Abstract
The genus Pneumocystis comprises potential pathogens that reside normally in the lungs of a wide range of mammals. Although they generally behave as transient or permanent commensals, they can occasionally cause life-threatening pneumonia (Pneumocystis pneumonia; PCP) in immunosuppressed individuals. Several decades ago, the presence of Pneumocystis morphotypes (trophic forms and cysts) was described in the lungs of normal cats and cats with experimentally induced symptomatic PCP (after immunosuppression by corticosteroids); yet to date spontaneous or drug-induced PCP has not been described in the clinical feline literature, despite immunosuppression of cats by long-standing retrovirus infections or after kidney transplantation. In this study, we describe the presence of Pneumocystis DNA in the lungs of normal cats (that died of various unrelated causes; n = 84) using polymerase chain reactions (PCRs) targeting the mitochondrial small and large subunit ribosomal RNA gene (mtSSU rRNA and mtLSU rRNA). The presence of Pneumocystis DNA was confirmed by sequencing in 24/84 (29%) cats, with evidence of two different sequence types (or lineages). Phylogenetically, lineage1 (L1; 19 cats) and lineage 2 (L2; 5 cats) formed separate clades, clustering with Pneumocystis from domestic pigs (L1) and carnivores (L2), respectively. Results of the present study support the notion that cats can be colonized or subclinically infected by Pneumocystis, without histological evidence of damage to the pulmonary parenchyma referable to pneumocystosis. Pneumocystis seems most likely an innocuous pathogen of cats’ lungs, but its possible role in the exacerbation of chronic pulmonary disorders or viral/bacterial coinfections should be considered further in a clinical setting.
Keywords: Pneumocystis, cat, mtLSU rRNA, mtSSU rRNA
Introduction
The genus Pneumocystis is used to describe related fungi that have evolved to live in the lower respiratory tract of mammals.1 They are transmitted by aerosols, with animals typically becoming infected during the neonatal period, resulting in persistent, sometimes lifelong infections.2,3 They generally are believed to be transient or permanent commensals, colonizing very limited portions of the lungs, while causing minimal or no damage to the host.4,5Pneumocystis is highly adapted for an existence in host lung, with strict dependence on its mammalian host for nutrients and a stable environment, while utilizing efficient strategies for immune evasion, thereby facilitating persistence in its host. The Pneumocystis genomes sequenced to date have all demonstrated a contracted genome compared to those of other closely related fungi, revealing adaptative mechanisms to live exclusively in mammalian hosts, very similar to a parasite/host relationship.6,7
This group of fungi does, however, have the potential to behave as an opportunistic pathogen in certain settings, such as severe malnutrition (e.g., in child refugees after the Second World War), immunosuppressive drug therapy (e.g., after solid organ transplantation) or inherited or acquired immunodeficiency states (including AIDS due to long-standing human immunodeficiency virus (HIV) infection).3,8 Generally, Pneumocystis species show great host selectivity, such that every mammalian group studied has only one or two specific Pneumocystis species with which it is strongly associated.6,9
Pneumocystis has the capacity to cause life-threatening pneumonia (Pneumocystis pneumonia; PCP) in immunosuppressed individuals, including human transplant recipients, patients receiving immunosuppressive drugs (corticosteroids, methotrexate, azathioprine, calcineurin inhibitors, tumor necrosis factor α [TNFα] antagonists, etc.) and patients with HIV/AIDS.10–12 The parallelism between HIV/AIDS in human patients and feline leukemia virus (FeLV) infection in cats has been considered in relation to the pathogenesis of feline pneumocystosis, although no association could be established.13 Perhaps more surprisingly, the same is true of the feline immunodeficiency virus (FIV).14 Spontaneous PCP in cats has not been reported. Cats receiving renal transplants, for example, have never developed PCP despite being susceptible to numerous other opportunistic pathogens such as Toxoplasma gondii and Cryptococcus neoformans/ Cryptococcus gattii because of prednisone and cyclosporine administration.15 PCP can, however, be produced in contrived experimental settings.16
Pneumocystis in cats was first described by investigators in Mexico and Denmark from 1950 to 198017–19 (reviewed in Table 1) based on characteristic morphology of trophic forms and cysts in feline lung specimens. There was no evidence of symptomatic PCP in these cats, which therefore were considered to be colonized or infected subclinically. Later, a small number of studies investigated cats as potential animal models for Pneumocystis infection, probably drawing upon the notion that cats might be susceptible to recrudescent Pneumocystis infection, as they are to toxoplasmosis following reactivation of Toxoplasma gondii bradyzoite cysts. In these studies, cats were administered extremely high doses of corticosteroids, and as a result, a proportion of them developed PCP pneumonia16,20 (Table 1).
