector control expressing Green Fluorescent Protein (rAd5-GFP) as shown in Figure 1.
Figure 1.
Schematic representation of Middle East respiratory syndrome coronavirus vaccine candidates. The proposed recombinant adenovirus 5 (rAd5) construct (rAd5-S1/F/CD40L) was engineered to express a fusion protein with the S1 subunit containing the S protein signal peptide at the N-terminal, followed by a trimerization motif derived from T4 bacteriophage fibritin (F) fused with the ectodomain of murine CD40 ligand at the C-terminus (CD40L). Thus, the S1 and CD40L are expressed as a trimerized fusion protein via F (S1/F/CD40L). The construct was placed under the control of the Cytomegalovirus promoter (CMV) and in front of the Bovine Growth Hormone–poly A site (Poly A). Other control constructs included rAd5-S1 and a control rAd5 vector expressing Green Fluorescent Protein (rAd5-GFP).
Cells and MERS-CoV Virus
Vero E6 and Huh7.5 cells were cultured and maintained in complete Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum. MERS-CoV-EMC/2012 was provided by Heinz Feldmann (National Institutes of Health [NIH], National Institute of Allergy and Infectious Diseases, Rocky Mountain Laboratories, Hamilton, Montana) and Ron A. Fouchier (Erasmus Medical Center, Rotterdam, Netherlands). MERS-CoV titration was done in Vero E6 cells using Median Tissue Culture Infectious Dose (TCID50) assay as previously described [11]. All work involving infectious MERS-CoV was conducted within approved biosafety level 3 (BSL-3) at the Galveston National Laboratory (GNL) at the University of Texas Medical Branch (UTMB) in Galveston.
Animal Experiments
The immunogenicity and the protective efficacy of the rAd5-based vaccine candidates were evaluated against MERS-CoV infection in the hDPP4 Tg+ mouse model. A total of 45 hDPP4 Tg+ mice aged 4–5 months were used (15 mice/group). Mice were intramuscularly immunized twice, 28 days apart, with 109 of rAd5 vaccine candidates (Figure 2). Blood samples were collected 3 weeks after each immunization via retro-orbital bleeding. Four weeks after the second immunization, mice were intranasally challenged with 103 TCID50 (~100 Median Lethal Dose [LD50]) of MERS-CoV. On days 3 and 6 postinfection, 4 mice from each group were killed to assess the virus titer and lung pathology. The remaining mice were monitored for morbidity and mortality on a daily basis for up to 18 days. All animal studies involving infectious MERS-CoV were conducted within approved animal BSL-3 laboratories at GNL in accordance with the Guide for the Care and Use of Laboratory Animals of the NIH and Association for Assessment and Accreditation of Laboratory Animal Care, and with institutional animal protocol approval from UTMB. Animals were housed in on-site animal facilities under a 12:12 light:dark cycle with room temperature and humidity kept between 21°C and 25°C and 31%–47%, respectively, with ad libitum access to food and water.
Figure 2.
Animal study design. Abbreviations: i.m., intramuscular; LD50, Median Lethal Dose; PFU, plaque-forming units.
Lung Pathology
At day 3 and 6 after challenge, 4 mice in each group were euthanized and their lungs were collected and examined for pathological changes after immunization and viral challenge. In brief, lung tissues were fixed in 10% buffered formalin for 72 hours, transferred to 70% ethanol, and later paraffin embedded. Histopathological evaluation was performed on deparaffinized sections stained by routine hematoxylin and eosin staining. Evaluations for histopathology were done by pathologists who were blinded to each specimen source. Numeric scores were assigned to assess the extent of pathological damage as follows: 0, no observed pathology (undetectable infiltration); 1, mild pathology (up to 5% infiltration); 2, moderate pathology (up to 20% infiltration); 3, severe pathology (>20% infiltration).
Viral Titration by TCID50
Lungs collected from challenged mice on day 3 postchallenge were used to determine viral titer by TCID50. In brief, pieces of collected lung tissue were weighed and homogenized in phosphate-buffered saline containing 2% fetal calf serum with a TissueLyser (Qiagen). After clarification of the cellular and tissue debris by centrifugation, the titers of infectious virus in the suspensions of infected tissues were determined using TCID50 assay. The virus titers of individual samples were expressed as log10 TCID50 per gram of tissue and the minimal detectable level of virus was 2.5 log10 TCID50 as determined by lung size.
