ABSTRACT
Our systems, thinking, training, legislation, and policies are lagging far behind momentous changes in science, and leaving us vulnerable in biosecurity. Synthetic viruses and genetic engineering of pathogens are a reality, with a rapid acceleration of dual-use science. The public availability of methods for dual-use genetic engineering, coupled with the insider threat, poses an unprecedented risk for biosecurity. Case studies including the 1984 Rajneesh salmonella bioterrorism attack and the controversy over engineered transmissible H5N1 influenza are analyzed. Simple probability analysis shows that the risks of dual-use research are likely to outweigh potential benefits, yet this type of analysis has not been done to date. Many bioterrorism agents may also occur naturally. Distinguishing natural from unnatural epidemics is far more difficult than other types of terrorism. Public health systems do not have mechanisms for routinely considering bioterrorism, and an organizational culture that is reluctant to consider it. A collaborative model for flagging aberrant outbreak patterns and referral from the health to security sectors is proposed. Vulnerabilities in current approaches to biosecurity need to be reviewed and strengthened collaboratively by all stakeholders. New systems, legislation, collaborative operational models, and ways of thinking are required to effectively address the threat to global biosecurity.
INTRODUCTION
Rapid developments in science and unprecedented accessibility of bioweapons have outpaced the capacity of our systems to deal effectively with bioterrorism threats. Acceleration in dual-use research of concern (DURC) and public availability of methods for DURC are gamechangers in biosecurity. DURC is research intended to benefit human health, but which can also inadvertently or deliberately harm people. Other vulnerabilities include lack of systems for differentiating natural and unnatural outbreaks, low awareness of the insider threat, and lack of intersectoral collaboration. The aim of this article is to examine traditional approaches to bioterrorism in the context of new challenges; and to outline potential approaches to improving biopreparedness in the era of DURC.
RECOGNIZING BIOTERRORISM
If a building collapses, even a child can differentiate between a bomb and an earthquake as the cause. Traditional approaches to bioterrorism are underpinned by the assumption that acts of bioterrorism, like a collapsing building, will be easily recognized. However, unless the event is related to an eradicated organism such as smallpox, even the most skilled experts cannot readily differentiate between unnatural and natural outbreaks when it involves a disease that occurs naturally. Experts need to firstly be open to the possibility, and secondly to have systems for flagging aberrant outbreak patterns.
There has been a surge in emerging infectious diseases in recent years, including Ebola, Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and avian influenza, but public health response has defaulted to an assumption of natural emergence. Ebola is a category A bioterrorism agent, and the 2014 West African outbreak displayed highly unusual features.1–3 For example, Ebola has never occurred in more than one country simultaneously previously, nor in capital cities.4 Further, two unrelated Ebola epidemics occurred at the same time in West Africa and The Democratic Republic of Congo in 2014, while an outbreak of Marburg virus simultaneously broke out in Uganda. Locals speculated about the origin of the 2014 Ebola epidemic being bioterrorism,5,6 yet this has been ignored in the public health response. Few nations have systematic mechanisms to assess re-emerging or emerging infections for potential bioterrorism, despite acceleration of science and accessibility of means for bioterrorism. An analysis of MERS-CoV shows that the pattern of disease fits with deliberate release,7 yet this possibility has not been seriously entertained. The geographic spread of the recently emerged H7N9 avian influenza is puzzling. In contrast to H5N1, H7N9 does not correspond to either wild bird flyways or poultry trading routes.8 The virus contains the same genetic mutations (which confers adaptation to humans), as engineered influenza viruses.8 Yet this H7N9 has also been assumed to be natural.
The science of epidemiology9 is about pattern recognition and classification of disease into the three major patterns, epidemic, endemic, and sporadic. Unnatural patterns which do not fit the predicted behavior of infectious diseases,10 may be a signal for potential bioterrorism. Field epidemiology is the science of investigating outbreaks, pioneered by the US Centers for Disease Control with its Epidemic Intelligence Service.11 The International Health Regulations (IHR),12 which specify standards in disease surveillance, notification and response, rely field epidemiology for implementation. However, skills, laws, and resources do not guarantee correct interpretation of disease data, as illustrated by the following case study.
PATTERN RECOGNITION: NATURAL OR UNNATURAL OUTBREAK?
