Abstract
Respiratory complications, in particular infections, are common in the setting of hematological malignancy and after hematopoetic stem cell transplant. The symptoms can be nonspecific; therefore, it can be difficult to identify and treat the cause. However, an understanding of the specific immune defect, clinical parameters such as speed of onset, and radiological findings, allows the logical diagnostic and treatment plan to be made. Radiological findings can include consolidation, nodules, and diffuse changes such as ground glass and tree-in-bud changes. Common infections that induce these symptoms include bacterial pneumonia, invasive fungal disease, Pneumocystis jirovecii and respiratory viruses. These infections must be differentiated from inflammatory complications that often require immune suppressive treatment. The diagnosis can be refined with the aid of investigations such as bronchoscopy, computed tomography (CT) guided lung biopsy, culture, and serological tests. This article gives a schema to approach patients with respiratory symptoms in this patient group; however, in the common scenario of a rapidly deteriorating patient, treatment often has to begin empirically, with the aim to de-escalate treatment subsequently after targeted investigations.
Keywords: respiratory infection, hematological malignancy, invasive mould disease
Introduction
Hematological malignancy is relatively common, with a prevalence of 549 per 100,000 and approximately 328,000 cases in the United Kingdom1 at any one time. They consist of a heterogenous group of diseases that are treated with high dose chemotherapy, often followed by hematopoetic stem cell transplant (HSCT). The diseases themselves, as well as the treatments, lead to significant immunosuppression, leaving the patients susceptible to infections that often affect the respiratory system. As a consequence, approximately 50% of patients with a hematological malignancy develop respiratory infections during the course of their treatment.2 Although this article focuses on the infective complications of hematological malignancy, noninfectious disorders account for approximately half of respiratory complications post HSCT3 and must always be actively considered in the differential diagnosis. Table 1 shows some of the more common and serious noninfectious problems that arise post HSCT. As treatment for noninfectious disorders often requires increased immunosuppression, significant infection usually has to be excluded prior to commencing treatment for a noninfectious pulmonary complication of hematological disease.
Table 1.
Acute and subacute non-infectious respiratory complications in the immunosuppressed patient.
| Clinical problem | Common radiological features |
|---|---|
| Acute presentation (hours to days) | |
| Pulmonary edema | Cardiomegaly, upper lobe diversion, interstitial oedema and pleural effusions |
| Acute respiratory distress syndrome (ARDS) | Bilateral ground glass, dependent consolidation, traction bronchiectasis |
| Diffuse alveolar hemorrhage | Rapidly progressive ground glass changes |
| Engraftment syndrome | Interstitial oedema and pleural effusions |
| Thoracic air leak syndrome | Pneumothorax, pneumomediastinum, subcutaneous emphysema |
| Leukostasis | Interstitial infiltrates and/or alveolar opacification |
| Subacute presentation (days to weeks) | |
| Idiopathic pneumonia syndrome | Diffuse bilateral infiltrates |
| Organizing pneumonia | Peribronchial and peripheral air space opacification |
| Radiation pneumonitis | Ground glass and consolidation within the radiation field developing into pulmonary fibrosis |
| Drug toxicity | Bilateral alveolitis (ground glass infiltrates), developing into pulmonary fibrosis |
| Chronic presentations (weeks to months) | |
| Pulmonary veno-occlusive disease | Enlarged pulmonary arteries, smooth interlobular septal thickening, ground glass opacities |
| Lung graft versus host disease (GvHD) | Mosaickism, progressive airway dilatation |
| Post transplant lymphoproliferative disorder (PTLD) | Pulmonary nodules and mediastinal lymphadenopathy |
| Pleuroparenchymal fibroelastosis | Fibrotic thickening of pleura and subpleural parenchyma |
| Nonclassifiable interstitial pneumonia (pulmonary fibrosis) | Ground glass, peribronchial crazy paving, reticulation and traction-bronchiectasis |
Sources of infecting organisms
Organisms causing infections reach the lung from a variety of sources. These pathogens include both common gram positive and negative pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa, respectively, as well as anaerobes (see Table 2).4 Many bacterial pathogens are nasopharyngeal commensals, which immunosuppressed individuals are less able to effectively clear from the lungs after aspiration. Respiratory pathogens are also commonly inhaled from infected contacts by droplet spread. The commonest causative organisms in this group are the respiratory viruses (see Table 3), which usually only cause mild, self-limiting infections in immunocompetent individuals but in patients with hematological malignancy present with relatively severe symptoms, prolonged infection, and higher rates of pneumonia and death.5–7 Less common causes of inhaled droplet lung infections are Mycoplasma pneumoniae, Chlamydia pneumonia, and Mycobacterium tuberculosis (Table 3). Inhalation of environmental organisms that do not usually cause infection in an immunocompetent host is another significant source of respiratory infection. These include Aspergillus species, other filamentous fungi, Nocardia, and nontuberculous mycobacteria. Aspergillus in particular can affect up to 10% of patients with hematological malignancy.8 Immunosuppression associated with hematological malignancy may also allow reactivation of organisms that are either dormant or persist at low numbers within the lung. These pathogens include Pneumocystis jirovecii, which seems to be a lung commensal that replicates to cause disease in certain types of immunosuppression unless patients are given appropriate prophylaxis.9 Reactivation is also the mode of infection for pneumonitis caused by cytomegalovirus (CMV) and other herpes viruses, and for some cases of M. tuberculosis occurring in subjects with latent infection. Finally, infections from other parts of the body can spread to the lung via hematogenous spread, for example, Candida species and bacterial seeding as septic emboli from indwelling catheters and lines.
Table 2.