Viral Titration by Reverse-Transcription Quantitative Polymerase Chain Reaction
Lung samples from each group of mice (n = 4 per group) were transferred to individual vials containing RNAlater solution and subsequently homogenized and subjected to total RNA isolation using TRIzol Reagent. To determine the viral titer in the lung, MERS-CoV-specific upstream E gene (upE) and endogenous control gene (mouse β-actin) were quantified using 1-step reverse-transcription quantitative polymerase chain reaction (RT-qPCR). Cycle threshold (Ct) values for each sample were analyzed against Ct values from a standard curve of MERS-CoV messenger RNA (mRNA) copy number. Relative MERS-CoV upE mRNA expression value was calculated for each replicate and expressed as the equivalent of log10 equivalents per gram (TCID eq/g) of the tissue by the standard Ct (∆∆Ct) method using Bio-Rad CFX Manager 3.0 software.
Enzyme-Linked Immunosorbent Assay
The end-point titers of anti-S1 total immunoglobulin G (IgG) as well as its isotypes including IgG1, IgG2a, and IgG2b from immunized mice were determined by enzyme-linked immunosorbent assay as described previously [11]. Serum samples were tested in a 2-fold serial dilution starting from 1:100. End-point titers were determined and expressed as the reciprocals of the highest dilution that gave an optical density signal higher than the cutoff value, defined as the mean of prebleed samples plus 3 standard deviations (SD).
MERS-Pseudotyped Neutralization Assay
MERS-pseudotyped viral particles (MERSpp) were produced and titrated using the Huh7.5 cell line as described previously [39]. Plasmids for generating MERSpp were kindly provided by Dr Nigel Temperton at the University of Kent, United Kingdom. Heat-inactivated serum samples were prepared in a 3-fold serial dilution starting from 1:20 and tested in duplicates in 96-well Nunc white plates. A standard amount of MERSpp (~200 000 Relative Light Units) was added to each well and plates were incubated for 1 hour at 37°C. Then, approximately 10 000 Huh7.5 cells were added to each well and incubated for 48 hours. Cells only and cells with MERSpp only were included in quadruplicate as controls in all plates. Following incubation, cells were lysed and luciferase activity was developed and measured using the Bright-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions. Log10 Median Inhibitory Concentration (IC50) neutralization titers were calculated for each serum sample using GraphPad Prism software.
Live Virus Microneutralization Assay
The standard live virus microneutralization (MN) assay was used as previously described [11]. In brief, starting at a dilution of 1:10, 60 μL volumes of serial 2-fold dilutions of heat-inactivated sera were mixed with equal volume of media containing 120 TCID50 of MERS-CoV. Each dilution was tested in duplicate in 96-well plates. The antibody-virus mixtures were incubated for 1 hour at 37°C before transfer of 100 μL into confluent Vero E6 cell monolayers in 96-well plates. Vero E6 cells cultured with DMEM medium with or without virus were included as positive and negative controls, respectively. After 72 hours of incubation, the nAb titer of each serum sample was determined as the reciprocal of the highest dilution capable of completely preventing virus-induced cytopathic effect in 50% of the wells (MN50).
Data Analysis
Statistical analysis was conducted using 1-way or 2-way analysis of variance with Bonferroni posttest to adjust for multiple comparisons between groups using GraphPad Prism software.