In September 1984, a large epidemic of salmonella occurred in the United States, with 751 cases arising from 10 restaurants. Eating at the salad bar was identified as the risk factor. Health authorities concluded this was a food-borne outbreak, and the cause, unsanitary food handlers. The salad bars were closed down, the outbreak subsided, and the matter would have ended there, but for a local politician, Jim Weaver, who accused a local religious cult of deliberately contaminating salad bars.13
He went to the media with his claims, but health authorities refuted him, and he was branded paranoid and prejudiced against the religious cult. Six months later, the leader of the cult, Bhagwan Shree Rajneesh, confessed to the attack. Initially, his confession was not believed, but a year after the outbreak the Federal Bureau of Investigation (FBI) found an exact genetic match of salmonella in the cult's laboratories.
The outbreak occurred in Oregon in 1984. Rajneesh had purchased a ranch for 4,000 followers, and was in conflict with Wasco County over land use, and sought to take control of the County by making enough locals sick so that they would not be able to vote. The restaurant attack was a practice run, with the final plan to contaminate the town water supply before election day. Numerous cult members were involved, including 2 registered nurses. This case was not discussed publicly or written up in medical literature for another 12 years.14
There are only two explanations for 10 restaurants being simultaneously affected by an identical strain of salmonella—either a common contaminated ingredient in all affected restaurants, or deliberate contamination. Yet neither explanation was considered, despite Jim Weaver's warning. Even when Rajneesh confessed, he was not believed. This case study illustrates the inability of experts to interpret the data correctly, and the active resistance to considering bioterrorism as a cause. The normal human tendency is to force available facts to fit the dominant paradigm of thinking. Most food borne outbreaks are because of unsafe food handling, and this is the dominant paradigm for a field epidemiologist. Even experts may default to the dominant paradigm and override fact with belief. There is a need to train field epidemiologists to recognize unusual patterns and consider bioterrorism as a possibility. If the possibility is never entertained, it will never be detected. The Rajneesh case would have remained undetected if not for the unsolicited confession. The ridiculing of the 1 person who recognized bioterrorism and the silence around the case for more than a decade illustrate important lessons.
THE INSIDER THREAT: BIOLOGISTS AS TERRORISTS?
U.S. Commission on the Prevention of Weapons of Mass Destruction Proliferation and Terrorism recognizes the risk posed by the insider—“Given the high level of know-how needed (we) should be less concerned that terrorists will become biologists and far more concerned that biologists will become terrorists.”15 The FBI investigation of the U.S. 2001 Anthrax letter bombs led to Dr Bruce Ivins, a researcher in the U.S. Army Medical Research Institute of Infectious Disease.16 Ivins, who initially helped the FBI with the investigation, eventually became a suspect and was investigated for almost 7 years, committing suicide before he was to be charged. In 2003, Professor Thomas Butler, a university scientist who had served in U.S. Naval Medical Research Unit, was convicted for illegal transportation of plague to Tanzania, and other charges.17 The aggressive prosecution of an eminent scientist was met with disapproval from the scientific community.18
Case studies of insiders show a range of personal motives19,20—greed, revenge, divided loyalty, blackmail, ego, work or family problems, or ideology. Insider threat has been traditionally viewed as stealing secrets to give to others, but where scientists are concerned, additional vested interests which could lead to personal gain such as patents on therapeutics, desire for fame, and potential for high impact research publications may all play a role in illegal research. Following the Ivins case, the United States came up with the Fink report in 2004,21 which made key recommendations for oversight of DURC, and recommended the creation of a National Science Advisory Board for Biodefense (NSABB), established in 2005.
For infectious diseases, the 2 major categories of DURC are synthetic genomics and genetic modification of pathogens. The first synthetic virus was created in 2002,22 and there are more than 50 unregulated private companies in the United States, China, and Europe in this industry. Genetic modification of pathogens has been documented since the Soviet bioweapons program, including insertion of genes from Ebola into smallpox.23 The most instructive case of DURC is research into transmissible avian influenza strains.