Table 1.
Review of feline Pneumocystis studies reported in the literature, including investigations in "norma” cats, FeLV-positive cats, and experimentally immunosuppressed cats.
| Prevalence of Pneumocystis positivity | Number of cats studied | FeLV | Immune-suppression | Histology (H&E-stained lung tissue specimens) | Cysts (asci) | Trophic forms | PCP | Reference |
|---|---|---|---|---|---|---|---|---|
| 4/100 (4.0%) | 100 | N/R | – | Normal | + | N/R | – | 17 |
| 10/79 (12.6%) | 79 | N/R | – | Normal | + | + | – | 18 |
| 3/75 (4.0%) | 75 | N/R | – | Normal | + | N/R | – | 19 |
| 0/5 (0%) | 5 | + | – | Acute bronchopneumonia | – | – | – | 13 |
| 0/5 (0%) | 5 | + | – | Leukemic infiltrates | – | – | – | 13 |
| 0/3 (0%) | 3 | + | – | Interstitial pneumonitis | – | – | – | 13 |
| 0/1 (0%) | 1 | + | – | Adenomatosis | – | – | – | 13 |
| 0/19 (0%) | 19 | – | – | Interstitial pneumonitis | – | – | – | 13 |
| 6/10 (60%) | 10 | – | Betamethasone Na PO4 2mg/cat twice weekly | Inconspicuous inflammatory changes, except alveolar macrophages often seen. Foamy or vacuolated alveolar exudate was not observed. | + | + | + Light PCP infection | 16 |
| 6/7 (86%) | 7 | – | Prednisolone acetate 10–12 mg/cat weekly | Inconspicuous inflammatory changes, except alveolar macrophages often seen. Foamy or vacuolated alveolar exudate was not observed. | + | + PCP infection | 16 | |
| 3/3 (100%) | 3 | N/R | Methyl-prednisolone 30 mg for 2 weeks, follow by 15 mg for 6 weeks | N/R | – | – | – | 28 |
| 5/5 (100%) | 5 | N/R | Methyl-prednisolone 10 mg/kg for 5–10 weeks | N/R | –* | –* | – | 20 |
Reference numbers in the table correspond to the references at the end of the manuscript. N/R, not recorded.
*DiffQuik-stained.
Although lower airway disease in cats has been studied extensively in companion animal medicine using modalities including radiology, computed tomography, bronchoalveolar lavage (BAL) cytology, microbiology, and examination of lung tissues at biopsy or necropsy, no clinical cases of pneumonia due to Pneumocystis occurring in cats have been described in the clinical veterinary literature to the best of our knowledge. There are, however, currently no molecular tools to assist the diagnosis of pneumocystosis in cats with atypical lung disease, or to determine if Pneumocystis may play a role in exacerbating clinical signs in cats with comorbid lung disease, for example, viral pneumonia (feline calicivirus, feline herpesvirus type 1, pox virus, etc.), “feline asthma” and mycoplasma disease of the lower airways. In addition, studying Pneumocystis in cats affords us the opportunity of determining the taxonomy of feline Pneumocystis in relation to Pneumocystis species encountered in the full range of mammalian species.
