Abstract
Background
With increasing international travel and historically high numbers of residents visiting friends and relatives overseas, travel-associated illnesses are frequent in Marseille, France. We report the changing epidemiology of travel-related illnesses over a 12-year period.
Methods
A single site GeoSentinel surveillance analysis was undertaken for 3460 ill returned travellers presenting to two public hospitals in Marseille, France from March 2003 to October 2015, with travel-related illnesses. Demographic characteristics, travel history, presenting symptoms and information on pre-travel consultations were collected.
Results
There was a predominance of travel to sub-Saharan Africa, in particular to Comoros archipelago. Tourism was the main reason for travel (1591/3460, 46%), followed by visiting friends or relatives (VFR) (895/3460, 26%), with a mean duration of 29 days; 35% (1212/3460) of travellers reported a pre-travel health consultation. The most common syndromic diagnoses were febrile systemic illness (1343, 39%), dermatologic (716, 21%), gastrointestinal (340, 10%) and respiratory/ear–nose–throat (331, ENT) (10%). Hospitalization rates were highest amongst travellers from sub-Saharan Africa (858/ 1632, 53%), and VFR (573/ 895, 64%, P < 0.001). Frequent diagnoses included malaria (797, 23%), dengue (96, 2.77%) and chikungunya (75, 2.17%), reflecting global trends. Comparison of two periods (2003–10 to 2011–15) demonstrated an increase in chikungunya and decrease in malaria and influenza-like illness. We report an increase in ill travellers from the Caribbean, Middle East and South-East Asia.
Conclusion
Surveillance of travellers provides relevant sentinel information on the changing epidemiology of infectious diseases across the globe, most notably for malaria, dengue and chikungunya. We demonstrate the use of travel surveillance in improving pre-travel consultation needs and to address autochthonous vector-borne viral risks.
Keywords: Travel medicine, sentinel surveillance, malaria
Background
International travel is increasing, with more than 23 million visits overseas by French tourists in 2015, compared to over 84 million international tourists arriving in France over the same period.1 Marseille, the main town in Mediterranean France, includes an urban and peri-urban population of over 1.5 million inhabitants. The economy of Marseille and its region is still partly linked to its commercial port and has always been one of the main gateways into France.2 Migrants, originating notably from North Africa, Senegal and Comoros, have settled in Marseille over several decades.2,3 Considering this context, travel-associated diseases are frequent in the local population.
We aim to report surveillance data to document the spectrum of infections in patients admitted for travel-related illness over a 12-year period to two referral centres in Marseille, in order to compare with other international data on ill returning travellers and to inform public health surveillance and local prevention practices.
Material and Method
Study design and procedures
The GeoSentinel Surveillance Network is a global clinic based surveillance system to track travel-related illnesses within 66 clinics in 29 countries.4 Physicians in travel/tropical medicine electronically submit demographic, clinical and diagnostic information on patients seeking medical care for a travel-related illness having crossed an international border within the past 10 years.5 Every patient has at least one confirmed or probable diagnostic code, and may be assigned several. Diagnoses are based on recognition of a syndrome and/or a specific pathogen. Other data includes travel characteristics, in/outpatient status, and whether a pre-travel consultation took place.
We report all patients recorded on the GeoSentinel database in Marseille over a 12-year period. Eligible patients had been referred to two hospitals in Marseille, ‘Assistance Publique—Hôpitaux de Marseille’ from March 2003 to October 2015 and ‘Hôpital d’Instruction des Armées Laveran’ from May 2007 to October 2015.
Statistical analysis
Two time periods of interest were compared, 2003–10 and 2011–15. All summary descriptive statistics were carried out using Microsoft Access and R statistical software program. Continuous variables were described with mean and SD or median and IQR as appropriate. Categorical variables were described with numbers and percent. Unpaired t-tests or Wilcoxon signed rank test was used to compare continuous quantitative variables, depending on the distribution. Non-parametric Fisher tests were used to compare qualitative variables. Due to multiple tests being performed a conservative two-sided significance level of P < 0.01 was chosen. Time series analysis of malaria cases (the most frequent diagnosis) was performed and multiplicative decomposition of monthly data was used to examine the trend, after removing seasonality and random error.
Results
A total of 3460 patients were included during the 12-year study period with a M/F sex ratio of 1.2 and a median age of 38 years (range: 1–86 years) (Table 1). Tourism was the main reason for travel (1591/3460, 46%), followed by visiting friends or relatives (VFR) (895, 26%). The mean travel duration was 29 days and 35% (1212) of all travellers reported a pre-travel health consultation. Among 1931 travellers (56%) to Africa, 557 (16%) travelled to the Comoros archipelago alone, 1632 to sub-Saharan Africa, and 299 (9%) to North Africa. Only 634 (18%) travelled to Asia, 407 (12%) to Latin America and 136 (4%) to Europe (Figure 1). The majority of patients were seen after travel (3264, 94%) and 38% (1326) were hospitalized (Table 1). No deaths were recorded. The percentage of hospitalized patients was highest amongst travellers to sub-Saharan Africa (858/ 1632, 53%), with similar rates seen in the Middle East (41/75, 55%, P = 0.8) and North America (42/66, 64%, P = 0.1), but much lower rates when compared to travellers from Asia (122/ 634, P < 0.001), Central America (46/ 202, P < 0.001) and Europe (35/ 136, P < 0.001). Higher proportions of patients visiting friends and family (573/ 895, 64%) were hospitalized, in comparison to tourists (409/ 1591, 26%, P < 0.001) or business travellers (163/488, 33%, P <0.001). The majority of VFRs had visited sub-Saharan Africa (689/895, 77%) and did not report any pre-travel consultation (561/895, 63%).