DURC: THE CASE OF ENGINEERED TRANSMISSIBLE AVIAN INFLUENZA VIRUSES
Influenza can affect many species of mammals and birds. H5N1 is a highly pathogenic avian influenza virus which emerged in 1997 and affects birds. It has caused sporadic human cases, mostly in people with close contact with sick or dead birds. Over 386 people have been infected worldwide since 1997.24,25 H5N1 is not easily transmissible between humans, but the potential of a random genetic mutation which could result in human transmissibility has driven global pandemic planning since 2005. There is an unknown, but real probability that a pandemic may arise from genetic mixing or a random mutation of avian H5N1. It was anticipated in 2005 that the next pandemic virus may be related to avian H5N1.26 In 2011, two research groups completed research on engineering the H5N1 to make it transmissible in mammals. The rationale was to anticipate and understand the emergence of a H5N1 pandemic virus, and to enable vaccines and treatments. The risks include bioterrorism or a laboratory accident, either of which could spark an unnatural pandemic. These research groups sought to make their methods public in leading journals.27
The scientific community remains deeply divided about DURC.28 The opposing view was that the risk of a laboratory accident or bioterrorism sparking a pandemic was unacceptable, and that the benefits were minimal.29 Influenza vaccines must be exactly matched to a virus to be protective, so creating an engineered virus ahead of a potential pandemic, should a pandemic with that exact virus eventuate naturally, is flawed, as the exact virus which causes a pandemic cannot be predicted. This unpredictability is illustrated the fact that the 2009 pandemic was not the anticipated H5N1, but an entirely different virus, H1N1, rendering all vaccines prepared for H5N1 useless.29
In 2011, the NSABB initially unanimously recommended censorship of methods for H5N1 DURC, as it was deemed a biosecurity risk.30 Yet in March 2012, following outspoken objections from scientists engaged in DURC, the NSABB reversed their position, and publication was allowed.31–33 Questions have been raised about bias and conflicts of interest within the NSABB in this reversal, and have yet to be satisfactorily resolved.34 Since then, DURC has accelerated, including engineered H7N9 and creation of a 1918 pandemic-like influenza virus.35,36
Inexplicably, the NSABB failed to meet again for more than 2 years, despite acceleration of DURC.35,37 The NSABB has further had its charter narrowed, with a reduced focus on DURC, and a reduction of funding. In the same time frame, major safety breaches occurred involving anthrax, avian influenza, and Ebola in the U.S. Centers for Disease Control and Prevention (CDC) laboratories; and unsecured vials of smallpox were found at the National Institute of Health.38–40 In July 2014, the NSABB dismissed all remaining members who had been part of the H5N1 controversy. The cumulative effect of these changes is to further erode protections for society against DURC.41
If other stakeholders such as law enforcement, defense and intelligence agencies have had input into these decisions, they have not had influence. The public debate has narrowly focused on medical research, without acknowledgment of other sectors or input from wider perspectives. Further, there has been no attempt to quantify risks versus benefits of DURC.
RISK–BENEFIT ANALYSIS AS A TOOL FOR BIOSECURITY: THE EXAMPLE OF H5N1 DURC
Naturally occurring H5N1 influenza is not easily transmitted between humans. Methods for engineering transmissible H5N1 are now publicly available, yet the risk–benefit arguments are not routinely quantified. To quantify these, it is first necessary to estimate probabilities of a natural pandemic occurring (preparedness for this as the argument in favor of DURC) and of an unnatural pandemic occurring as the result of a laboratory accident or deliberate release (the argument against DURC).
If the risk of a natural pandemic was low and the risk of an unnatural one high, this would favor risk over benefit, and vice versa. Figure 1 outlines the context of H5N1 dual-use research, and shows the two pathways through which a pandemic could occur—either naturally, with a probability P1, or unnaturally through a laboratory accident or deliberate release with a probability, P2.
FIGURE 1.
Summary of the dilemma of potential pandemic influenza and dual-use research.
A rough estimation of P1 versus P2 can be made as follows. We know that natural pandemics occur every 40 years or so, so P1 may be 1/40 or 0.025. The probability of an engineered pandemic strain is 1 (100%), because such viruses have already been created.32,33 The probability of a pandemic arising as a result, P2 = 1 × K, where K is either the probability of bioterrorism or a laboratory accident. We have seen four major safety breaches with dangerous pathogens in leading institutes in 2014,38–40 so we could assume the probability of a laboratory accident is high. For bioterrorism, it may be the case that K = 1. It is accepted in law enforcement that if a reproducible method for creating a 3-dimensional printer gun is published on the Internet, the probability of it being used by criminals is 1. It would be naïve to assume the probability as anything other than 1 for terrorists undertaking genetic engineering of viruses once the methods are published.