Bacteria that cause respiratory infection in patients with hematological malignancy.
| Gram positive | Gram negative | Anaerobes | Atypical |
|---|---|---|---|
| Streptococcus pneumoniae Streptococcus pyogenes Staphylococcus aureus Nocardia asteroides Rhodococcus equi | Pseudomonas spp. Klebsiella pneumoniae Escherichia coli Enterobacter cloacae Stenotrophomonas maltophilia Citrobacter spp. Serratia marcescens Acinetobacter baumanii Hemophilus influenzae Proteus spp. Burkholderia spp. Achromobacter spp. Moraxella catarrhalis | Prevotella spp. Fusobacterium spp. Bacteroides spp. | Mycoplasma pneumoniae Chlamydophila pneumoniae Legionella spp. |
Modified from Evans and Ost.4
Table 3.
Fungi, viruses, and mycobacteria that cause respiratory infection in patients with hematological malignancy.
| Fungi | Viruses | Mycobacteria |
|---|---|---|
| Candida spp.Aspergillus spp.Other filamentous fungi:Fusarium spp.Scedosporium spp.Mucor spp.Rhizopus spp.Pneumocystis jiroveciiEnvironmental fungi:HistoplasmosisCoccidiomycosisCryptococcus neoformans | Respiratory viruses:Influenza A and BParainfluenza 1–-3Human metapneumovirusAdenovirusCoronavirusRespiratory syncytial virusRhinovirusHerpesviruses:CytomegalovirusVaricella zosterHerpes simplexHuman herpes virus 6 | Mycobacterium tuberculosisNontuberculous mycobacteria:Mycobacterium avium- intracellulare complexMycobacterium abscessusMycobacterium fortuitumMycobacterium kansasiiMycobacterium chelonae |
Clinical approach
The multiple potential infecting organisms, with a corresponding variety in antimicrobial treatment options, can make selection of the appropriate management strategy difficult. Fortunately, an understanding of the specific immune deficiencies that act as specific risk factors for specific organisms (Table 4) in combination with clinical parameters such as speed of onset (Table 5) and radiological appearance usually allows the differential diagnosis to be narrowed down. This in turn then allows the formation of a logical targeted diagnostic and treatment plan. In patients who do not improve rapidly with first-line therapy with broad spectrum antibiotics, cross-sectional thoracic CT imaging is essential as it provides much better definition of the pattern of radiological changes than a chest radiograph. These radiological patterns can be broken down into three main groups: consolidation, nodules (micro- and macro-), and diffuse changes, which can be further subdivided into ground glass and tree-in-bud patterns. We discuss the likely causes for each of these radiological patterns and how this guides the appropriate initial investigations and treatment options.
Table 4.
Common infective causes of respiratory symptoms in patients with hematology malignancy categorised by immune defect.
| Immune defect and common associations | Common pathogens |
|---|---|
| Neutropenia / functional neutrophil defects:LeukemiaAplastic anemia / bone marrow infiltrationsHSCTChemotherapy | Bacterial pneumoniaAspergillus spp.Other filamentous fungiInvasive candidiasis |
| Impaired T-cell functionHSCTImmunosuppressive therapiesLymphoma | P. jiroveciiRespiratory virusesCytomegalovirusOther herpesvirusesMycobacteriaNocardia |
| Immunoglobulin deficiency (mainly IgG)CLLMyelomaHSCTB-cell depletion therapies | Bacterial pneumoniaBacterial exacerbations of bronchiectasisRespiratory viruses |
| Prolonged high dose corticosteroids | P. jiroveciiAspergillus spp.Respiratory virusesCytomegalovirusMycobacteriaBacterial pneumonia |
| Kinase inhibitorsJAK inhibitors (e.g., Ruxolitinib)BCR pathway inhibitors (e.g., Ibrutinib) | Aspergillus spp.P. jiroveciiBacterial pneumoniaAspergillus spp.P. jirovecii |
Table 5.
Causes of respiratory symptoms in hematological malignancy categorised by speed of onset.
| Speed of onset | Infective causes | Noninfective causes* |
|---|---|---|
| 1–3 days | Bacterial pneumonia | Pulmonary edemaDiffuse alveolar hemorrhageAdult respiratory distress syndromeEngraftment syndrome |
| 3–7 days | Bacterial pneumoniaRespiratory virusesM. pneumoniae | Adult respiratory distress syndromeEngraftment syndrome |
| 1–2 weeks | Respiratory virusesM. pneumoniaeCMV / other herpesviruses | Drug / radiation pneumonitisIdiopathic pneumonitis |
| 2–6 weeks | Aspergillus spp.Other filamentous fungiNocardia spp.M. tuberculosisPneumocystis jirovecii | Drug / radiation pneumonitisIdiopathic pneumonitisLung GvHDOrganizing pneumoniaLymphoma / malignant infiltrationPTLD |
| Months | M. tuberculosisNontuberculous mycobacteria | Lymphoma / malignant infiltrationDrug / radiation pneumonitis (fibrotic phase)BronchiectasisOrganizing pneumoniaPTLDLung GvHDPost-allograft restrictive lung disease / Pleuroparenchymal fibroelastosis |
*Pulmonary emboli can present in any time category.