The aim of this study was to establish if Pneumocystis DNA could be detected in the lungs of normal cats using a molecular protocol targeting mitochondrial gene targets and to describe its prevalence and significance as a potential pathogen. Further, we sought to determine the genetic relatedness of feline Pneumocystis isolates with those of rodents, dogs, ferrets, pigs, and other species.
Methods
Sampling
Lung tissue from 84 cats (Felis catus) were screened for Pneumocystis DNA at the Mycology Unit of the Parasitology Laboratory, Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe–Italy). Lung specimens were obtained from necropsy examinations by the Diagnostic Department of IZSVe, from cats that died because of cancer, acute viral disease, chronic kidney disease, or following vehicular trauma. The right lung of each cat was weighed. The mean weight of the right lung was 30 g (range 15–45 g). The specimen size investigated further (1.0–1.1 g in total) represented approximately 3% of the weight of the right lung, including an equal portion from each of the cranial, middle and caudal lung lobes, respectively. Bronchoalveolar lavage (BAL) specimens were obtained from cats presented to the Clinica Veterinaria San Marco (Padua–Italy), including patients with “labored breathing” or “respiratory distress.” BAL fluid and lung tissue samples were preserved at −20°C prior to DNA extraction and polymerase chain reaction (PCR) testing. Signalment and clinical findings of cats are summarized in Table 2, Supplementary Table 1 (lungs), and Supplementary Table 2 (BAL fluid specimens).
Table 2.
Clinical and necropsy data from PCP PCR-positive cats presented for necropsy examination. DSH = domestic short-haired cat; M = male; F = female; MN = male neutered; FN = female neutered; Young animals (age ≤ 1year); Adult (age > 1 year); BCS – body condition score; n/a = not available; nd = not done. –ve = negative; +ve = positive.
| No. | Lifestyle | Origin (Province) | Breed | Age* | Sex | BCS | Lung - Macroscopic | Lung - Histology (H&E) | Corona-virus (qPCR) | FPV (IHM) | FeLV | FIV | Presumptive cause of death | Pneumocystis lineage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Family | Padua | Maine Coon | Young | F | Good | Inflamed areas with pulmonary consolidation in both lungs. Fibrin exudation. Presence of foam and whitish material in bronchi. | Focal granulomatous pneumonia with fibrinous exudation, abundant bacterial and congestive pneumonia. Rare fibrin thrombus in blood vessels. | nd | +ve | nd | nd | Gastro-enteritis | Lineage 1 |
| 2 | Family | Padua | Scottish Fold | Young | M | Poor | Oedema and pulmonary congestion at apical lobes | Atelectasis. Emphysema and alveolar oedema. | +ve | -ve | nd | nd | Peritonitis | Lineage 1 |
| 3 | Street | Padua | Italian DLH | Young | F | Good | Congestion of parenchyma. | Congestion of parenchyma. Alveolar oedema foci. | -ve | -ve | nd | nd | Catarrhal enteritis | Lineage 1 |
| 4 | Street | Padua | Italian DSH | Young | M | Poor | Lung collapsed. Fibrino-purulent bronchopneumonia | Fibrino-purulent necrotic bacterial pneumonia | nd | +ve | nd | nd | Pneumonia | Lineage 1 |
| 5 | Street | Padua | Italian DSH | Young | M | Good | Pulmonary congestion | nd | nd | nd | nd | nd | Catarrhal enteritis | Lineage 1 |
| 6 | Street | Padua | Italian DSH | Young | M | Poor | Absence of lung alterations | nd | +ve | +ve | nd | nd | Catarrhal-haemorrhagic enteritis | Lineage 1 |
| 7 | Street | Padua | Italian DSH | Young | M | Poor | Not reported | Autolysis. Presence of numerous bacteria. | -ve | +ve | nd | nd | Catarrhal haemorrhagic gastritis and enteritis | Lineage 1 |