If we assume this to be true, then P1 = 0.025 and P2 = 1. Even if P2 is not 1, P2 is likely to be higher than P1. A more refined approach to calculating probabilities may result in different estimates, but probability of risk is unlikely to be less than that of benefit. Despite the potentially catastrophic impacts on society and the high stakes involved, risk analysis is not globally mandated for this question. Other approaches to quantifying risk show that DURC carries unacceptable risk to society.42 Following a review of DURC in 2015, the U.S. National Institute of Health will contract a private firm to conduct a risk analysis on paused DURC. This is a step in the right direction, but critics have raised conflicts of interest in the process, and global governance is still lacking.43
ETHICS, CONFLICT OF INTEREST, AND DEFINING THE STAKEHOLDERS
There are a broad range of stakeholders in biosecurity, from the general public, to scientists, health professionals, food and agriculture sectors, government, law enforcement, and the military, each with their own interests. Scientists have an interest in their own research careers, in publishing research, and gaining research grants. Law enforcement and military, on the other hand, are concerned with protecting the public from threats. The interests of different stakeholders may come into conflict, yet conflicts of interests around DURC have not been well addressed. Nor has there been any move to force declaration of conflicts of interest.34 Using accepted principles of dealing with conflict of interest in medical research as defined by the National Institutes of Health,44 decisions about DURC should be made by people free of conflicts of interest. Further, such decisions impact on all humanity, not just on the scientists involved, and it is important that all stakeholders be involved in the decision-making.
The ethics of dual-use research is more complex than usually considered by institutional ethics review boards and research ethics committees. These have developed from the 1964 declaration of Helsinki, and been refined around studies of therapeutic agents.45 Principles of beneficence, nonharm, informed consent, and privacy apply equally to populations as they do to individuals, yet the ethical implications of DURC have not been well considered. The impact of an epidemic can affect people who do not make an informed decision or consent to accepting the risk, so harm at a population level caused by DURC with epidemic potential is unique, and not something which ethics committees consider routinely. Although some organizations and countries have specific policies on DURC and mechanisms for oversight, this is by no means uniform or universal, and many gaps in accountability exist. It would be important to develop universal and enforceable guidelines that address DURC, considering questions of risk, benefit, and informed consent as it applies to the community as a stakeholder.46
BETTER THREAT RECOGNITION AND RESPONSE: A PROPOSED INTERSECTORAL MODEL
Public health surveillance systems and organizational culture within public health tend to assume natural causes, as illustrated by the Rajneesh salmonella outbreak.14 On the other hand, relying on infectious diseases expertise within other agencies such as defense or law enforcement is not adequate, as the critical mass of expertise required is only present in the health sector.
As I have shown in my analysis of MERS-CoV, simple principles of epidemiology can be applied to analyze patterns as expected or unexpected, and to use this as a flag for potential deliberate release.7 However, a paradigm shift is required to utilize this science for flagging aberrant patterns. Although we have excellent surveillance systems in many countries, the question about natural or unnatural patterns of disease is not routinely asked in public health. When asked, it is rarely accepted and often vocally opposed.47
If systems incorporate aberrant pattern analysis as a flag for potential bioterrorism,7 this could then act as a referral point to security, law enforcement or defense sectors, who are far better equipped to investigate the possibility of deliberate release. In fact, the evidence required to prove bioterrorism cannot be acquired by health authorities. A laboratory isolation of a pathogen or an epidemiologic pattern cannot prove deliberate release—the best contribution that health authorities can make is early identification of aberrant patterns, and referral to law enforcement agencies. As illustrated by the Rajneesh salmonella case, the evidence for bioterrorism was finally obtained by a FBI raid on the Rajneesh laboratories and the finding of the identical outbreak strain in their cultures. Figure 2 shows a proposed model for incorporating pattern analysis into outbreak response, and for referral to law enforcement and security agencies of aberrant patterns, whereupon a collaborative approach between health and security agencies is required to investigate further.
FIGURE 2.
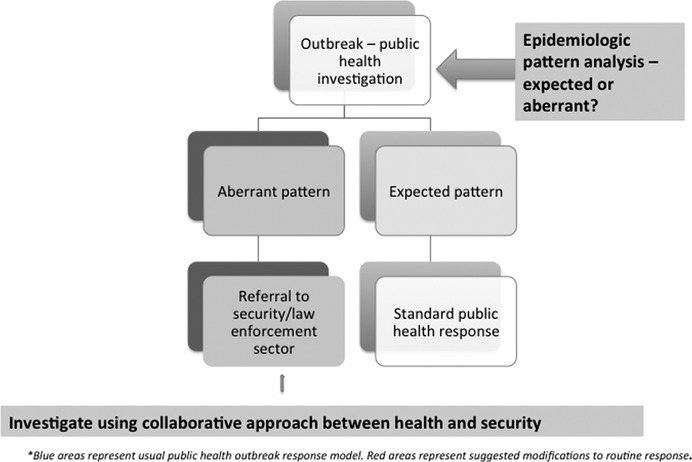
Proposed enhanced model for systematic consideration of unnatural causes of outbreaks in public health response*.