Table 1.
Overall patient demographics and travel characteristics (n = 3460), and comparisons between two periods (2003–10 and 2011–15)
| N (%) | 2003–10, n = 2144 | 2011–15, n = 1316 | |
|---|---|---|---|
| Gender | |||
| Male | 1852 (53.53) | 1158 (54.01) | 694 (52.74) |
| Female | 1598 (46.18) | 977 (45.57) | 621 (47.19) |
| Not documented | 10 (0.29) | 9 (0.42) | 1 (0.08) |
| Median age in years (IQR) | 38 (27–52) | 39 (27–51)* | 40 (28–54)* |
| Country of birth | |||
| France | 2324 (67.17%) | 1369 (63.85) | 955 (72.57) |
| Reason for travel | |||
| Tourism | 1591 (45.98) | 958 (44.68) | 633 (48.10) |
| Visiting friends and relatives | 895 (25.87) | 599 (27.94)* | 296 (22.49)* |
| Business | 488 (14.1) | 286 (13.34) | 202 (15.35) |
| Missionary/volunteer/researcher/aid work | 197 (5.69) | 128 (5.97) | 69 (5.24) |
| Military | 122 (3.53) | 67 (3.12) | 55 (4.18) |
| Immigration | 68 (2.91) | 34 (1.59) | 17 (1.29) |
| Student | 60 (1.74) | 48 (2.24)* | 12 (0.91)* |
| Medical tourism | 34 (0.98) | 19 (0.89) | 13 (0.99) |
| Not documented | 5 (0.14) | 5 (0.23) | 0 |
| Travel duration (median, IQR) | 29 (15–61) | 29 (15–61) | 27 (15–60) |
| Pre-travel encounter | |||
| Yes | 1212 (35.03) | 782 (36.47) | 430 (32.67) |
| No | 1672 (48.32) | 999 (46.6) | 673 (51.14) |
| Don’t know | 576 (16.65) | 327 (15.25) | 213 (16.19) |
| Clinical setting | |||
| Immigration travel only | 68 (1.96) | 34 (1.59) | 17 (1.29) |
| Seen during travel | 126 (3.64) | 86 (4.01) | 40 (3.04) |
| Seen after travel | 3264 (94.34) | 2022 (94.31) | 1242 (94.38) |
| Not documented | 2 (0.06) | 2 (0.09) | 0 |
| Patient type | |||
| Inpatient | 1326 (38.32) | 908 (42.35)* | 418 (31.76)* |
| Outpatient | 2124 (61.39) | 1226 (57.18)* | 898 (68.24)* |
| Not documented | 10 (0.29) | 10 (0.47) | 0 |
| Region of exposure | |||
| Australia/New Zealand | 6 (0.17) | 6 (0.28) | 0 (0) |
| Caribbean | 146 (4.22) | 71 (3.31)* | 75 (5.7)* |
| Central America | 56 (1.62) | 35 (1.63) | 21 (1.6) |
| Eastern Europe | 40 (1.16) | 21 (0.98) | 19 (1.44) |
| Middle East | 75 (2.17) | 26 (1.21)* | 49 (3.72)* |
| North Africa | 299 (8.64) | 168 (7.84) | 131 (9.95) |
| North America | 66 (1.91) | 56 (2.61)* | 10 (0.76)* |
| North East Asia | 43 (1.24) | 31 (1.45) | 12 (0.91) |
| Oceania | 35 (1.01) | 15 (0.7) | 20 (1.52) |
| South America | 205 (5.92) | 123 (5.74) | 82 (6.23) |
| South Central Asia | 164 (4.74) | 102 (4.76) | 62 (4.71) |
| South East Asia | 427 (12.34) | 204 (9.51)* | 223 (16.95)* |
| Sub-Saharan Africa | 1632 (47.17) | 1129 (52.66)* | 503 (38.22)* |
| Western Europe | 96 (2.77) | 69 (3.22) | 27 (2.05) |
| Not ascertainable | 170 (4.91) | 88 (4.1)* | 82 (6.23)* |
| Presenting syndromes | |||
| Fever | 1343 (38.82) | 903 (42.12) | 440 (33.43)* |
| Respiratory/ENT | 331 (9.57) | 202 (9.42) | 129 (9.80) |
| Gastrointestinal (except acute diarrhoea) | 340 (9.83) | 173 (8.07) | 167 (12.69)* |
| Acute diarrhoea | 267 (7.72) | 203 (9.47) | 64 (4.86)* |
| Dermatological | 716 (20.69) | 447 (20.85) | 269 (20.44) |
| Etiological conditions | |||
| Malaria | 797 (23.03) | 607 (28.31)* | 190 (14.44)* |
| Dengue | 96 (2.77) | 58 (2.71) | 38 (2.89) |
| Chikungunya | 75 (2.17) | 29 (1.35)* | 46 (3.50)* |
| ILI | 148 (4.28) | 109 (5.08)* | 39 (2.96)* |
| Giardia | 49 (1.42) | 33 (1.54) | 16 (1.22) |
*Significant difference, at the alpha 0.01 level.