Consolidation
Dense focal consolidation (Fig. 1A) often develops rapidly in the context of fevers, dyspnoea and elevated C-reactive protein (CRP). This clinical pattern is highly suggestive of pneumonia caused by pyogenic bacterial pathogens10 associated with community and hospital acquired pneumonias, often originating from microaspiration of nasopharyngeal commensals. Blood and sputum cultures are essential, and treatment with broad-spectrum antibiotics incorporating gram negative cover should be commenced, and most patients will respond to these making invasive investigation with bronchoscopy unnecessary. However, if the patient does not respond rapidly, that is, within 48 to 72 hours, infection with a highly resistant organism such as methicillin-resistant S. aureus or multiresistant P. aeruginosa (resistant to three of the following: carbapenem, ceftazidime, tobramycin, or ciprofloxacin) should be considered. This will necessitate escalation to second-line antibiotics, and if the patient can tolerate bronchoscopy, bronchoalveolar lavage (BAL) of the affected lobe should be performed to try and obtain a clear microbiological diagnosis.
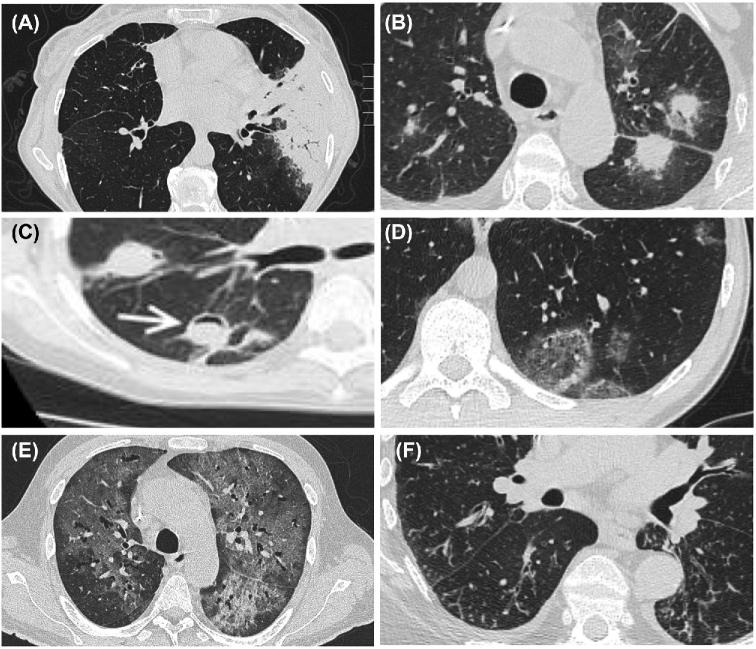
Figure 1.
Cross-sectional radiological images in respiratory complications of hematological disease. (A) Consolidation due to bacterial pneumonia, (B) halo with surrounding ground glass in invasive mould disease, (C) air crescent sign (white arrowhead demonstrates crescent) in partially treated invasive mould disease after neutrophil recovery, (D) ground glass changes due to P. jirovecii, (E) tree in bud changes due to respiratory viral infection, (F) atoll/reverse halo sign due to organizing pneumonia.
Focal consolidation with a subacute onset has a broader differential diagnosis; these include bacterial pneumonia, Aspergillus species, and Nocardia species (usually asteroides), and noninfectious causes such as organizing pneumonia and recurrence of hematological malignancy. Diagnostic tests including BAL for culture, galactomannan,11 and cytology are necessary. While transbronchial biopsy has low yield and is not recommended for the diagnosis of invasive fungal disease12 given the complication rate of pneumothorax in particular, it may be useful in confirming alternative diagnoses. Dense peripheral lesions adjacent to the pleura are amenable to CT guided percutaneous biopsy. Histology can rapidly confirm a diagnosis of invasive fungal disease (IFD), Nocardia infection, organizing pneumonia, or malignant infiltrations (e.g., lymphoma), and the biopsy material can also be sent for culture.
Pulmonary nodules
Pulmonary nodules are rounded lesions within the lung with a diameter greater than 4 mm in diameter, but in the hematological malignancy population they are often substantially larger than this and can be termed macronodules. The presence of macronodules should always raise the suspicion of an IFD, the commonest of which is invasive aspergillosis, the majority of which are caused by A. fumigatus. Several other Aspergillus and filamentous fungi species such as mucormycetes can cause IFD and have similar clinical and radiological findings.8 The CT scan has several distinct appearances that increase the likelihood that a macronodule is caused by IFD, though are not necessarily very specific. A surrounding halo of ground glass (Fig. 1B) is a classical sign of angioinvasive fungal disease, with the halo representing hemorrhage, and the air crescent sign (Fig. 1C) due to the formation of a fungal ball within a cavity caused by fungal destruction of lung tissue is also highly suggestive of IFD.13,14 Macronodules caused by IFD undergo a classic evolution of changes on CT as the infection is controlled, with the nodule developing the air crescent sign, followed by thinning of the cavity wall and shrinkage of its overall size, associated with clearance of the associated surrounding consolidation.15
A recently described CT sign that points to IFD is the occluded vessel sign,16 where pulmonary arteries are interrupted within areas of consolidation. This had an 89% sensitivity and 52% specificity for proven or probable IFD by EORTC criteria17 but does require a CT pulmonary angiogram protocol with contrast injection, with its attendant risks of renal toxicity and allergic reactions. Similarly, the hypodense sign, central hypoattenuation within a macronodule, has recently been shown to have a similar sensitivity (46%) and superior specificity (83%) to the halo sign for IFD.16,18 The reverse halo (also termed the atoll sign) is a strong indicator for mucormycosis early in the disease course of neutropenic patients.19
Although CT appearances of macronodules can be highly suggestive of IFD, microbiological confirmation gives additional confidence in the diagnosis and ensures the patient receives antifungal treatment that is effective against the specific infecting fungal pathogen. Unfortunately, all existing microbiological tests for IFD have significant drawbacks. Culture of BAL20,21 or sputa is insensitive,22 although when positive in the immunosuppressed patient is highly suggestive of active infection. Antigen testing using the serum galactomannan has a sensitivity of 41–78% and specificity of 60–95% when two sequential samples have an optical density >0.5 giving a negative predictive value of up to 95% in azole naive patients in the highest risk groups (neutropenic patients)15,23,24 but does not confirm IFD species. Furthermore, serum galactomannan is less accurate in patients receiving triazole prophylaxis,24–26 which is now in widespread use in hemato-oncology patients. Measuring galactomannan in BAL instead has a much greater sensitivity of 87% and specificity of 89% even in the setting of triazole prophylaxis,24 and hence a negative BAL galactomannan can allow de-escalation of treatment with antifungals. Mucormycetes have little galactomannan in their cell walls rendering serum and BAL analysis for this test insensitive.27Aspergillus polymerase chain reaction (PCR) should be sensitive but may not be specific due to widespread presence of Aspergillus species in the environment, and as yet there is little standardization between kits and is not in widespread use.28 The lateral flow device provides a point of care test for fungal wall antigens that is as sensitive and specific as PCR29 and has significant clinical promise but is not yet in wide commercial use.