Other than incorporating pattern analysis into outbreak response as a reactive step, there is scope to develop proactive, automated algorithms for analyzing intelligence gathered on public infectious diseases surveillance websites and social media. Proactive, regular information sharing (such as operational level working groups) between sectors rather than reactive, situation-specific meetings of top-level committees would enhance biosecurity and confer the benefit of diverse perspectives on outbreak analysis.
Transforming our approach to bioterrorism across health and security sectors needs to be made from the grassroots and be incorporated into training programs. Risk analysis is very rarely used in public health, but could be incorporated into public health and epidemiology training. It may also be useful to have cross-sectoral training programs, where members of defense, police, and intelligence sectors spend time training in public health organizations and vice versa, as no other sector can replicate the critical mass of expertise in health. Placements in other agencies for training (such as defense and police trainees being placed in public health organizations and vice versa), will enhance mutual understanding and build enduring links to improve biosecurity. Vertical biosecurity programs in each sector are ineffective because the level of expertise required to deal with the public health aspects of bioterrorism is only available in large public health agencies.
CHALLENGES FOR BIOSECURITY INTO THE FUTURE
The category A-B-C framework for bioterrorism was developed long before the problem of DURC, and reflect predominant concerns in the cold war era around the Soviet Bioweapons program.23 Use of the A-B-C framework often assumes that perpetrators will be identified hostile states or terrorist groups. It also often assumes an ideological, political or religious motive, when the example of the insider threat shows that many other motives, including personal gain, may be at play. An important question to consider, particularly when reflecting on the insider threat and lessons from notable cases, is “Who profits?” Infectious disease is an industry, with many vested interest groups who could potentially profit from outbreaks. During an infectious diseases epidemic (such as Ebola), health agencies, pharmaceutical companies, researchers and other stakeholders, despite assumed good intentions, all stand to gain from the generation of business around the epidemic. It is naïve to assume that all stakeholders in an infectious diseases emergency are driven universally by altruistic motives. For example, in the Ivins case, which remains unresolved, he shot to prominence as an expert initially assisting the FBI in their investigation of the 2001 anthrax letter bombs in the United States, and had patents on anthrax vaccines. Human nature is universal, and the factors involved in the insider threat as described for crime, apply equally to bioterrorism.19,20 It is timely to review simplistic assumptions about motives for bioterrorism, as well as the category A-B-C framework for bioterrorism preparedness, its relevance for current issues and threats, and to preparedness policy.
In terms of models of biopreparedness, the United States takes a more integrated approach than many other countries, combining human, animal and agricultural threats, as well as man-made and natural threats. The current administration has adopted a preventive approach including soft diplomacy,48 supported by a national CDC with operational response capacity, and numerous expert committees.49 However, the underlying assumption remains that bioterrorism will be recognized as such, and that perpetrators will be hostile states or terrorist groups.
The concept of global health security was first introduced in 2001,50 and relaunched in 2014 as the “Global Health Security agenda” led by the U.S. CDC.51 The focus of this initiative is on improving international disease surveillance and response on the basis of IHR.12 The IHR focus on disease surveillance, detection, notification, reporting, response, and containment is health centric, with minimal recognition of other stakeholders or the need for aberrant pattern analysis. The IHR is also underpinned by the assumption of predominantly natural threats, and does not identify bioterrorism as a major threat. It is also somewhat ironic that following the launch of the Global Health Security initiative that the U.S. CDC was embroiled in a series of serious safety breaches involving anthrax, avian influenza, and Ebola.38–40
Legislative frameworks for public safety from bioterrorism may also need to be reviewed. It is sobering to reflect that in the Rajneesh case, the salmonella cultures were seized 12 months after the event, and that the Ivins investigation took almost 7 years. What if Rajneesh had been harboring smallpox, instead of Salmonella, in his laboratory? A pandemic or epidemic of a lethal infection would take days to weeks to spread around the world. It poses an unacceptably high risk to society if suspects are in possession of dangerous pathogens (such as Ebola, smallpox, or avian influenza) while investigations drag on for years. Given the reality of the insider threat and the demonstrated resistance of the scientific community to investigation of their own,18 traditional law enforcement approaches may not be suitable for investigating scientists suspected of bioterrorism. The insider threat must be acknowledged as a serious one in the era of DURC, and the rights of scientists should not outweigh the rights of the public if there is a risk of harm. It may be necessary to consider legislation which allows urgent intervention and precedence of the rights of the community over individual rights for suspected bioterrorism.