RESULTS
Targeting MERS-CoV S1 via CD40L Enhances Circulating S1-Specific IgG and Its Isotypes
To evaluate the immunogenicity of the generated rAd5 vector-based MERS vaccines, we immunized mice intramuscularly with 2 doses of individual vaccines and measured the levels of circulating S1-specific IgG and its isotypes before and at 3 weeks after each immunization. Here, we observed that both rAd5-S1/F/CD40L and rAd5-S1 induced S1-specific Abs from all isotypes in hDPP4 Tg+ mice after primary or secondary immunization (Figure 3). As expected, control vector (rAd5-GFP) failed to elicit any S1-specific Ab response. Notably, a single dose or 2 doses of rAd5-S1/F/CD40L consistently elicited significantly higher levels of total IgG as well as IgG1, IgG2a, and IgG2b isotypes compared with the control group immunized with rAd5-GFP (Figure 3), whereas immunization with a single dose of rAd5-S1 induced significant titers of total IgG, IgG1, and IgG2a but not IgG2b compared to the control group, with a second dose of rAd5-S1 eventually inducing significantly higher levels of IgG2b compared to the control group. Interestingly, immunizing mice with a second dose of rAd5-S1/F/CD40L enhanced the levels of circulatory IgG and all tested isotypes compared with the rAd5-S1–vaccinated group. Furthermore, 2 doses of rAd5-S1 only enhanced IgG2b significantly compared to 1 dose, with mean IgG1:IgG2a ratios being 3.8 (SD, 3.2) and 3.9 (SD, 3.2) after priming and boosting, respectively. In contrast, boosting with a second dose of rAd5-S1/F/CD40L resulted in a significant increase in total IgG, particularly in IgG2a and IgG2b isotypes but not IgG1, compared with a single dose of rAd5-S1/F/CD40L, suggesting a bias toward Th1 response as demonstrated by mean IgG1:IgG2a ratios of 3.4 (SD, 2.4) and 1.8 (SD, 1.4) after priming and boosting, respectively. Together, these data showed that targeting the secreted S1 to CD40-expressing APCs via CD40L (rAd5-S1/F/CD40L) not only enhanced S1-specific IgG Abs levels but also boosted Th1 responses, as demonstrated by markedly elevated titers of S1-specific IgG2a and IgG2b antibodies, especially after boosting.
Figure 3.
Circulating immunoglobulin G (IgG) antibodies. End-point titers of circulating Middle East respiratory syndrome coronavirus S1-specific total IgG, IgG1, IgG2a, and IgG2b antibodies induced by recombinant adenovirus 5 (rAd5)–based vaccines were determined using serum samples collected 3 weeks after priming and boosting. Data are shown as mean titer with standard deviation from 1 of 2 independent experiments, with n = 10–11 mice per treatment group. *P < .0332, **P < .0021, ***P < .0002, ****P < .0001 (2-way analysis of variance with Bonferroni posttest); ns, not significant. See the Figure 1 legend for explanation of the rAd5 constructs.
A Single Dose of CD40-Targeted MERS-CoV S1 Induces High Levels of nAbs in Immunized Mice
To extend our analysis and to evaluate the effector function of induced Abs, we measured their neutralizing activities before and after each immunization. As expected, all immunized mice from all groups showed no detectable levels of nAbs in samples collected before immunization. As shown in Figure 4, a single dose of either rAd5-S1/F/CD40L or rAd5-S1 elicited high levels of nAbs compared to the control group (rAd5-GFP), with rAd5-S1/F/CD40L, but not rAd5-S1, being consistently capable of inducing significantly higher levels of nAbs against both live and pseudotyped MERS-CoV virus, suggesting that rAd5-S1/F/CD40L is better than rAd-S1 vaccine in promoting strong humoral response and neutralizing activity against MERS-CoV. However, after boosting once, all rAd5-S1/F/CD40L– and rAd5-S1–immunized animals elicited robust and significant levels of nAbs, indicating that at least 2 doses of rAd-S1 are required to induce nAb levels similar to those obtained by a single dose of rAd5-S1/F/CD40L. These findings clearly confirm that incorporation of CD40L as molecular adjuvant could effectively enhance the immunogenicity of S1-based vaccines and may represent a very promising vaccine platform to induce protective immunity with a single dose.
Figure 4.
Middle East respiratory syndrome coronavirus spike recombinant adenovirus 5 (rAd5) vaccine induced neutralizing antibodies. In the live virus microneutralization assay, neutralization titers were determined as the highest serum dilutions from each individual mouse that completely protected Vero E6 cells in at least 50% of the wells (MN50), and titers are shown as mean with standard deviation (SD) from 15 mice per group from 1 experiment. In the pseudotyped virus neutralization assay, Median Inhibitory Concentration (IC50) was calculated for each serum sample, and titers are shown as mean of log10 IC50 from 10 mice per group (SD) from 1 experiment. *P < .0332, **P < .0021, ***P < .0002, ****P < .0001 (1-way analysis of variance with Bonferroni posttest); ns, not significant. See the Figure 1 legend for explanation of the rAd5 constructs.