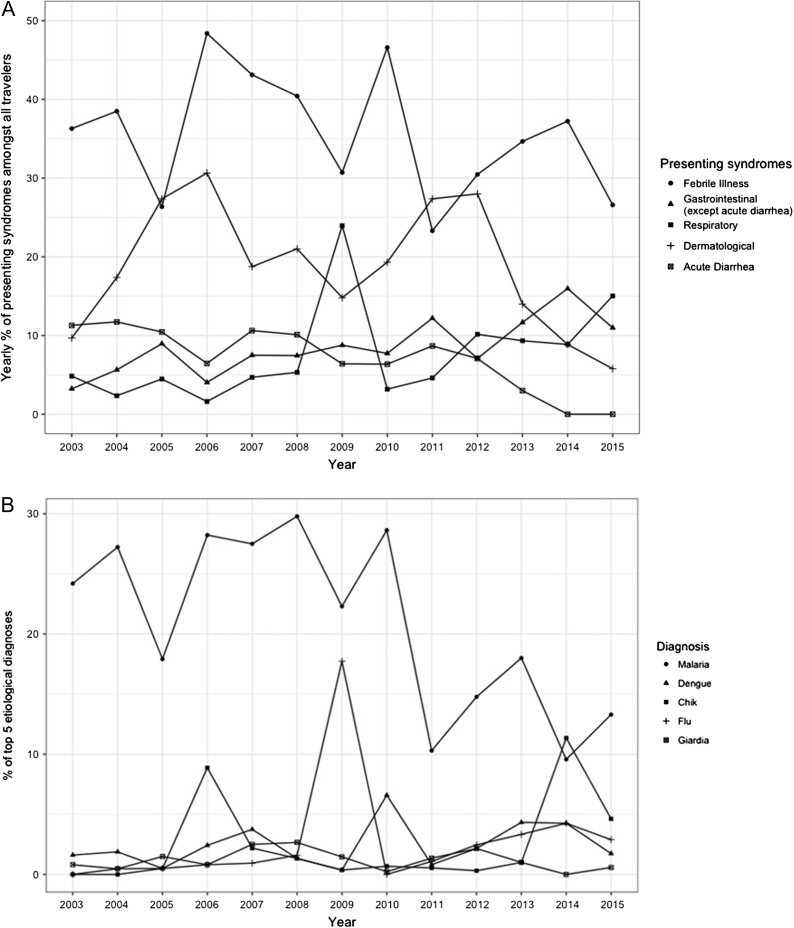
Figure 1.
(A) Main rates of presenting syndromes by year 2003–2015 in travellers returning to Marseille. (B) Main rates of etiological diagnosis for Malaria, Dengue, Chikungunya, Influenza-like illness and Giardia by years 2003–15 in travellers returning to Marseille.
Comparing patient characteristics between the two periods (2003–10 and 2011–15), there was a significant difference in the age of travellers, with an increase in the median age from 37 to 40. There was a significant decline in VFR as the reason for travel (599/2144, 27.94% to 296/1316, 22.49%). There was a decrease in hospitalization rates between the two periods (908/2144, 42.35% to 418/1316, 31.76%). Travel trends between the two periods differed, with increasing travel to the Caribbean (71/2144, 3.31% to 75/1316, 5.7%), the Middle East (26/2144, 1.21% to 49/1316, 3.72%), South East Asia (204/2144, 9.51% to 223/1316, 16.95%). Declining rates of travellers were seen from North America and sub-Saharan Africa (Table 1).
The most common syndromic diagnoses were febrile systemic illness (1343/3460, 39%), dermatologic (716, 21%), gastrointestinal (340, 10%) and respiratory/ear–nose–throat (331, ENT) (10%) (Figure 2A). A large number of respiratory infections (148/331, 44.7%) and acute diarrhoeal cases were undocumented (105 / 267, 39.3%). In comparison, the proportion of fever cases without an etiological cause was lower (209/1356, 15.6%). Unspecified skin infections and arthropod bites accounted respectively for 17.2% (n = 125) and 7.4% (n = 54) of dermatological presentations. Influenza-like illness (ILI), pneumonia, bronchitis and unspecified pharyngitis accounted respectively for 44.7% (n = 148), 29.6% (n = 98), 8.2% (n = 27) and 5.1% (n = 17) of respiratory and ENT presentations. Confirmed influenza cases included 28 influenza A, 12 H1N1 and eight influenza B. Overall, 49 patients with acute diarrhoea had confirmed25 or suspected24 Giardia infection.