Finally, it is clear that systems, thinking, training in public health, legislation, and policies are lagging far behind momentous changes in science in the context of game-changing advances in DURC. The insider threat, conflict of interest, and ethical considerations in dual-use research are poorly addressed, with the debate to date heavily weighing in favor of the rights of scientists.46 These issues are not the exclusive domain of the health and medical research sectors—all sectors, including the community, health, science, law enforcement, and defense agencies need an equal seat at the table when making critical decisions about biosecurity. There is also a need for revision of human research ethics guidelines around dual-use research, as decisions made in 1 country may impact the rest of the world, and individuals who were not consulted nor consented to the research may be harmed by DURC.46
Bioterrorism is difficult to recognize, particularly if caused by pathogens that may also occur naturally. Current paradigms of epidemic response do not routinely consider unnatural causes of outbreaks, and need to be reformed. The critical mass and skills required to respond to natural or unnatural epidemics cannot be replicated in nonhealth sectors, necessitating better collaboration between sectors. The ability to respond rapidly to bioterrorism threats once recognized, is further hampered by inadequate legislative frameworks. These problems combine to create major vulnerability in biosecurity within the current environment. Approaches to reducing risk include cross-sectoral training and capacity building in threat recognition, mechanisms for routine referral of aberrant epidemic patterns from public health agencies to law enforcement, better governance of DURC, awareness, and management of the insider threat, use of risk analysis calculations for regulating DURC, and improved legislation to allow rapid intervention for suspected bioterrorism. A quantum change in approach, collective, informed, transparent decision-making, and integrated cross-sectoral models of operation are required to improve global biopreparedness in a dangerous new era.
ACKNOWLEDGMENT
This study was funded by University of New South Wales, Australia.
Footnotes
This article was presented by CR MacIntyre (Australia's Biosecurity–Will Inter-Sectoral Co-operation Make a Difference?), National Security Australia Conference, Sydney, Australia May 26, 2014.
REFERENCES
- 1. World Health Organization Ebola Virus Disease in Guinea. Geneva: World Health Organization, 2014. Available at http://www.afro.who.int/en/clusters-a-programmes/dpc/epidemic-a-pandemic-alert-and-response/outbreak-news/4063-ebola-hemorrhagic-fever-in-guinea.html; accessed August 23, 2014. [Google Scholar]
- 2. Osterholm M. What we need to fight Ebola. The Washington Post. 2014. Available at http://www.washingtonpost.com/opinions/what-we-need-to-fight-ebola/2014/08/01/41f4dbb8-182d-11e4-9349-84d4a85be981_story.html; accessed August 23, 2014.
- 3. Krever M. Scientist Who Discovered Ebola: “This is unprecedented”. Amanpour CNN; 2014. Available at http://amanpour.blogs.cnn.com/2014/07/02/scientist-who-discovered-ebola-this-is-unprecedented; accessed August 23, 2014.
- 4. MacIntyre CR, Chughtai AA, Seale H, Richards GA, Davidson PM. Uncertainty, risk analysis and change for Ebola personal protective equipment guidelines. Int J Nurs Stud 2015; 52(5): 899–903. doi: 10.1016/j.ijnurstu.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nossiter A. Fear of Ebola breeds a terror of physicians. The New York Times. 2014. Available at http://www.nytimes.com/2014/07/28/world/africa/ebola-epidemic-west-africa-guinea.html?_r=0; accessed August 23, 2014.
- 6. Diarra A, Hussain M. Mob attacks Ebola treatment centre in Guinea, suspected cases reach Mali. Reuters. July27, 2014. Available at http://www.reuters.com/article/2014/04/04/us-guinea-ebola-mali-idUSBREA331ZP20140404; Accessed August 23, 2014.
- 7. MacIntyre CR. The discrepant epidemiology of Middle East respiratory syndrome coronavirus (MERS-CoV). Environ Syst Decis 2014; 34(3): 383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bui C, Bethmont A, Chugtai A, et al. A systematic review of the comparative epidemiology of avian and human influenza A H5N1 and H7N9–lessons and unanswered questions. Transbound Emerg Dis 2015. doi: 10.1111/tbed.12327. (Epub ahead of print January 29) [DOI] [PubMed]
- 9. Hennekens CH, Buring JE. Epidemiology in Medicine, Ed 1 Little Brown and Co, 1987. Reprinted by Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 10. Anderson R, May R. Infectious diseases of humans. Dynamics and control. Norfolk, Great Britain, Oxford University Press, 2008. [Google Scholar]
- 11. US Centers for Disease Control and Prevention Epidemic Intelligence Service (EIS). Available at http://www.cdc.gov/EIS/; accessed July 15, 2014.