As discussed above in the consolidation section, a CT guided percutaneous biopsy is a rapid way of identifying IFD in macronodules, as well as some other pathogens, and noninfective diagnoses. The biopsy material can also be sent for culture to identify the infecting species and antimicrobial resistance profile. Hemorrhage and pneumothorax are the main complications of percutaneous CT guided biopsies, with the former being a particular problem in hematological malignancy due to the prevalence of significant thrombocytopenia. However, targeting peripheral lesions and using platelet transfusions minimizes these risks.
Overall, a specific diagnosis of invasive fungal disease can be difficult to achieve and microbiological diagnosis of IFD remains unreliable. Diagnosis is usually made with a consideration of multiple elements: clinical risk factors, radiological changes, biomarkers, and the use of triazole prophylaxis. As mortality without treatment is high,30,31 empirical treatment is usually started in high-risk patients as soon as the clinical picture is compatible with an IFD. Although published data suggest that azoles such as voriconazole and posaconazole are as effective as amphotericin (if not more so),15,32 liposomal amphotericin is often the first-line therapy in patients receiving azole prophylaxis due to fears about fungal resistance.33,34 If azoles are used, ensuring that therapeutic levels are achieved by monitoring serum levels improves outcomes.35–38 Newer azoles are being developed, and one of these isavuconazole has recently been shown to be noninferior to voriconazole and has the advantage of being effective against mucormycosis.39 Dual agent antifungal may have superior outcomes in IFD and could be considered in critically ill patients.40,41
Other causes of nodules include septic emboli, Nocardia, mycobacterial infections, and noninfectious causes. Septic bacterial emboli cause distinctive radiological appearances of multiple cavitating nodules, usually in the lung periphery and often eroding into the pleural space to cause infected hydropneumothoraces. The most common sources are infected indwelling catheters, so line infection needs considering in any patient with radiological evidence of lung nodules, necessitating paired blood and line cultures. Multiple well-defined micronodules in the context of cell-mediated immune deficiency can be caused by Nocardia42 and mycobacterial species.43 Nocardial infection is associated with myeloablative conditioning and steroids, with a median time to infection of 10 months post HSCT.42 Pulmonary infection has a mortality rate up to 53% and requires treatment for 6–12 months with oral trimethoprim/sulphamethoxazole or parenteral treatment with carbapenems and/or amikacin. Prophylactic trimethoprim/sulphamethoxazole for pneumocystis also protects against Nocardia. Nontuberculous mycobacteria infection post HSCT has an incidence of between 0.4 and 10%,44 associated with GvHD and further immunosuppression, and has a 7–19% mortality rate.45,46
Noninfectious causes of nodules such as lymphoma, other malignancies, and post transplant lymphoproliferative disorder (PTLD) need histological diagnosis. However, smaller size nodules may not be amenable to percutaneous biopsy, the yield of BAL remains poor, and in the nonresponding patient the diagnosis may require video assisted thoracoscopic biopsy. In these situations it is important to try and identify potential extrathoracic sites of disease that are more amenable to biopsy than the lung.
Diffuse disease
The differential diagnosis for diffuse, less dense, bilateral infiltrations on the CT scan is broad. These changes encompass two main patterns, ground glass infiltrates and tree-in-bud changes, which differ in their likely causes and are discussed separately below. The important microbiological tests are blood and sputum cultures, serum β-D-glucan antigen testing (a fungal cell wall component), blood CMV viral load, and multiplex PCR for respiratory viruses on nasopharyngeal aspirate. Inflammatory markers such as CRP can help differentiate between infectious and noninfectious causes, although CRP can also be significantly elevated in noninfective hyperinflammatory states. Serial full blood counts and coagulation status can help identify patients at risk of engraftment syndrome (clinical syndrome occurring at time of neutrophil recovery) or pulmonary hemorrhage. Obtaining BAL for cytology and microbiological testing is very helpful, but these patients are often too hypoxic to undergo a bronchoscopy.
Ground glass infiltrates
Bilateral ground glass infiltrates (Fig. 1D) can be caused by a wide range of microbial pathogens including pyogenic bacteria, respiratory viruses, cytomegalovirus, Pneumocystis jirovecii, and multiple noninfective causes. This pattern is unlikely to be caused by an IFD. Often ground glass infiltrations are associated with areas of denser consolidation creating a mixed appearance on the CT scan. The likely causes of rapid onset of bilateral ground glass infiltrates over a few days include bacterial infections, pulmonary edema, and acute respiratory distress syndrome (ARDS), and less commonly alveolar hemorrhage or engraftment syndrome. Engraftment syndrome presents with widespread infiltrates associated with fever, rash, and other organ dysfunction within 4 days of granulocyte recovery post-HSCT.47 A subacute onset of respiratory symptoms over days and weeks with associated ground glass changes has similar causes as acute presentations, but the differential diagnosis needs to be expanded to include P. jirovecii, CMV, respiratory viruses, and drug- or radiotherapy-induced pneumonitis. There are some aspects of the clinical presentations of the above diseases that can suggest the underlying cause, and these are discussed below.