| 8 | Street | Padua | Italian DSH | Young | M | Poor | Congestion of parenchyma. Atelectasis apical lobe | Pyogranulomatous pneumonia. | nd | nd | nd | nd | Pneumonia | Lineage 1 |
| 9 | Family | Rovigo | Italian DSH | Young | M | Good | Pneumonia and emphysema | Lymphocytic Interstitial pneumonia and fibrinopurulent thrombo-embolic dissemination. | -ve | nd | nd | nd | Pneumonia | Lineage 1 |
| 10 | Street | Padua | Italian DSH | Young | F | Good | Abdominal haemorrhage and hemoperitoneum | nd | nd | nd | nd | nd | Vehicular trauma | Lineage 1 |
| 11 | Family | Palermo | Italian DSH | Young | F | Good | Bilateral pleural effusion | Alveolar atelectasis, presence of bacteria in the alveoli. | +ve | -ve | -ve | -ve | Necrotic enteritis | Lineage 1 |
| 12 | n/a | n/a | n/a | Young | F | Good | Presence of coagulated blood in trachea. Haemorrhagic areas. | nd | nd | nd | nd | nd | Vehicular trauma | Lineage 1 |
| 13 | Street | Padua | Italian DSH | Adult | MN | Good | Pulmonary congestion | nd | nd | nd | nd | nd | Hepatic adenocarcinoma | Lineage 1 |
| 14 | Family | Venice | Italian DSH | Adult | MN | Good | Not reported | Alveolar oedema. | nd | nd | -ve | -ve | Hepatitis and catarrhal haemorrhagic enteritis | Lineage 1 |
| 15 | Street | Rovigo | Italian DSH | Adult | F | Poor | Not reported | nd | nd | nd | nd | nd | Vehicular trauma | Lineage 1 |
| 16 | Street | Venice | Italian DSH | Adult | F | Good | Pulmonary congestion and oedema | Oedema and alveolar congestion. | -ve | -ve | nd | nd | Necrotic superficial enteritis. Nephritis. | Lineage 1 |
| 17 | Street | Venice | Italian DSH | Adult | F | Poor | Pulmonary congestion and oedema | nd | nd | +ve | nd | nd | Chronic nephritis | Lineage 1 |
| 18 | Street | Padua | Italian DLH | Adult | F | Good | Congestion of parenchyma. | Alveolar oedema. | nd | nd | nd | nd | Vehicular trauma | Lineage 1 |
| 19 | Family | Padua | Italian DSH | Adult | M | Good | Pulmonary congestion and haemorrhage at apical lobes | Diffuse haemorrhage. | nd | nd | nd | nd | Vehicular trauma | Lineage 1 |
| 20 | Street | Padua | Italian DSH | Young | F | Good | Pleural and pericardial serum-hematic effusion; pulmonary congestion. | nd | -ve | -ve | nd | nd | Enteritis | Lineage 2 |
| 21 | Street | Venice | Italian DSH | Young | M | n/a | Pulmonary congestion | Lymphocytic interstitial pneumonia. | +ve | -ve | nd | nd | Vehicular trauma | Lineage 2 |
| 22 | Street | Padua | Italian DSH | Adult | M | Poor | Pulmonary congestion | Partial destruction of the parenchyma consisting of interstitial infiltration of round cells of lymphoid origin. | -ve | nd | +ve | -ve | Lymphoma | Lineage 2 |
| 23 | Family | Rovigo | Italian DSH | Adult | MN | Good | Pulmonary consolidation | Low preservation of cells due to freezing storage. | nd | nd | nd | nd | Enteritis | Lineage 2 |
| 24 | Street | Venice | Italian DSH | Adult | FN | n/a | Absence of lung alterations | nd | -ve | +ve | -ve | -ve | Haemorrhagic gastritis and catarrhal enteritis | Lineage 2 |
DNA extraction from lung tissue and BAL fluid specimens
Lung tissue
For each cat, lung specimens were placed into a 2 ml tube with 800 μl phosphate-buffered saline (PBS) and 1 tungsten bead (5 mm diameter), and lysed in a Tissue-Lyser (Qiagen, Hilden, Germany) for 1.5 min at 30 Hz. From each tube, 50 μl was transferred into a new 1.5 ml tube. A total of 150 μl of homogenated tissue (made up from the three portions of right lung) was used for DNA extraction.