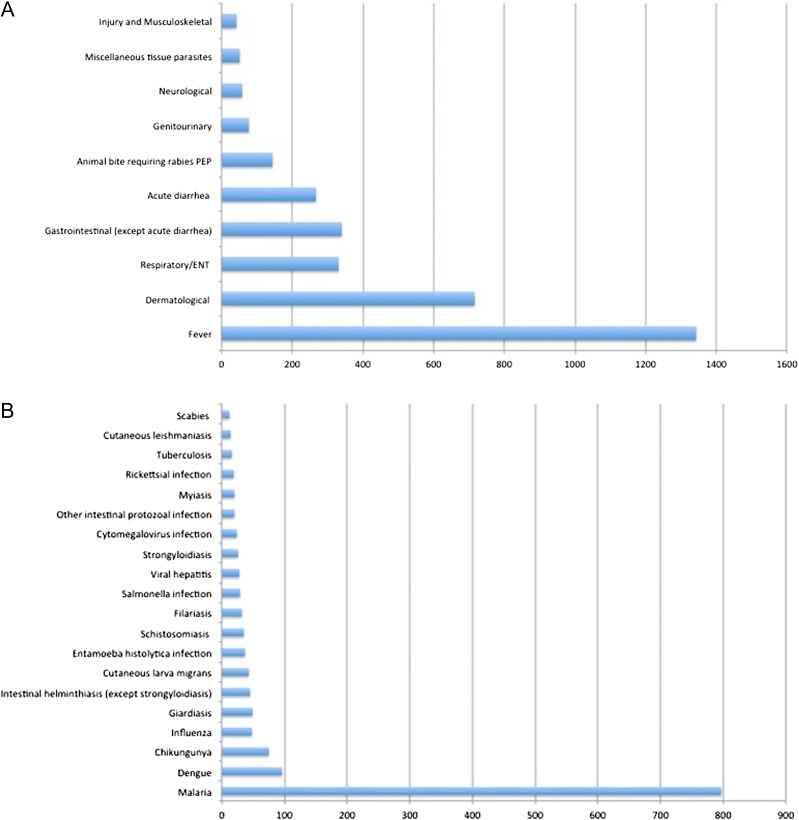
Figure 2.
(A) Top 10 syndromic diagnoses (n = 3369 of 3794 diagnoses). (B) Top 20 diagnosis with etiological agent identified (n = 1465). Further information: Dengue (uncomplicated, 94, severe 2), Influenza (28 confirmed cases of influenza A, 12 H1N1 and eight influenza B), intestinal helminthic infections (Ascaris, 2; Pinworm, 1; Heterophyes, 1; hookworm, 12; tapeworm, 4; whipworm, 2; unspecified, 23), schistosomiasis (S. haematobium; 9, S. mansoni, 15, species unknown, 11), filariasis (Bancroft, 1; loiasis, 26, other, 1; species unknown, 4), viral hepatitis (hepatitis A acute, 13; hepatitis B carrier, 1; hepatitis B acute, 2; hepatitis B chronic, 2, hepatitis C chronic, 3, hepatitis E, 7), Salmonella infections (S. paratyphi, 8; S. typhi, 8, other, 13), tuberculosis (pulmonary, 13; extrapulmonary, 3; MDR or XDR, 5), rickettsial infection (Rickettsia orientia, 1; tickborne spotted fever, 12; murine typhus, 2, other, 4), other intestinal protozoal infections (amoebas other than Entamoeba histolytica, 7; Blastocystis, 5; Dientamoeba, 2; Isospora, 1; other (no further precision), 5).
The top 20 etiological diagnoses are presented in Figure 2B. Parasitic infections were largely predominant and disproportionately dominated by malaria cases followed distantly by intestinal protozoal and helminthic infections, skin parasitic infections (cutaneous larva migrans, filariasis, myasis, leishmaniasis and scabies) and schistosomiasis. Arthropod-borne viral infections including dengue and chikungunya were also frequent. Influenza virus was the most frequently identified pathogen responsible for respiratory tract infections, followed by tuberculosis. Viral hepatitis, salmonella and cytomegalovirus infections were also among the most common diagnoses. In total, 19 rickettsial infections were identified (Figure 3).
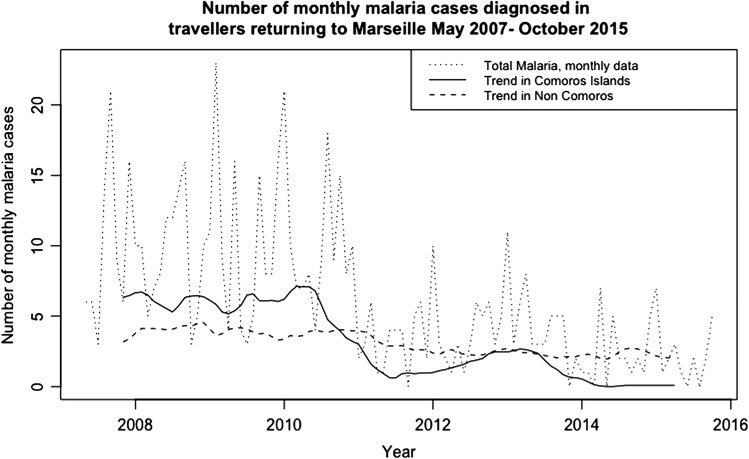
Figure 3.
Number of monthly malaria cases by region of exposure. Black dotted line: monthly number of total malaria cases reported; red line: overall trend after multiplicative decomposition in Comoros; green line: overall trend after multiplicative decomposition in regions of exposure other than the Comoros.
Less frequent etiological diagnoses included bacterial infections (n = 60), parasitic and fungal infections (n = 32) and viral infections (n = 26) (Table 2).
Table 3.