Pneumocystis pneumonia (PJP, previously referred to as PCP in older publications) often has a distinct clinical presentation of progressive dyspnoea over several weeks associated with desaturation on exertion and then eventually hypoxemia. This is usually associated with only low-grade fevers and moderate increases in CRP. The incidence is as low as 0.1% in patients receiving prophylaxis.48 Pulmonary coinfection is common, particularly with CMV, and mortality rates have been reported to be as high as 30–60% in hematological malignancy,49 although in our experience it is considerably less than this. CT findings are often highly suggestive of PJP, classically showing diffuse bilateral ground glass shadowing with a predilection for the upper lobes and marked subpleural sparing. Serum antigen testing for β-D-glucan is very helpful, with a published sensitivity of 95% and specificity of 86% for PJP.50,51 However, β-D-glucan levels can also be elevated with other fungi, in particular with candidemia, so need to be interpreted in the context of the overall clinical picture. The diagnosis of PJP can also be confirmed in some patients by identification of cysts in bronchoalveolar lavage using immunofluorescence, although this is often negative in hematology patients. Overall, in patients with a classical clinical and radiological presentation the diagnosis of PJP can be confirmed by the response to empirical treatment, usually with high dose co-trimoxazole or clindamycin and primaquine. Adjunct systemic corticosteroids are used in hypoxic patients but do complicate assessing the response to empirical treatment as noninfective causes of a pneumonitis can also improve with corticosteroid treatment.
CMV pneumonitis is most often due to reactivation of latent infection during periods of impaired cell mediated immunity and T-cell depletion rather than primary infection, and has a high mortality of up to 50%.52 CT findings in CMV pneumonia are not that distinctive and include bilateral ground glass infiltrates and symmetrical micronodules.53 The diagnosis is suggested by highly elevated blood CMV viral load, especially if this has increased rapidly, and can be confirmed by obtaining BAL fluid for quantitative PCR54 and cytology to look for viral inclusion bodies. However, the patients are often too hypoxic for a safe bronchoscopy. Treatment is with intravenous ganciclovir, followed by conversion to valganciclovir, with foscarnet and cidofovir as second and third line agents.55
Although there can be clinical (e.g., rapid weight gain suggesting fluid retention and pulmonary oedema), and radiological features (Table 1) that suggest specific causes, making a confirmed diagnosis of noninfective etiologies of bilateral infiltrates is often difficult. The diagnosis often partially depends on microbiological testing to try and exclude infective causes, including bronchoscopy if the patient is able to tolerate the procedure. Bronchoscopy can also be diagnostic for alveolar hemorrhage with similar or increasing recovery of bloody fluid with sequential lavage. The main clinical decision is whether to introduce systemic corticosteroids as a treatment for suspected noninfective causes such as drug- or radiation-pneumonitis, alveolar hemorrhage, or rarer complications of specific therapies such as all-trans retinoic acid differentiation syndrome.
Tree-in-bud changes
Bilateral tree-in-bud (Fig. 1E) changes are suggestive of acute respiratory viral infections (Table 3) or widespread bacterial bronchiolitis. This can sometimes be seen in patients with bronchiectasis as a complication of hematological disease (e.g., secondary to hypogammaglobulinemia). Respiratory viral infections are very common in patients with hematological disease and can now be readily diagnosed by PCR on a nasopharyngeal aspirate. The CT often demonstrates widespread, diffuse, symmetrical tree-in-bud changes, although these infections can also cause ground glass infiltrates. In comparison to immunocompetent individuals, respiratory viral infections in patients with hematological malignancy (particularly after HSCT) are more prolonged, lasting weeks and even months, and lead to an increased risk of respiratory compromise due to the development of viral or secondary bacterial pneumonia.56 The viruses recognised to cause respiratory infection in hemato-oncology patients are noted in Table 3. Some have specific treatments though the data for efficacy are largely limited to case series. Ribavirin is used for respiratory syncytial virus (RSV), although it appears to have little effect once patients develop respiratory failure.57 Adenovirus is often cultured, though less commonly causing infection, can be treated with Cidofovir.58,59 Neuraminidase inhibitors reduce mortality due to influenza infection,60 although they are less effective in patients who are immunosuppressed, have GvHD, lymphopenia, or older age;61 preemptive vaccination is key in preventing infection.62 There are no recognized organism-specific treatments for parainfluenza,63 human metapneumovirus,7 and rhinovirus.64
Bronchiectasis is a common complication of many hematological diseases including multiple myeloma, chronic lymphocytic leukemia (CLL), B-cell depletion therapies, and HSCT, and can result in subacute bacterial bronchial infections. These cause patchy tree-in-bud infiltrates associated with bronchial wall thickening and dilatation and are usually caused by Gram negative pathogens such as K. pneumoniae or P. aeruginosa that will require prolonged therapy with appropriate antibiotics. Too short an antibiotic course will allow the infection to recur, and this can lead to a vicious cycle of recurrent infections with an inability to gain weight or fully recover before the next infection occurs. Antibiotic prophylaxis and correction of hypogammaglobulinemia with supplementary immunoglobulins is important for these patients and is also recommended in other patients with hematological malignancy and secondary antibody deficiency in the setting of recurrent infections.65