MERS-CoV S1-Based Vaccines Protect hDPP4 Tg+ Mice From MERS-CoV Challenge
Having demonstrated that CD40-targeted vaccine was superior in eliciting immune responses in hDPP4 Tg+ mice, we next investigated if these S1-based vaccines could effectively protect these highly MERS-CoV permissive mice from viral challenge. To this end, the vaccinated mice were challenged with 100 LD50 of MERS-CoV and subsequently monitored for 3 weeks. It was clear that mice immunized twice with either rAd5-S1 or rAd5-S1/F/CD40L were completely protected based on clinical signs of disease (weight loss) and mortality (Figure 5), whereas rAd5-GFP–immunized animals expectedly succumbed to lethal infection within days, likely due to encephalitis [28, 40]. These findings suggest that both rAd5-S1 and rAd5-S1/F/CD40L vaccines are protective in this mouse model.
Figure 5.
S1-based recombinant adenovirus 5 (rAd5) vaccines provide complete protection against lethal Middle East respiratory syndrome coronavirus (MERS-CoV) challenge. Survival curve and body weight loss of human dipeptidyl peptidase 4 transgenic mice immunized with 2 doses of 109 plaque-forming units of the indicated rAd5 constructs and challenged with 100 Median Lethal Dose (LD50) of MERS-CoV (EMC2012 strain) 5 weeks after secondary immunization. Data are shown from 1 experiment with n = 11 mice per treatment group. See the Figure 1 legend for explanation of the rAd5 constructs.
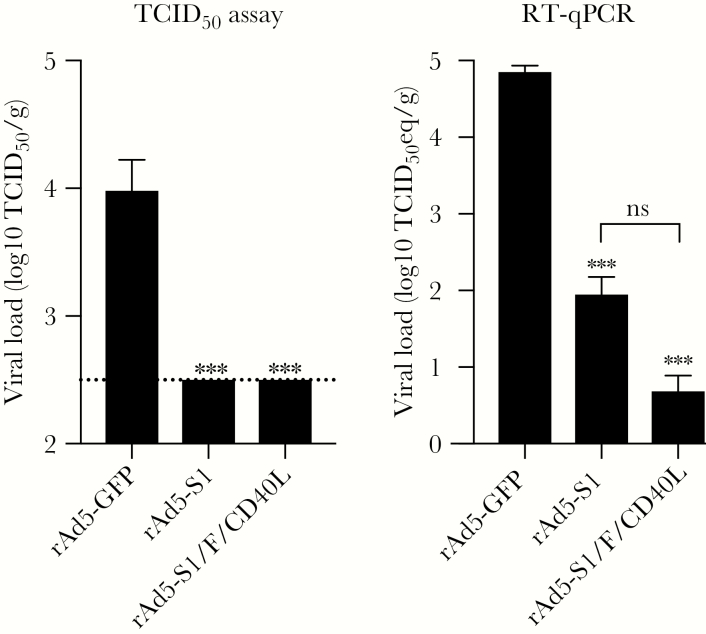
MERS-CoV S1-Based Vaccines Inhibit Pulmonary Viral Replication in hDPP4 Tg+ Mice
To further confirm that immunized hDPP4 Tg+ mice were protected from MERS-CoV infection after challenge, we measured lung viral titer, both infectious progeny virus and viral RNA, on day 3 postchallenge by Vero E6-based infectivity assay and RT-qPCR, respectively. Analysis of infectious viral titer revealed that immunization with both vaccines significantly protected against viral replication to undetectable levels compared with rAd5-GFP–immunized and –challenged mice (Figure 6). These results were further confirmed by an average of 3- to 4-log reduction in pulmonary viral RNA, compared to that of the rAd5-GFP–immunized group (Figure 6). It should be mentioned that, albeit not statistically significant, rAd5-S1/F/CD40L–immunized animals had a viral RNA load lower by 1 log compared with the rAd5-S1 group, consistent with the overall stronger immune response we observed in this group.
Figure 6.
Pulmonary viral load. Viral load was determined in the lungs of immunized and challenged mice with Middle East respiratory syndrome coronavirus (MERS-CoV). Viral load was determined as Median Tissue Culture Infectious Dose per gram (TCID50/g) and TCID50 equivalent per gram (TCID50 eq/g) by TCID50 and reverse-transcription quantitative polymerase chain reaction (RT-qPCR) assays, respectively. Limit of detection in the TCID50 assay was 2.5 log10 (dashed line). TCID50 eq/g was determined using a standard curve generated from dilutions of RNA from a MERS-CoV with known virus titer. Titers are shown as mean with standard deviation from 4 mice per group from 1 experiment. ***P < .0002 (1-way analysis of variance with Bonferroni posttest); ns, not significant. See the Figure 1 legend for explanation of the recombinant adenovirus 5 (rAd5) constructs.