BAL fluid specimens
BAL fluid (1 ml) was transferred to a 2 ml microcentrifuge tube containing 1 tungsten bead (5 mm diameter). A bead-beating step was applied for 1 min at 30 Hz to maximize DNA yield.
DNA extraction
For both lung tissue and BAL fluid, DNA extraction was performed using the DNAeasy Blood & Tissue kit (Qiagen, Courtaboeuf, France) following the manufacturer's instructions, with minor modifications. Each homogenized sample (150 μl of homogenated lung tissue and 100 μl of BAL fluid) was transferred into a 1.5 ml tube with 200 μl buffer (ATL lysis buffer) and 20 μl proteinase K and incubated overnight at 56°C. DNA was eluted in 100 μl of elution buffer. A negative control (sterile water) was systematically included in each series of DNA extractions.
PCR amplification protocols
Lung tissues and BAL fluid specimens were investigated for Pneumocystis DNA by using:
mtSSU rRNA nested-PCR
A 320–340 bp fragment of the mtSSU rRNA gene was amplified by nested PCR with two primer pairs (Paz 112-10F and Paz 112-10R as external primers; Paz 112–13 and Paz112-14 as internal primers) and PCR conditions as reported previously.21
mtLSU rRNA real-time PCR
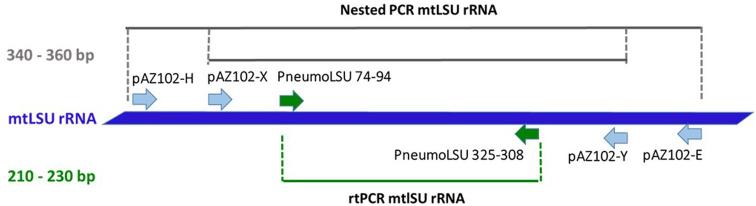
The nested mtLSU rRNA PCR protocol reported previously21 did not amplify Pneumocystis DNA from any feline lung specimens (including mtSSU rRNA Pneumocystis PCR-positive cats). Accordingly, a new set of primers (PneumoLSU 74–94 FOR 5′-AGGATATAGCTGGTTTTCTGC-3′and PneumoLSU 325-308 REV 5′-TRTTCTGGGCTGTTYCCC-3′) was designed, targeting a short segment (210–230 bp) of the mtLSU rRNA gene. This region is included between the internal primers of the nested mtLSU rRNA PCR (pAZ102-X 5′-GTGAAATACAAATCGGACTAGG-3′ and pAZ102-Y 5′-TCACTTAATATTAATTGGGGAGC-3′22 (Fig. 1). Primers were designed manually, according to general primer design rules,23 based on a sequence alignment of reference sequences from different Pneumocystis spp. available from the NCBI database (http://www.ncbi.nlm.nih.gov). The sequence specificity of the primers used was confirmed in a BLASTn search24 against GenBank.
Figure 1.
Schematic diagram indicating qPCR protocol and primers designed for the mtLSU rRNA fragment. The names of the new forward and reverse primers are provided in green (arrows). On the left, length of amplification products (bp) is provided. This Figure is reproduced in color in the online version of Medical Mycology.
Negative and positive “Pneumocystis carinii DNA” controls were included in each PCR experiment, to monitor for possible contamination and determine the correctness of the amplification. Part of the Felis catus interphotoreceptor retinoid-binding protein (IRBP) gene was amplified as an internal control to monitor for DNA-inhibition.25 Samples were considered as positive for Pneumocystis presence when a PCR product of the expected size was amplified and sequenced. Nested PCR was performed using AmpliTaq Gold DNA Polymerase (Life Technologies, Foster City, CA, USA) on the Veriti Thermal Cycler (Life Technologies). Real-time PCR (qPCR) was performed using the StepOnePlus™ Real-time PCR Systems (Applied Biosystems, Foster City, CA, USA).