- 12. World Health Organization International health regulations. 2005. Available at http://www.who.int/topics/international_health_regulations/en/; accessed August 23, 2014.
- 13. Flynn D. Salmonella bioterrorism: 25 years later. Food Safety News. Available at http://www.foodsafetynews.com/2009/10/for-the-first-12/; accessed June 2014.
- 14. Török TJ, Tauxe RV, Wise RP, et al. A large community outbreak of salmonellosis caused by intentional contamination of restaurant salad bars. JAMA 1997; 278(5): 389–95. [DOI] [PubMed] [Google Scholar]
- 15. Graham B, Talent J, Allison G, et al. World at risk. The Report of US Commission on the Prevention of Weapons of Mass Destruction Proliferation and Terrorism. Ed 1 New York, Vintage Books, Random House, 2008. [Google Scholar]
- 16. Bhattacharjee Y. FBI closes anthrax case, says Bruce Ivins was sole culprit behind letter attacks. Science Insider February2010. Available at http://news.sciencemag.org/policy/2010/02/fbi-closes-anthrax-case-says-bruce-ivins-was-sole-culprit-behind-letter-attacks; accessed August 23, 2014.
- 17. Tanne JH. Infectious diseases expert convicted over missing plague bacteria. BMJ 2003; 327(7427): 1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Genougreh WB. Update on Dr. Thomas Butler. Clin Infect Dis 2006; 43(2): 259–60. [DOI] [PubMed] [Google Scholar]
- 19. Federal Bureau of Investigation FBI counterintelligence: the insider threat. An introduction to detecting and deterring an insider spy. 2014. Available at http://www.fbi.gov/about-us/investigate/counterintelligence/the-insider-threat; accessed June 2014.
- 20. National Cybersecurity and Communications Integration Center Combating the insider threat. Washington, DC, US Department of Homeland Security, 2014. Available at https://www.us-cert.gov/sites/default/files/publications/Combating%20the%20Insider%20Threat_0.pdf; accessed August 23, 2014. [Google Scholar]
- 21. National Research Council Biotechnology Research in an Age of Terrorism. Washington, DC, The National Academies Press, 2004. [PubMed] [Google Scholar]
- 22. Samuel G, Selgelid M, Kerridge I. Managing the unimaginable. Regulatory responses to the challenges posed by synthetic biology and synthetic genomics. EMBO Rep 2009; 10(1): 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alibek K, Handelman S. Biohazard: The Chilling True Story of the Largest Covert Biological Weapons Program in the World: Told from Inside by the Man Who Ran it, reprint ed New York, Dell Publishing, 1999. [Google Scholar]
- 24. Kerkhove V. Brief literature review for the WHO global influenza research agenda-highly pathogenic avian influenza H5N1 risk in humans. Influenza Other Respir Viruses 2013; 7(Suppl 2): 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization Cumulative number of confirmed human cases for avian influenza A (H5N1) reported to WHO, 2003–2014, 2014. Available at http://www.who.int/influenza/human_animal_interface/EN_GIP_20140124CumulativeNumberH5N1cases.pdf; accessed August 23, 2014.
- 26. World Health Organization Pandemic Influenza Preparedness and Response: A WHO Guidance Document. Geneva, 2009. Available at http://www.who.int/influenza/resources/documents/pandemic_guidance_04_2009/en/; accessed August 23, 2014. [PubMed] [Google Scholar]
- 27. Fouchier RAM, Garcia-Sastre A, Kawaoka Y. Pause on avian flu transmission studies. Nature 2012; 481(7382): 443. [DOI] [PubMed] [Google Scholar]
- 28. Prominent Virologists Want U.S. Advisory board to take a second look at controversial flu papers. Science Insider 2012. Available at http://news.sciencemag.org/people-events/2012/01/prominent-virologists-want-u.s.-advisory-board-take-second-look-controversial?ref=ra; accessed August 23, 2014.
- 29. Osterholm MT, Henderson DA. Life sciences at a crossroads: respiratory transmissible H5N1. Science 2012; 335(6070): 801–2. [DOI] [PubMed] [Google Scholar]
- 30. National Institutes of Health Biosecurity: National Science Advisory Board for Biosecurity (NSABB). 2014. Available at http://osp.od.nih.gov/office-biotechnology-activities/biosecurity/nsabb; accessed July 15, 2014.