Characteristics of the five main etiological diagnoses
| Malaria (N = 797) | Dengue (N = 96) | Chikungunya (N = 75) | Influenza-like illness (N = 148) | Giardia (N = 49) | |
|---|---|---|---|---|---|
| Male (%) | 522 (65.5) | 49 (51.04) | 35 (46.67) | 71 (47.97) | 24 (48.98) |
| Median age (IQR) | 26 (17–36) | 19 (13–29.5) | 23 (12.25–31.75) | 32 (1–62) | 11 (5–27) |
| French born | 254 (31.87%) | 79 (82.29) | 67 (89.33) | 102 (68.92) | 46 (93.88) |
| Top 3 reason for travel, N (%) | Visiting Friends and | Tourism 60 (62.5) | Tourism 45 (60.00) | Tourism 108 (72.97) | Tourism 21 (42.86) |
| Relatives 474 (59.47) | Visiting Friends and | Business 15 (20) | Visiting Friends and | Business 11 (22.45) | |
| Business 109 (13.67) | Relatives 16 (16.67) | Visiting Friends and | Relatives 19 (12.84) | Student = 7 (14.29) | |
| Military 68 (8.53) | Business 13 (13.54) | Relatives 8 (10.67) | Business 17 (11.49) | Missionary/volunteer/research/aid work = 7 (14.29) | |
| Top 3 regions of exposure, N (%) | Sub-Saharan Africa 729 (91.47) | Asia 34 (35.42) | Caribbean 33 (44) | North America 36 (24.32) | Sub-Saharan Africa 16 (32.65) |
| South America 51 (6.4) | Caribbean 24 (25) | Sub-Saharan Africa 29 (38.67) | Western Europe 25 (16.89) | Asia 15 (30.61) | |
| Asia 6 (0.75) | Sub-Saharan Africa 21 (21.88) | Asia 10 (13.33) | Sub-Saharan Africa 22 (14.86) | South America 6 (12.24) | |
| Top 5 countries of exposure, N (%) | Comoros 411 (51.57) | Guadeloupe 15 (15.62) | Martinique 17 (22.67) | United States 35 (23.65) | India 10 (20.41) |
| Côte d’Ivoire 66 (8.28) | Indonesia 11 (11.46) | Reunion 17 (22.67) | Saudi Arabia 21 (14.19) | Madagascar 5 (10.20) | |
| French Guana 44 (5.52) | Thailand 11 (11.46) | Guadeloupe 11 (14.67) | Spain 14 (9.46) | Benin 3 (6.12) | |
| Cameroon 35 (4.39) | Comoros 9 (9.38) | Madagascar 4 (5.33) | Comoros 11 (7.43) | Burkina Faso 3 (6.12) | |
| Burkina Faso 33 (4.14) | Martinique 8 (8.33) | India/Indonesia/Mauritius/Saint Martin = 3 (4) | United Kingdom 9 (6.08) | China 3 (6.12) | |
| Indonesia 3 (6.12) | |||||
| Senegal 3 (6.12) | |||||
| Pre-travel encounter | No 409 (51.32) | No 51 (53.12) | No 53 (70.67) | No 59 (39.86) | No 12 (24.49) |
| Yes 305 (38.27) | Yes 29 (30.21) | Yes 16 (21.33) | Yes 57 (38.51) | Yes 23 (46.94) | |
| Don’t know (9.54) | Don’t know 16 (16.67) | Don’t know 6 (8) | Don’t know 32 (21.62) | Don’t know (26.53) |
Table 2.
Less frequent infections acquired by travellers, according to main presenting complaints and type of pathogen
| Presenting complaints | Bacterial infections | Parasitic and fungal infections | Viral infections |
|---|---|---|---|
| Respiratory/URTI |
|
||
| Gastrointestinal |
|
|
|
| Dermatological |
|
|
|
| STI |
|
|
|
| Neurological |
|
|
|
| Wide range of symptoms |
|
|
|
The most common etiological diagnoses in patients presenting with a systemic febrile illness (n = 1343) were malaria (797, 59.3%), dengue (96, 7.1%), chikungunya (75, 5.5%) and cytomegalovirus (24, 1.8%). Among 797 diagnoses of malaria, Plasmodium falciparum accounted for 603 (76%) cases, P. vivax for 82 (10%), P. ovale for 50 (6%) and P. malariae for 24 (3%), while 38 were unspecified. Severe malaria affected 33 of these patients. Most malaria cases were in migrant VFRs (474/797, 59.5%) and were acquired in sub-Saharan Africa (729, 91.5%), notably in the Comoros archipelago (411, 51.6%) (Table 3). Figure 3 compares the overall trends after multiplicative decomposition of monthly malaria cases in the Comoros archipelago and in other exposure regions.
A total of 96 dengue cases, including two severe cases, and 75 chikungunya cases were recorded. Most cases were in French-born tourists returning from Asia, the French Caribbean islands and Africa (Table 3). Most patients with malaria, dengue and chikungunya infections did not seek pre-travel advice (409, 51.32%; 51, 53.12% and 53, 70.67%, respectively).
Regarding the presenting syndromes and diagnoses between the two periods (2003–10 and 2011–15), there was a significant increase in the number of patients with gastrointestinal symptoms, apart from acute diarrhoea, (173/2144, 8.07% to 167/1316, 12.69%) with a decrease in fever (903/2144, 42.12% to 440/1316, 33.43%), and acute diarrhoeal presentations (203/2144, 9.47% to 64/1316, 4.86%) (Table 1). There was a significant decrease in patients with malaria (607/2144, 28.31% to 190/1316, 14.44%) and ILI (109/2144, 5.08% to 39/1316, 2.96%) between the two periods with a significant increase in the rates of chikungunya (29/2144, 1.25% to 46/1316, 3.50%).