Sequencing and phylogenetic analysis
Sequencing reactions were performed from both ends, as reported previously.26 The sequence alignment was performed using the ClustalW algorithm, integrated in MEGA 6.1,27 which was then refined manually. Molecular phylogeny was performed using the Maximum likelihood (ML) approach on the mtLSU rRNA and mtSSU rRNA data sets. Hasegawa-Kishino-Yano or Tamura 3 parameter and G distribution were determined as best model in MEGA 6.1. We also added to our dataset, Pneumocystis sequences isolated from dogs, pigs, non-human primates, humans and rodents available from GenBank. Sequences of P. wakefieldiae, P. carinii, and P. murina isolated from rodents were used as an outgroup. Accession numbers of the Pneumocystis sequences used, including sequences produced in the study, are presented in Table 3.
Table 3.
GenBank accessions of Pneumocystis used for phylogeny.
| Host species | Pneumocystis species | mtLSU rRNA | mtSSU rRNA |
|---|---|---|---|
| Canis lupus familiaris (dog) | Pneumocystis sp. | KU985306 | KU986906 |
| Felis catus (cat) | Pneumocystis sp. (L1) | MH818398 | MH818401 |
| Pneumocystis sp. (L1) | MH818399 | MH818402 | |
| Pneumocystis sp. (L2) | MH818400 | MH818403 | |
| Homo sapiens (man) | Pneumocystis jirovecii | KX017566 | HQ228547 |
| Indian Macaca rhesus (Rhesus monkey) | Pneumocystis sp. | AF461784 | AF395573 |
| Mus musculus (house mouse) | Pneumocystis murina | – | AB626627 |
| Mustela putorius furo (ferret) | Pneumocystis sp. | S42921 | – |
| Rattus norvegicus (brown rat) | Pneumocystis wakefieldiae | FJ475121 | – |
| Pneumocystis carinii | M58604 | – | |
| Sus scrofa domesticus (pig) | Pneumocystis sp. | JN887823 | KC454437 |
Dash (–), sequence not available; mtLSU rRNA, mtSSU rRNA, mitochondrial large and small subunit of ribosomal RNA, respectively. Sequences produced in the current study are reported in boldface.
Histology and cytology
Lung tissue specimens (a small portion of cranial right lung lobe) were obtained from unfrozen feline carcasses presented to our institute. After fixation in neutral buffered formalin, paraffin embedding and routine sectioning at 4 μm, selected tissue sections were stained with hematoxylin and eosin (H&E), according to clinical suspicions. Additional histological preparations, stained with Grocott-Gomori's methenamine silver stain (GMS) and Toluidine Blue, were performed from lungs of 15 cats confirmed as being Pneumocystis DNA-positive by molecular investigations. Cytology of cytocentrifuged BAL specimens were prepared and stained using Diff-Quik® at the Clinica Veterinaria San Marco, a specialist centre where cats were admitted for veterinary investigation and treatment.
Statistical analysis
Epidemiologic data pertaining to cats (age, breed, sex, neuter status, provenance) and other data collected concerning their health status (nutritional history, lung alterations, presence of parasites or other infectious diseases, causes of death) were tested for statistical association with Pneumocystis prevalence using the Chi-square test or Fisher's exact test, as appropriate (software SPSS for Windows, version 13.0).
Results
PCR data
Pneumocystis DNA was amplified only from the lung tissue specimens. All feline BAL fluid specimens tested negative for Pneumocystis DNA. Overall, Pneumocystis DNA was detected in 24/84 (29%) of fresh feline lung specimens using both the mtSSU rRNA nested PCR and the mtLSU rRNA real-time PCR (qPCR).