- 31. Malakoff D. NSABB reverses position on flu papers. Science Insider. 2012. Available at http://news.sciencemag.org/people-events/2012/03/breaking-news-nsabb-reverses-position-flu-papers; accessed August 23, 2014.
- 32. Herfst S, Schrauwen EJA, Linster M, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012; 336(6088): 1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Imai M, Watanabe T, Hatta M, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012; 486(7403): 420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maher B. Bias accusation rattles US biosecurity board. Nature 2012. Available at http://www.nature.com/news/bias-accusation-rattles-us-biosecurity-board-1.10454?WT.ec_id=NEWS-20120417; accessed August 23, 2014.
- 35. Watanabe T, Zhong G, Russell Colin A, et al. Circulating avian influenza viruses closely related to the 1918 virus have pandemic potential. Cell Host Microbe 2014; 15(6): 692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cui L, Liu D, Shi W, et al. Dynamic reassortments and genetic heterogeneity of the human-infecting influenza A (H7N9) virus. Nat Commun 2014; 5: 3142. [DOI] [PubMed] [Google Scholar]
- 37. McCoy T. Was it ‘crazy’ for this scientist to re-create a bird flu virus that killed 50 million people? The Washington Post 2014. Available at http://www.washingtonpost.com/news/morning-mix/wp/2014/06/12/good-idea-or-crazy-re-creating-a-bird-flu-virus-that-once-killed-50-million-people/?tid=hp_mmcrazy; accessed August 23, 2014.
- 38. Kaiser J. Lab incidents lead to safety crackdown at CDC. Science Insider 2014. Available at http://news.sciencemag.org/biology/2014/07/lab-incidents-lead-safety-crackdown-cdc; accessed August 23, 2014.
- 39. Schnirring L. More problems shutter CDC labs, prompt review, July 11, 2014. 2014. Available at http://www.cidrap.umn.edu/news-perspective/2014/07/more-problems-shutter-cdc-labs-prompt-review2014; accessed August 23, 2014.
- 40. Sun LH, Achenbach J. CDC reports potential Ebola exposure in Atlanta lab. The Washington Post 2014. Available at http://www.washingtonpost.com/national/health-science/cdc-reports-potential-ebola-exposure-in-atlanta-lab/2014/12/24/f1a9f26c-8b8e-11e4-8ff4-fb93129c9c8b_story.html?postshare=7491419455093730; accessed January 8, 2015.
- 41. Reardon S. Safety lapses in US government labs spark debate. Nature News 2014. Available at http://www.nature.com/news/safety-lapses-in-us-government-labs-spark-debate-1.15570; accessed August 23, 2014.
- 42. Lipsitch M, Inglesby TV. Moratorium on research intended to create novel potential pandemic pathogens. mBio 2014; 5(6): e02366–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaiser J. NIH moving ahead with review of risky virology studies. Science Insider February2015. Available at http://news.sciencemag.org/biology/2015/02/nih-moving-ahead-review-risky-virology-studies.
- 44. The National Academies Institute of Medicine (US) Committee. Conflict of Interest in Medical Research, Education, and Practice. Washington, DC, National Academies Press (US), 2009. [PubMed] [Google Scholar]
- 45. Shuster E. Fifty years later: the significance of the Nuremberg code. N Engl J Med 1997; 337(20): 1436–40. [DOI] [PubMed] [Google Scholar]
- 46. MacIntyre CR. Re-thinking the ethics of dual-use research of concern on transmissible pathogens. Environ Sys Decis 2015; 35(1): 129–32. [Google Scholar]
- 47. Mackay I, Murillo L, Majumder M, Evans N, Goldstein S. Middle East respiratory virus came from camels, not terrorists. The Conversation. 2014. Available at http://theconversation.com/middle-east-respiratory-virus-came-from-camels-not-terrorists-29772; accessed August 23, 2014.
- 48. Koblentz GD. From biodefence to biosecurity: the Obama administration's strategy for countering biological threats. Int Aff 2012; 88(1): 131–48. [DOI] [PubMed] [Google Scholar]
- 49. National Security Council National Strategy for countering biological threats. Washington, DC, The White House, 2009. [Google Scholar]
- 50. Anonymous Global Health Security Initiative. 2001. Available at http://www.ghsi.ca/english/index.asp2014; accessed August 23, 2014.
- 51. US Centers for Disease Control and Prevention Keeping the world safe and secure from infectious disease threats. 2014. Available at http://www.cdc.gov/globalhealth/security/; accessed July 15, 2014.