CD40-Targeted MERS-CoV S1 Prevents Vaccine-Associated Lung Pathology in Virally Challenged hDPP4 Tg+ Mice
Finally, investigations were carried out to assess safety of these vaccine candidates to rule out any vaccine-associated pulmonary injuries as observed with some vaccines against other coronaviruses. Evaluation of lung pathology in immunized and challenged mice showed up to 15% multiple monocytic and lymphocytic infiltrations (moderate pathology) in the lungs of the rAd5-GFP group 3 and 6 days postchallenge compared to other groups, which showed minimal to no inflammatory infiltration (Figure 7). Surprisingly, although rAd5-S1 immunization protected animals from death and weight loss and prevented the lung infiltration similar to rAd5-S1/F/CD40L, we observed perivascular hemorrhage in the whole lung of all examined mice immunized with rAd5-S1 but not those vaccinated with rAd5-S1/F/CD40L (Figure 7). Together, these results suggest that although S1 alone could protect the animals from viral infection, it may inadvertently induce lung pathology, and that using CD40L as targeting molecule and molecular adjuvant does not only enhance immunogenicity and protective efficacy but also prevents vaccine-associated pulmonary pathology.
Figure 7.
Lung pathology after challenge. Histopathologic evaluation was performed on deparaffinized sections stained by routine hematoxylin and eosin staining. Arrows indicate monocytic and lymphocytic infiltrates as well as perivascular hemorrhage. The depicted lung pathology on days 3 and 6 is a representative of 2 independent experiments. See the Figure 1 legend for explanation of the recombinant adenovirus 5 constructs.
DISCUSSION
The rapid spread and persistence of MERS-CoV in the Arabian Peninsula, in addition to the associated high mortality rates, represent a serious global public health concern, particularly that the elimination of the zoonotic reservoir is extremely unlikely at the current setting. This threat is further complicated by the absence of prophylactic or therapeutic measures. Therefore, development of an effective and safe vaccine is urgently needed to prevent or contain MERS-CoV. Several groups have investigated various MERS-CoV vaccine platforms in several animal models [9–18, 28]. However, serious safety concerns are associated with vaccines for several coronaviruses including MERS-CoV and need to be elucidated and better understood [19–28].
We showed in this study that although rAd5 expressing S1 or CD40-targeted S1 were both capable of inducing significant levels of IgG and nAbs specific to MERS-CoV in immunized mice, incorporation of CD40L substantially enhances the immunogenicity of S1, as demonstrated by the effectiveness of a single immunization dose, which was sufficient to elicit stronger and robust immune responses compared to control groups, consistent with our previous reports [37, 38]. Importantly, both S1 and CD40-targeted S1 vaccines provided complete protection, prevented virus replication significantly, and prevented monocytic and lymphocytic lung infiltration as compared to control group. However, the non-CD40-targeted vaccine (rAd5-S1) uniformly induced varying degrees of severe perivascular hemorrhage within the lungs of all examined mice following viral challenge, regardless of the exhibited full protection against lethal MERS-CoV challenge. While these data suggest that there might be yet to be explained lung pathology associated with rAd5 vector– and/or MERS-CoV S1–based vaccines, our results clearly show that including CD40L as a fusion protein of S1-based MERS-CoV vaccine could prevent or at least mitigate the safety concern of undesirable immunopathology while affording complete protection in hDPP4 Tg+ mice.
At least 3 groups have developed different rAd-vectored MERS-CoV vaccines [14, 41, 42]. However, these studies have mainly focused on the immunogenicity and/or the protective efficacy, and may have overlooked any possible vaccine-associated pathology, especially after viral challenge. Our finding that rAd5-S1 immunized animals were completely protected despite the observed pathology is reminiscent of the immunopathology documented using WIV for MERS-CoV, SARS-CoV, and respiratory syncytial virus after viral challenge [19–28, 43–45]. It is of note that lung pathology was also observed in some previous studies involving rhesus macaques immunized with high doses of DNA vaccines expressing MERS-CoV S protein [16], as well as in mouse models vaccinated with RBD or vectored vaccines encoding S protein [46–48]. Nevertheless, this is the first report of vaccine-associated severe pulmonary perivascular hemorrhage after viral challenge. Although the exact molecular mechanisms underlying such vaccine-induced pulmonary injury associated with rAd5-S1 vaccination remain to be determined, several factors could have been involved. Specifically, the likely higher levels of residual infectious viruses in rAd5-S1–immunized mice as detected by RT-qPCR, and the quantitative and perhaps qualitative difference of specific antibody and T-cell responses, as evidenced by the lower levels of Ig2a and IgG2b found in this group, could have played a role. Therefore, future studies should include systematic analyses of T-cell responses as we have previously conducted [37, 38], to fully explore the mechanisms of immunization-induced hemorrhage of the lungs. Collectively, these previous data, along with our report here, indicate that viral components (ie, within the S protein) could be involved in inadvertent adverse reactions similar to those associated with the different S-based SARS vaccines [19–28].