Treatment strategies
Almost all hematology patients presenting acutely with fever and dyspnoea will require broad-spectrum antibiotics. Starting antifungals with the initial fever does not improve outcomes compared to delaying to day 4 if the fever does not settle.66 Similarly, cross-sectional CT is only necessary if the symptoms do not resolve rapidly with antibiotics.67 If the fever persists, then characteristic CT changes in the clinical context (speed of onset, immune defects, other clinical features) will often indicate the need for specific treatments, for example, liposomal amphotericin or voriconazole in neutropenic patients with a macronodule with surrounding halo. However, the wide differential diagnosis means that empirical treatment targeting different infectious and noninfectious causes is often required. Microbiological confirmation remains variably successful; culture techniques are slow and sensitivity can be poor, hence the development of biomarkers and PCR to increase sensitivity. While invasive procedures such as bronchoscopy or biopsy can give vital diagnostic information and in particular allow the de-escalation of antifungals and make alternative diagnoses, patients can deteriorate rapidly and be too hypoxic for such investigations. Furthermore, many cases of respiratory problems in hematology patients have a combination of causes, so even when a microbe has been identified, this may not prevent broader treatment. Another significant issue is when to stop therapy in patients treated empirically with multiple agents who then improve, as the cause of the underlying problem may remain unclear. Most bacterial infections resolve with a few days of antibiotics, but aspergillosis can require prolonged therapy to prevent recurrence. Exactly how long antifungals should be continued is not known; serum galactomannan levels may have some utility, with a ≥35% reduction after 1 week associated with a good clinical outcome,68,69 but mainly outcome is monitored by observing radiological responses. It is unclear at which stage during this evolution that it is safe to stop antifungals without leading to a significant risk of recurrence.
Patients with hematological malignancy can develop a range of immune defects during the course of their illness or associated with the necessary treatments. These allow various pathogens to cause disease, and the respiratory tract is commonly affected; this is associated with significant morbidity and mortality. Infections must be treated promptly, requiring empirical therapy chosen to cover the most likely pathogens given the clinical presentation. An understanding of the relevant immune defect, along with the recognition of patterns of clinical presentation and findings on cross-sectional CT imaging, allows logical deduction of likely culprits and targeted microbiological and molecular investigations to help narrow the differential diagnosis. This is with the caveat that there is significant cross-over between radiological findings, and a high prevalence of noninfective respiratory complications that are often diagnoses of exclusion. As such, there are many occasions when the specific diagnosis is never discovered, and critically ill patients have to be treated for multiple organisms and noninfective complications empirically. There is an urgent need for improved rapid diagnostics with better sensitivity and specificity to allow more directed treatment of respiratory infections in hematological malignancy. Ideally, future research should focus on the development of point of care tests that accurately identify specific organisms. If possible, these will be noninvasive and easy to perform even on critically ill patients, allowing pathogen-specific treatments and minimising unnecessary drug-related toxicity.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Li J, Smith A, Crouch S, Oliver S, Roman E. Estimating the prevalence of hematological malignancies and precursor conditions using data from Haematological Malignancy Research Network (HMRN). Cancer Causes Control. 2016; 27: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morrison V. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. 2009; 9: 365–370. [DOI] [PubMed] [Google Scholar]
- 3. Brodoefel H, Faul C, Salih H, Vogel W, Fenchel M, Horger M. Therapy-related noninfectious complications in patients with hematologic malignancies: high-resolution computed tomography findings. J Thorac Imaging. 2013; 28: W5–W11. [DOI] [PubMed] [Google Scholar]
- 4. Evans SE, Ost DE. Pneumonia in the neutropenic cancer patient. Curr Opin Pulm Med. 2015; 21: 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Englund JA. Diagnosis and epidemiology of community-acquired respiratory virus infections in the immunocompromised host. Biol Blood Marrow Transplant. 2001; 7: 2S–4S. [DOI] [PubMed] [Google Scholar]
- 6. Nichols W, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research. Biol Blood Marrow Transplant. 2001; 7: 11S–15S. [DOI] [PubMed] [Google Scholar]
- 7. Shah DP, Ghantoji SS, Mulanovich VE, Ariza-Heredia EJ, Chemaly RF. Management of respiratory viral infections in hematopoietic cell transplant recipients. Am J Blood Res. 2012; 2: 203–218. [PMC free article] [PubMed] [Google Scholar]
- 8. Pagano L, Caira M, Candoni A et al.. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006; 91: 1068–1075. [PubMed] [Google Scholar]
- 9. Caselli D, Petris MG, Rondelli R et al.. Single-day trimethoprim/sulfamethoxazole prophylaxis for pneumocystis pneumonia in children with cancer. J Pediatr. 2014; 164: 389–392. [DOI] [PubMed] [Google Scholar]