Discussion
Many demographic characteristics reported here are comparable to global GeoSentinel surveillance studies amongst returning travellers from the developing world and in travellers returning to the USA. Similar rates in terms of sex, median age, travel duration and predominance for tourism as the reason of travel are reported.6,7 However, our results are characterized by the predominance of travellers returning from Africa, in comparison to Asia in a previous global GeoSentinel study.4 This can be explained by the local demographics in Marseille, particularly related to the migrant African population. Marseille is historically associated with Comoros migrants, with a large Comoros-originated population estimated at 50 000–70 000 in early 2000s, although more recent figures are unknown.3 This results in differences of traveller characteristics in comparison to other GeoSentinel sites, notably higher rates of VFRs, more frequent hospitalizations and low levels of pre-travel consultations. We report a disproportionately high rate of hospitalizations in patients from sub-Saharan Africa and amongst VFRs. This highlights the need to target our travel health prevention messages towards VFRs, a sub-group with known barriers to accessing pre-travel health advice.8,9 The African migrant population in Marseille may have specific barriers to pre-travel consultations, including financial constraints for malaria prophylaxis and lack of information. Potential strategies include primary care physicians routinely questioning immigrant patients about future travel plans.8
Sentinel surveillance amongst travellers can be used to collect important epidemiological and virological information to alert international authorities to the changing epidemiology of infections, especially in countries with limited active public health surveillance.10,11 This is demonstrated by the decline of malaria in the Comoros. We confirm that P. falciparum malaria remains the most frequent cause of febrile illness in international travellers returning to Marseille, particularly from sub-Saharan Africa. This is similar to both global studies4 and previous local studies in Marseille from 1999 to 2003.12 An overall decreasing trend in the number of monthly malaria cases can be noted from 2007 to 2015 (Figure 3). This is consistent with a global general decreasing trend since 2000.4 However, in our study, the sudden drop in 2011 is most likely due to a decrease in the numbers of cases acquired in the Comoros archipelago (Figure 3); decomposition of monthly non-Comoros malaria cases demonstrated a gradual decreasing trend in comparison to the sudden drop of cases in Comoros travellers from 2011. This is in line with local malaria surveillance data demonstrating a major decline in malaria morbidity and mortality in the Union of Comoros between 2010 and 2014, which is attributed to malaria prevention and control interventions.13 This is an example of a local single site analysis in Marseille providing accurate sentinel surveillance information for malaria epidemiology in Comoros. Alongside sentinel analysis for diagnosis, important information on rates of malarial drug resistance can also be collected from travellers.14
Our results highlight VFRs as disproportionately at risk for malaria, which corroborates recent French national data and from a London tropical hospital.15,16 Other at risk groups in our series were business travellers and military personnel, as previously observed.17–19
Other examples of the use of travellers as sentinels for public health surveillance includes the identification of dengue outbreaks in Luanda and Dar es Salaam11 and for the Indian Ocean and West Africa.20,21 Due to a large number of our patients returning from Africa, travellers returning to Marseille may be useful sentinels for future arboviral African outbreaks. Rapid testing for dengue is essential in patients presenting with a fever from Africa. Similarly, the geographical profile and number of travellers presenting with chikungunya in Marseille (Table 3 and Figure 1B) is a reflection of increasing global expansion, providing further examples of vital sentinel surveillance in travellers.22,23 As our survey ended in October 2015, we only report one case of Zika virus; however, this is now an important differential amongst febrile travellers.24 Marseille may be a vital sentinel site to document possible Zika epidemics on the African continent.25
Public health surveillance of these arboviral infections are of particular interest in the Mediterranean region, due to the local emergence of arthropod-borne diseases given the presence of the Aedes albopictus vector in the Mediterranean region and risk of autochthonous transmission, particularly around hospitals.26 Arthropod-borne diseases accounted for 68% of the top 20 diagnoses. Dengue was the second most common diagnosis during our study period, with 35% of patients from Asia, consistently with global data.4,7 Furthermore, we report a significant increase in chikungunya cases between the two periods. This high rate of imported arboviral diseases is likely to increase, given the trend of increasing numbers of unwell travellers returning from the Caribbean and South East Asia. This indicates the need for improved prevention strategies, both before travel, by reinforcing pre-travel advice toward prevention of insect bites and malaria chemoprophylaxis, and after, with improved epidemiological surveillance and healthcare professional training. Pre- and post-travel advice should also include advice on reducing respiratory transmission (such as local emergence of multi-resistant tuberculosis or Middle East Respiratory Syndrome (MERS) coronavirus), and improved hygiene practices to reduce nosocomial transmission of multi-resistant bacteria. Furthermore, improved diagnostic tools after travel, such as multiplex PCR, may help increase the number of etiological diagnoses amongst syndromic diarrhoeal and respiratory illnesses.