Among the 24 PCR-positive cats, 14 were young, and 16 cases were from cats living in “cat colonies.” Domestic short haired cats comprised 22 of these cats, with a single Main Coon and a single Scottish Fold. Statistically, no feline characteristics were associated with occurrence of Pneumocystis, except for age, that is, cats younger than 1 year showed higher PCP prevalence (54%) compared to older individuals (13%) (Pearson χ2 15.2; P < .0001). There was no statistical association between Pneumocystis DNA presence (colonization) and sex, neuter status, plane of nutrition/body condition score, or lifestyle (family vs street cats).
The causes for death or euthanasia in this group was enteritis (11 cats), vehicular trauma (6 cats), feline infectious peritonitis (1 cat), pneumonia (3 cats), cancer (2 cats), and nephritis (1 cat). A total of 6/12 cats tested were infected by feline panleukopenia virus, while 4/11 tested were feline enteric Coronavirus-positive. Of the 24 PCP-positive cats, only four cats were tested for retroviruses (i.e., FIV and FeLV). Of these 4 cats, there was one FeLV-positive individual (no. 22; Table 2).
Pulmonary coinfection with bacteria was detected in 9/24 cats, the isolates consisting of a Corynebacterium spp. and Enterobacter spp. (1 cat), Staphylococcus spp. (3 cats), Escherichia coli and Enterococcus (2 cats), Enterococcus alone (1 cat), Streptococcus canis (1 cat), and polymicrobial (1 cat). Three of 24 PCP-positive cats were coinfected by intestinal helminths; two cats were coinfected with Taenia taeniaeformis and Toxocara cati, whereas ascarids alone were identified in a single cat.
At necropsy, lungs for the 24 PCP PCR-positive cats were described as having no gross alterations (2 cats), congestion (13 cats), fibrinopurulent bronchopneumonia (1 cat), pulmonary consolidation (1 cat), fibrin exudation and presence of foam and whitish material in bronchial lumen (1 cat), and pneumonia and emphysema (1 cat). Pleural effusion was reported in two cats with severe enteritis (no. 20 and no. 11; Table 2), and data was unavailable for 3 cats. Abdominal and thoracic hemorrhage were evident in cats that died following vehicular trauma.
Lung histology
Lung histology largely reflected the cause of death for cats recruited opportunistically for this study. H&E- stained sections were available for 15 of 24 cats PCR-positive for Pneumocystis. Of the younger cats (n = 9), two cats had granulomatous pneumonia with fibrinous exudation and abundant bacterial (no. 1, no. 8; Table 2), two had pulmonary congestion and alveolar edema (no. 2, no. 3; Table 2), one had fibrinopurulent necrotizing bacterial pneumonia (no. 4; Table 2), and two cats had lymphocytic interstitial pneumonia (no. 9, no. 21; Table 2). Atelectasis and autolysis with presence of numerous bacteria was described in two cats (no. 7, no. 11; Table 2).
Of the six adult cats, one had diffuse hemorrhage following vehicular trauma (no. 19; Table 2), another was uninterpretable due to freezing artifact (no. 23; Table 2), and three cats had alveolar edema (no. 14, no. 16, no. 18; Table 2). Histology of the cat (no. 22; Table 2) with lymphoma showed partial invasion of the parenchyma consisting of interstitial infiltration with round neoplastic lymphoid cells.
Critically, none of the cats had microscopic changes consistent with PCP pneumonia, although only representative sections of lung were examined. Additional Gomori methenamine silver (GMS) and Toluidine Blue stained preparations were performed from blocks of lung tissues PCR-positive for Pneumocystis DNA, but there was no evidence for asci (cysts) or trophic forms (trophozoites) in any of the lung specimens examined from these seven cats.