- 10. Zembower TR. Epidemiology of infections in cancer patients. Cancer Treat Res. 2014; 161: 43–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maertens J, Van Eldere J, Verhaegen J, Verbeken E, Verschakelen J, Boogaerts M. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis. 2002; 186: 1297–1306. [DOI] [PubMed] [Google Scholar]
- 12. Patterson TF, Thompson GR, Denning DW et al.. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016; 63: e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee YR, Choi YW, Lee KJ, Jeon SC, Park CK, Heo JN. CT halo sign: the spectrum of pulmonary diseases. Br J Radiol. 2005; 78: 862–865. [DOI] [PubMed] [Google Scholar]
- 14. Greene RE, Schlamm HT, Oestmann J-W et al.. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007; 44: 373–379. [DOI] [PubMed] [Google Scholar]
- 15. Herbrecht R, Denning DW, Patterson TF et al.. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002; 347: 408–415. [DOI] [PubMed] [Google Scholar]
- 16. Stanzani M, Sassi C, Lewis RE et al.. High resolution computed tomography angiography improves the radiographic diagnosis of invasive mold disease in patients with hematological malignancies. Clin Infect Dis. 2015; 60: 1603–1610. [DOI] [PubMed] [Google Scholar]
- 17. De Pauw B, Walsha TJ, Donnellya JP et al.. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive. Clin Infect Dis. 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sassi C, Stanzani M, Lewis RE et al.. The utility of contrast-enhanced hypodense sign for the diagnosis of pulmonary invasive mould disease in patients with haematological malignancies. Br J Radiol. 2018; 91: 20170220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Legouge C, Caillot D, Chrétien ML et al.. The reversed halo sign: Pathognomonic pattern of pulmonary mucormycosis in leukemic patients with neutropenia? Clin Infect Dis. 2014; 58: 672–678. [DOI] [PubMed] [Google Scholar]
- 20. Arendrup MC, Bille J, Dannaoui E, Ruhnke M, Heussel CP, Kibbler C. ECIL-3 classical diagnostic procedures for the diagnosis of invasive fungal diseases in patients with leukaemia. Bone Marrow Transpl. 2012; 47: 1030–1045. [DOI] [PubMed] [Google Scholar]
- 21. Maschmeyer G, Carratalà J, Buchheidt D et al.. Diagnosis and antimicrobial therapy of lung infiltrates in febrile neutropenic patients (allogeneic SCT excluded): updated guidelines of the Infectious Diseases Society of Hematology and Medical Oncology (DGHO). Ann Oncol. 2015; 26: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ho DY, Lin M, Schaenman J et al.. Yield of diagnostic procedures for invasive fungal infections in neutropenic febrile patients with chest computed tomography abnormalities. Mycoses. 2011; 54: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maertens JA, Klont R, Masson C et al.. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clin Infect Dis. 2007; 44: 1329–1336. [DOI] [PubMed] [Google Scholar]
- 24. Miceli MH, Maertens J. Role of non-culture-based tests, with an emphasis on galactomannan testing for the diagnosis of invasive aspergillosis. Semin Respir Crit Care Med. 2015; 36: 650–661. [DOI] [PubMed] [Google Scholar]
- 25. Vena A, Bouza E, Alvarez-Uria A et al.. The misleading effect of serum galactomannan testing in high-risk hematology patients receiving prophylaxis with micafungin. Clin Microbiol Infect. 2017; 23: 1000.e1–1000.e4. [DOI] [PubMed] [Google Scholar]
- 26. Marr K a. Primary antifungal prophylaxis in hematopoietic stem cell transplant recipients: clinical implications of recent studies. Curr Opin Infect Dis. 2008; 21: 409–414. [DOI] [PubMed] [Google Scholar]
- 27. Bitar D, Lortholary O, Le Strat Y et al.. Population-based analysis of invasive fungal infections. Emerg Infect Dis. 2014; 20: 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arvanitis M, Ziakas PD, Zacharioudakis IM, Zervou FN, Caliendo AM, Mylonakis E. PCR in diagnosis of invasive aspergillosis: a meta-analysis of diagnostic performance. J Clin Microbiol. 2014; 52: 3731–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoenigl M, Prattes J, Spiess B et al.. Performance of galactomannan, beta-d-glucan, aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol. 2014; 52: 2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996; 23: 608–615. [DOI] [PubMed] [Google Scholar]
- 31. Lin S-J, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001; 32: 358–366. [DOI] [PubMed] [Google Scholar]
- 32. Walsh TJ, Pappas P, Winston DJ et al.. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002; 346: 225–234. [DOI] [PubMed] [Google Scholar]
- 33. Cornely OA, Maertens J, Bresnik M et al.. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis. 2007; 44: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 34. Jørgensen KJ, Gøtzsche PC, Dalbøge CS, Johansen HK. Voriconazole versus amphotericin B or fluconazole in cancer patients with neutropenia. Cochrane Database Syst Rev. 2014; 2014: CD004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walsh TJ, Raad I, Patterson TF et al.. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007; 44: 2–12. [DOI] [PubMed] [Google Scholar]
- 36. Denning DW, Ribaud P, Milpied N et al.. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002; 34: 563–571. [DOI] [PubMed] [Google Scholar]
- 37. Guinea J, Escribano P, Marcos-Zambrano LJ et al.. Therapeutic drug monitoring of voriconazole helps to decrease the percentage of patients with off-target trough serum levels. Med Mycol. 2016; 54: 353–360. [DOI] [PubMed] [Google Scholar]
- 38. Smith J, Safdar N, Knasinski V et al.. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother. 2006; 50: 1570–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maertens JA, Raad II, Marr KA et al.. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016; 387: 760–769. [DOI] [PubMed] [Google Scholar]
- 40. Marr KA, Schlamm HT, Herbrecht R et al.. Combination antifungal therapy for invasive aspergillosis a randomized trial. Ann Intern Med. 2015; 162: 81–89. [DOI] [PubMed] [Google Scholar]