Our study suggests that although rAd5-S1 could be a potential vaccine candidate, it might be associated with severe lung pathology upon viral challenge or infection. These findings are of great importance in order to develop safe and effective human MERS-CoV vaccine. Moreover, there is a huge gap in our understanding of the correlates of protection in camels, and the waning nature of nAbs in these animals [49, 50] might hinder the “One Health” approach in tackling the MERS epidemic. Therefore, it is critical to better understand and elucidate any safety concerns that might be associated with MERS-CoV vaccine to develop a safe and effective human vaccine. Our previous studies have mainly focused on the development of candidate universal influenza vaccines using vectored vaccines by targeting antigens to CD40-expressing cells via CD40L. This platform was modified, optimized, and utilized to generate an immunogenic, protective, and safe MERS-CoV vaccine based on the S1 subunit of the MERS-CoV S protein, thus representing a promising platform for vaccine development against a broad range of pathogens.
Notes
Financial support. This work was supported by King Abdulaziz City for Science and Technology (grant number AT-35–171 to A. M. H.).
Potential conflicts of interest. A. M. H. is an inventor on a US patent application related to this work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814–20. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV)—Saudi Arabia disease outbreak news, 26 January 2018. http://www.who.int/csr/don/26-january-2018-mers-saudi-arabia/en/. Accessed 29 October 2018. [Google Scholar]
- 3. Park SH, Kim YS, Jung Y, et al. Outbreaks of Middle East respiratory syndrome in two hospitals initiated by a single patient in Daejeon, South Korea. Infect Chemother 2016; 48:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu G, Hu Y, Wang Q, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 2013; 500:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013; 495:251–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He Y, Zhou Y, Liu S, et al. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun 2004; 324:773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He Y, Li J, Du L, et al. Identification and characterization of novel neutralizing epitopes in the receptor-binding domain of SARS-CoV spike protein: revealing the critical antigenic determinants in inactivated SARS-CoV vaccine. Vaccine 2006; 24:5498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol 2009; 7:226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Shi W, Joyce MG, et al. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun 2015; 6:7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coleman CM, Liu YV, Mu H, et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 2014; 32:3169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Amri SS, Abbas AT, Siddiq LA, et al. Immunogenicity of candidate MERS-CoV DNA vaccines based on the spike protein. Sci Rep 2017; 7:44875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma C, Wang L, Tao X, et al. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments—the importance of immunofocusing in subunit vaccine design. Vaccine 2014; 32:6170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song F, Fux R, Provacia LB, et al. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J Virol 2013; 87:11950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo X, Deng Y, Chen H, et al. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology 2015; 145:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haagmans BL, van den Brand JM, Raj VS, et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science 2016; 351:77–81. [DOI] [PubMed] [Google Scholar]
- 16. Muthumani K, Falzarano D, Reuschel EL, et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med 2015; 7:301ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alharbi NK, Padron-Regalado E, Thompson CP, et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine 2017; 35:3780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou Y, Jiang S, Du L. Prospects for a MERS-CoV spike vaccine. Expert Rev Vaccines 2018; 17:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weingartl H, Czub M, Czub S, et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol 2004; 78:12672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tseng CT, Sbrana E, Iwata-Yoshikawa N, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One 2012; 7:e35421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang ZY, Werner HC, Kong WP, et al. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc Natl Acad Sci U S A 2005; 102:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Czub M, Weingartl H, Czub S, He R, Cao J. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine 2005; 23:2273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deming D, Sheahan T, Heise M, et al. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med 2006; 3:e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaume M, Yip MS, Kam YW, et al. SARS CoV subunit vaccine: antibody-mediated neutralisation and enhancement. Hong Kong Med J 2012; 18(Suppl 2):31–6. [PubMed] [Google Scholar]