Another specificity of Marseille are the large numbers of Muslim Hajj pilgrims each year returning from Mecca.27 We demonstrate the increasing trend of travellers returning from the Middle East, demonstrating the importance of Marseille as a sentinel surveillance site to the Middle East. Over the last few years, the vast majority of travellers with respiratory symptoms after returning from the Middle East consulted because of a fear of contracting MERS.28 Due to a protocolized management procedure, patients returning from the Middle East (n = 75) were disproportionately hospitalized (41, 55%), resulting in a hospitalization bias.
Finally, the characteristics of sentinel surveillance in Marseille are also related to the specifics of the medical community. We house the national reference centre for rickettsioses, and so provide an opportunity to document high risk geographic areas for these emerging, underestimated infections.29 Similarly, the centre for rabies vaccination is situated in Marseille, providing sentinel surveillance on patients seeking rabies post-exposure prophylaxis. We highlight the potential for rabid animal exposure in travellers, notably in Asia and the need to reinforce pre-exposure prophylaxis.30
Previous studies local to Marseille have used a prospective cohort design, providing a denominator, amongst Hajj pilgrims31 and travellers to Senegal.32 This approach allows an estimation of risk of travel-associated diseases, including mild self-limiting conditions but is biased by traveller selection. Here, we use a different approach that describes the spectrum of diseases in patients returning from any foreign destinations and being referred to a specialized structure. Limitations include an important recruitment bias, with an underestimate of self-limiting conditions, a lack of denominator and disease prevalence estimates. However, it captures more severe and uncommon travel-related illnesses and provides vital sentinel information on the changing epidemiology of infectious diseases across the globe, most notably for dengue and chikungunya, as well as identifying less common infectious disease outbreaks in the tropics where laboratory facilities may be limited.33
In conclusion, we demonstrate the need to understand the local population and corresponding travel risks, particular amongst those visiting friends and relatives who have higher hospitalization rates post-travel. Sentinel surveillance amongst travellers in Marseille provides essential information to guide both local practices, especially pre-travel consultation needs and addressing autochthone vector-borne risks, and informing institutional partners worldwide regarding the spread of arboviral epidemics and other arthropod borne infections.
Acknowledgements
We acknowledge the role of all healthcare professionals in the two infectious disease departments in Marseille in collecting data as part of the GeoSentinel surveillance network.
Conflict of interest: The authors have declared no conflicts of interest.
Funding
GeoSentinel is supported by a cooperative agreement (U50CK00189) between the Centres for Disease Control and Prevention and the International Society of Travel Medicine (ISTM) and by funding from the ISTM and the Public Health Agency of Canada. Mediterranée Infection is funded by the Agence Nationale de la Recherche « Investissements d’avenir » (Méditerranée Infection 10-IAHU-03).
Author contributions
K.G. analysed the data and wrote the article. H.S., F.S., P.B., P.P. collected data, and edited the article. P.G. collected and analysed the data and wrote the article.
References
- 1. Direction Générale des Entreprises Etudes économiques Chiffres clés du tourisme [Internet]. 2016 [cited 2017 Oct 6]. http://www.entreprises.gouv.fr/files/files/directions_services/etudes-et-statistiques/stats-tourisme/chiffres-cles/2016-Chiffres-cles-tourisme-FR.pdf
- 2. Bertoncello B, Bredeloup S. Le Marseille des marins africains. Rev Eur Migr Int 1999; 15(3):177–97. [Google Scholar]
- 3. Parola P, Gazin P, Pradines B et al. . Marseilles: a surveillance site for malaria from the Comoros Islands. J Travel Med 2004; 11(3):184–6. [DOI] [PubMed] [Google Scholar]
- 4. Leder K, Torresi J, Brownstein JS et al. . Travel-associated illness trends and clusters, 2000–2010. Emerg Infect Dis 2013; 19(7):1049–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harvey K, Esposito DH, Han P et al. . Surveillance for travel-related disease—GeoSentinel Surveillance System, United States, 1997–2011. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002 2013; 62: 1–23. [PubMed] [Google Scholar]
- 6. Hagmann SHF, Han PV, Stauffer WM et al. . Travel-associated disease among US residents visiting US GeoSentinel clinics after return from international travel. Fam Pract 2014; 31():678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freedman DO, Weld LH, Kozarsky PE et al. . Spectrum of disease and relation to place of exposure among Ill returned travelers. N Engl J Med 2006; 354(2):119–30. [DOI] [PubMed] [Google Scholar]
- 8. Leder K, Lau S, Leggat P. Innovative community-based initiatives to engage VFR travelers. Travel Med Infect Dis 2011; 9(5):258–61. [DOI] [PubMed] [Google Scholar]
- 9. Seale H, Kaur R, Mahimbo A et al. . Improving the uptake of pre-travel health advice amongst migrant Australians: exploring the attitudes of primary care providers and migrant community groups. BMC Infect Dis [Internet] 2016. [cited 2017 Jun 20]; 16(1). http://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-016-1479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gautret P, Botelho-Nevers E, Charrel RN, Parola P. Dengue virus infections in travellers returning from Benin to France, July-August 2010. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2010; 15(36):pii: 19657:1–2. [PubMed] [Google Scholar]