- 41. Candoni A, Caira M, Cesaro S et al.. Multicentre surveillance study on feasibility, safety and efficacy of antifungal combination therapy for proven or probable invasive fungal diseases in haematological patients: the SEIFEM real-life combo study. Mycoses. 2014; 57: 342–350. [DOI] [PubMed] [Google Scholar]
- 42. Shannon K, Pasikhova Y, Ibekweh Q, Ludlow S, Baluch A. Nocardiosis following hematopoietic stem cell transplantation. Transpl Infect Dis. 2016; 18: 169–175. [DOI] [PubMed] [Google Scholar]
- 43. Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015; 36: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Al-Anazi KA, Al-Jasser AM, Al-Anazi WK. Infections caused by non-tuberculous mycobacteria in recipients of hematopoietic stem cell transplantation. Front Oncol. 2014; 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doucette K, Fishman JA. Nontuberculous mycobacterial infection in hematopoietic stem cell and solid organ transplant recipients. Clin Infect Dis. 2004; 38: 1428–1439. [DOI] [PubMed] [Google Scholar]
- 46. Weinstock DM, Feinstein MB, Sepkowitz KA, Jakubowski A. High rates of infection and colonization by nontuberculous mycobacteria after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003; 31: 1015–1021. [DOI] [PubMed] [Google Scholar]
- 47. Cornell RF, Hari P, Drobyski WR. Engraftment syndrome after autologous stem cell transplantation: an update unifying the definition and management approach. Biol Blood Marrow Transplant. 2015; 21: 2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cordonnier C, Cesaro S, Maschmeyer G et al.. Pneumocystis jirovecii pneumonia: still a concern in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016; 71: 2379–2385. [DOI] [PubMed] [Google Scholar]
- 49. Torres HA, Kontoyiannis DP, Aguilera EA et al.. Cytomegalovirus infection in patients with lymphoma: an important cause of morbidity and mortality. Clin Lymphoma Myeloma. 2006; 6: 393–398. [DOI] [PubMed] [Google Scholar]
- 50. Karageorgopoulos DE, Qu J-M, Korbila IP et al.. Accuracy of β-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013; 19: 39–49. [DOI] [PubMed] [Google Scholar]
- 51. Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. β-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011; 52: 750–770. [DOI] [PubMed] [Google Scholar]
- 52. Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am. 2010; 24: 319–337. [DOI] [PubMed] [Google Scholar]
- 53. Horger MS, Pfannenberg C, Einsele H et al.. Cytomegalovirus pneumonia after stem cell transplantation: correlation of CT findings with clinical outcome in 30 patients. AJR Am J Roentgenol. 2006; 187: W636–643. [DOI] [PubMed] [Google Scholar]
- 54. Lee HY, Rhee CK, Choi JY, Lee HYL, Lee JW, Lee DG. Diagnosis of cytomegalovirus pneumonia by quantitative polymerase chain reaction using bronchial washing fluid from patients with hematologic malignancies. Oncotarget. 2017; 8: 39736–39745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Emery V, Zuckerman M, Jackson G et al.. Management of cytomegalovirus infection in haemopoietic stem cell transplantation. Br J Haematol. 2013; 162: 25–39. [DOI] [PubMed] [Google Scholar]
- 56. Abbas S, Raybould JE, Sastry S, de la Cruz O. Respiratory viruses in transplant recipients: more than just a cold. Clinical syndromes and infection prevention principles. Int J Infect Dis. 2017; 62: 86–93. [DOI] [PubMed] [Google Scholar]
- 57. Harrington RD, Hooton TM, Hackman RC et al.. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis. 1992; 165987–993. [DOI] [PubMed] [Google Scholar]
- 58. Sandkovsky U, Vargas L, Florescu DF. Adenovirus: Current epidemiology and emerging approaches to prevention and treatment. Curr Infect Dis Rep. 2014; 16: 416. [DOI] [PubMed] [Google Scholar]
- 59. Florescu DF, Hoffman JA. Adenovirus in solid organ transplantation. Am J Transplant. 2013; 13: 206–211. [DOI] [PubMed] [Google Scholar]
- 60. Kmeid J, Vanichanan J, Shah DP et al.. Outcomes of influenza infections in hematopoietic cell transplant recipients: application of an immunodeficiency scoring index. Biol Blood Marrow Transplant. 2016; 22: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reid G, Huprikar S, Patel G et al.. A multicenter evaluation of pandemic influenza A/H1N1 in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2013; 15: 487–492. [DOI] [PubMed] [Google Scholar]
- 62. Machado CM, Cardoso MRA, da Rocha IF, Boas LS V, Dulley FL, Pannuti CS. The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant. 2005; 36: 897–900. [DOI] [PubMed] [Google Scholar]
- 63. Wendt CH, Weisdorf DJ, Jordan MC, Balfour HH, Hertz MI. Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med. 1992; 326: 921–926. [DOI] [PubMed] [Google Scholar]
- 64. Milano F, Campbell AP, Guthrie KA et al.. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010; 115: 2088–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oscier D, Dearden C, Erem E et al.. Guidelines on the diagnosis, investigation and management of chronic lymphocytic leukaemia. Br J Haematol. 2012; 159: 541–564. [DOI] [PubMed] [Google Scholar]
- 66. Maschmeyer G, Heinz WJ, Hertenstein B et al.. Immediate versus deferred empirical antifungal (IDEA) therapy in high-risk patients with febrile neutropenia: a randomized, double-blind, placebo-controlled, multicenter study. Eur J Clin Microbiol Infect Dis. 2013; 32: 679–689. [DOI] [PubMed] [Google Scholar]
- 67. Hauggaard A, Ellis M, Ekelund L. Early chest radiography and CT in the diagnosis, management and outcome of invasive pulmonary aspergillosis. Acta Radiol. 2002; 43: 292–298. [DOI] [PubMed] [Google Scholar]
- 68. Maertens J, Buvé K, Theunissen K et al.. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer. 2009; 115: 355–362. [DOI] [PubMed] [Google Scholar]
- 69. Chai LYA, Kullberg BJ, Johnson EM et al.. Early serum galactomannan trend as a predictor of outcome of invasive aspergillosis. J Clin Microbiol. 2012; 50: 2330–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]