- 25. Olsen CW, Corapi WV, Ngichabe CK, Baines JD, Scott FW. Monoclonal antibodies to the spike protein of feline infectious peritonitis virus mediate antibody-dependent enhancement of infection of feline macrophages. J Virol 1992; 66:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weiss RC, Scott FW. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis 1981; 4:175–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He Y, Zhou Y, Wu H, et al. Identification of immunodominant sites on the spike protein of severe acute respiratory syndrome (SARS) coronavirus: implication for developing SARS diagnostics and vaccines. J Immunol 2004; 173:4050–7. [DOI] [PubMed] [Google Scholar]
- 28. Agrawal AS, Tao X, Algaissi A, et al. Immunization with inactivated Middle East respiratory syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother 2016; 12:2351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol 2000; 67:2–17. [DOI] [PubMed] [Google Scholar]
- 30. Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol 2009; 21:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev 2003; 14:297–309. [DOI] [PubMed] [Google Scholar]
- 32. Allen RC, Armitage RJ, Conley ME, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science 1993; 259:990–3. [DOI] [PubMed] [Google Scholar]
- 33. Cao J, Wang X, Du Y, Li Y, Wang X, Jiang P. CD40 ligand expressed in adenovirus can improve the immunogenicity of the GP3 and GP5 of porcine reproductive and respiratory syndrome virus in swine. Vaccine 2010; 28:7514–22. [DOI] [PubMed] [Google Scholar]
- 34. Gómez CE, Nájera JL, Sánchez R, Jiménez V, Esteban M. Multimeric soluble CD40 ligand (sCD40L) efficiently enhances HIV specific cellular immune responses during DNA prime and boost with attenuated poxvirus vectors MVA and NYVAC expressing HIV antigens. Vaccine 2009; 27:3165–74. [DOI] [PubMed] [Google Scholar]
- 35. Huang D, Pereboev AV, Korokhov N, et al. Significant alterations of biodistribution and immune responses in Balb/c mice administered with adenovirus targeted to CD40(+) cells. Gene Ther 2008; 15:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yao Q, Fischer KP, Li L, et al. Immunogenicity and protective efficacy of a DNA vaccine encoding a chimeric protein of avian influenza hemagglutinin subtype H5 fused to CD154 (CD40L) in Pekin ducks. Vaccine 2010; 28:8147–56. [DOI] [PubMed] [Google Scholar]
- 37. Hashem AM, Gravel C, Chen Z, et al. CD40 ligand preferentially modulates immune response and enhances protection against influenza virus. J Immunol 2014; 193:722–34. [DOI] [PubMed] [Google Scholar]
- 38. Fan X, Hashem AM, Chen Z, et al. Targeting the HA2 subunit of influenza A virus hemagglutinin via CD40L provides universal protection against diverse subtypes. Mucosal Immunol 2015; 8:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grehan K, Ferrara F, Temperton N. An optimised method for the production of MERS-CoV spike expressing viral pseudotypes. MethodsX 2015; 2:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tao X, Garron T, Agrawal AS, et al. Characterization and demonstration of the value of a lethal mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol 2016; 90:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim E, Okada K, Kenniston T, et al. Immunogenicity of an adenoviral-based Middle East respiratory syndrome coronavirus vaccine in BALB/c mice. Vaccine 2014; 32:5975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Munster VJ, Wells D, Lambe T, et al. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines 2017; 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson TR, Parker RA, Johnson JE, Graham BS. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J Immunol 2003; 170:2037–45. [DOI] [PubMed] [Google Scholar]
- 44. Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 1969; 89:405–21. [DOI] [PubMed] [Google Scholar]
- 45. Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422–34. [DOI] [PubMed] [Google Scholar]
- 46. Tai W, Zhao G, Sun S, et al. A recombinant receptor-binding domain of MERS-CoV in trimeric form protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. Virology 2016; 499:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Volz A, Kupke A, Song F, et al. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J Virol 2015; 89:8651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Malczyk AH, Kupke A, Prüfer S, et al. A highly immunogenic and protective Middle East respiratory syndrome coronavirus vaccine based on a recombinant measles virus vaccine platform. J Virol 2015; 89:11654–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Doremalen N, Hijazeen ZS, Holloway P, et al. High prevalence of Middle East respiratory coronavirus in young dromedary camels in Jordan. Vector Borne Zoonotic Dis 2017; 17:155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reusken CB, Haagmans BL, Müller MA, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis 2013; 13:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]