- 11. Neumayr A, Munoz J, Schunk M et al. . Sentinel surveillance of imported dengue via travellers to Europe 2012 to 2014: TropNet data from the DengueTools Research Initiative. Eurosurveillance [Internet] 2017. Jan 5 [cited 2017 Jun 20]; 22(1). http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parola P, Soula G, Gazin P et al. . Fever in travelers returning from tropical areas: prospective observational study of 613 cases hospitalised in Marseilles, France, 1999–2003. Travel Med Infect Dis 2006; 4(2):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kassim SA, James PB, Alolga RN et al. . Major decline in malaria morbidity and mortality in the Union of Comoros between 2010 and 2014: the effect of a combination of prevention and control measures. South Afr Med J Suid-Afr Tydskr Vir Geneeskd 2016; 106(7):709–14. [DOI] [PubMed] [Google Scholar]
- 14. Gharbi M, Flegg JA, Hubert V et al. . Longitudinal study assessing the return of chloroquine susceptibility of Plasmodium falciparum in isolates from travellers returning from West and Central Africa, 2000–2011. Malar J 2013; 12(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centre National de Référence du Paludisme Rapport d’activité 2015 [Internet] Centre National de Référence du Paludisme; 2015. http://cnrpaludisme-france.org/docs/rapport_activites_cnr_paludisme_2014.pdf
- 16. Marks M, Armstrong M, Whitty CJM, Doherty JF. Geographical and temporal trends in imported infections from the tropics requiring inpatient care at the Hospital for Tropical Diseases, London—a 15 year study. Trans R Soc Trop Med Hyg 2016; 110(8):456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou S, Li Z, Cotter C et al. . Trends of imported malaria in China 2010–2014: analysis of surveillance data. Malar J [Internet] 2016. [cited 2017 Jun 20]; 15(1). http://www.malariajournal.com/content/15/1/39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Newman RD, Parise ME, Barber AM, Steketee RW. Malaria-related deaths among U.S. travelers, 1963–2001. Ann Intern Med 2004; 141(7):547–55. [DOI] [PubMed] [Google Scholar]
- 19. Rapp C, Aoun O, Ficko C et al. . Infectious diseases related aeromedical evacuation of French soldiers in a level 4 military treatment facility: a ten year retrospective analysis. Travel Med Infect Dis 2014; 12(4):355–9. [DOI] [PubMed] [Google Scholar]
- 20. Gautret P, Simon F, Hervius Askling H et al. . Dengue type 3 virus infections in European travellers returning from the Comoros and Zanzibar, February-April 2010. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2010; 15(15):19541. [PubMed] [Google Scholar]
- 21. Ninove L, Parola P, Baronti C et al. . Dengue virus type 3 infection in traveler returning from West Africa. Emerg Infect Dis 2009; 15(11):1871–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wahid B, Ali A, Rafique S, Idrees M. Global expansion of chikungunya virus: mapping the 64-year history. Int J Infect Dis. 2017; 58: 69–76. [DOI] [PubMed] [Google Scholar]
- 23. Savini H, Gautret P, Gaudart J et al. . Travel-associated diseases, Indian Ocean Islands, 1997–2010. Emerg Infect Dis 2013; 19(8):1297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamer DH, Barbre KA, Chen LH et al. . Travel-associated zika virus disease acquired in the Americas through February 2016: a GeoSentinel analysis. Ann Intern Med 2017; 166(2):99–108. [DOI] [PubMed] [Google Scholar]
- 25. Nutt C, Adams P. Zika in Africa: the invisible epidemic? Lancet 2017; 389(10079):1595–6. [DOI] [PubMed] [Google Scholar]
- 26. Cotteaux-Lautard C, Berenger J-M, Fusca F et al. . A new challenge for hospitals in Southeast France: monitoring local populations of Aedes albopictus to prevent nosocomial transmission of dengue or chikungunya. J Am Mosq Control Assoc 2013; 29(1):81–3. [DOI] [PubMed] [Google Scholar]
- 27. Griffiths K, Charrel R, Lagier J-C et al. . Infections in symptomatic travelers returning from the Arabian peninsula to France: a retrospective cross-sectional study. Travel Med Infect Dis 2016; 14(4):414–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gautret P, Benkouiten S, Griffiths K, Sridhar S. The inevitable Hajj cough: surveillance data in French pilgrims, 2012–2014. Travel Med Infect Dis 2015; 13(6):485–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Delord M, Socolovschi C, Parola P. Rickettsioses and Q fever in travelers (2004–2013). Travel Med Infect Dis 2014; 12(5):443–58. [DOI] [PubMed] [Google Scholar]
- 30. Gautret P, Harvey K, Pandey P et al. . Animal-associated exposure to rabies virus among travelers, 1997–2012. Emerg Infect Dis 2015; 21(4):569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gautret P, Soula G, Delmont J et al. . Common health hazards in French pilgrims during the Hajj of 2007: a prospective cohort study. J Travel Med 2009; 16(6):377–81. [DOI] [PubMed] [Google Scholar]
- 32. Dia A, Gautret P, Adheossi E et al. . Illness in French travelers to Senegal: prospective cohort follow‐up and sentinel surveillance data. J Travel Med 2010; 17(5):296–302. [DOI] [PubMed] [Google Scholar]
- 33. Aubry C, Gautret P, Nougairede A, et al. Outbreak of acute haemorrhagic conjunctivitis in Indian Ocean Islands: identification of Coxsackievirus A24 in a returned traveller. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2012. May 31; 17(22):1–3. [DOI] [PubMed] [Google Scholar]