Molecular biology and phylogeny
The presence of Pneumocystis DNA was determined using two different mitochondrial genome targets, mtSSU rRNA amplified using nested PCR and mtLSU rRNA amplified by Sybr Green qPCR. Alignments discriminated two separate lineages (or sequence types [ST]) in the mtSSU rRNA Pneumocystis sequences and two lineages in the mtLSU rRNA sequences. This distinction between ST was consistent irrespective of which PCR protocol was adopted, for all 24 cats in which results for both PCR assays were available for comparison.
For the mtSSU rRNA gene, lineage 1 (SSU-L1) consisted of a 345 bp amplicon, which was identical in all instances (19 cats) with respect to sequence length, nucleotide composition, and position. Lineage 2 (SSU-L2) consisted of a 281 bp amplicon, identical in all instances (five cats) in terms of nucleotide composition and position. Comparison of the two lineages based on the mtSSU rRNA gene (SSU-L1 and SSU-L2) estimated a sequence divergence of 0.314.27
For the mtLSU rRNA gene, lineage 1 (LSU-L1) consisted of an identical sequence of 207 bp from 19 cats. Lineage 2 (LSU-L2) consisted of an identical 201 bp amplicon from the five remaining cats. Comparison of LSU-L1 and LSU-L2 estimated a sequence divergence of 0.398.27
Lineage 1 was identified in the same 19 cats for both mtSSU rRNA and mtLSU rRNA gene targets, while lineage 2 was identified in the same five cats, and again, for both gene targets. Thus, only a single lineage (lineage 1 or lineage 2) was identified in any given cat.
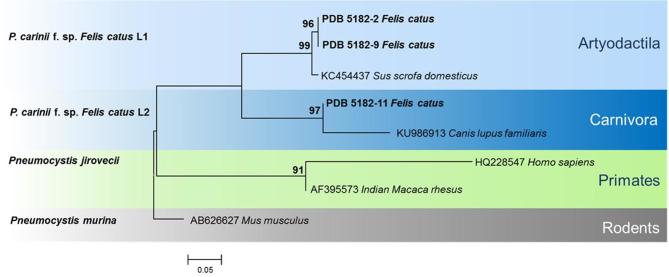
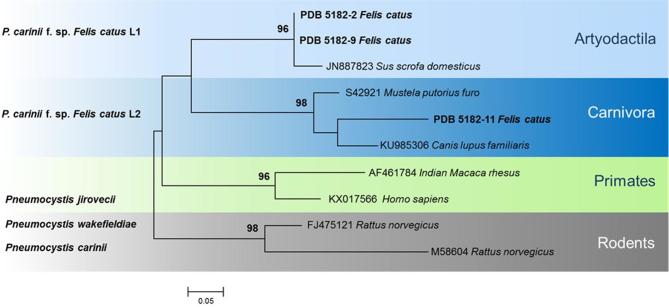
Two rooted trees were constructed using mtSSU rRNA and mtLSU rRNA sequences (Fig. 2, 3). In the mtSSU rRNA tree, lineages SSU-L1 and SSU-L2 grouped into separate clades of Pneumocystis, from the Artyodactila (SSU-L1) and the Carnivora (SSU-L2). The SSU-L1 lineage clustered in the same group as Pneumocystis isolated from pigs, whereas SSU-L2 clustered with Pneumocystis from dogs (Fig. 2). In the mtLSU rRNA tree, the ST LSU-L1 grouped in the clades isolated from the Artyodactila, while LSU-L2 was grouped with the Carnivora clade, showing greater similarity to dog Pneumocystis than ferret Pneumocystis (Fig. 3). In both mtSSU rRNA and mtLSU rRNA dendrograms, Carnivora and Artyodactila were paraphyletic and well separated from primate and rodent clades, used as outgroups. The backbone showed good support for the deepest nodes, but terminal clades were resolved at bootstrap values of ≥ 90%.
Figure 2.
Phylogenetic maximum likelihood tree of Pneumocystis sequences of mitochondrial small subunit of ribosomal RNA (mtSSU rRNA). Tamura 3 parameter and G distribution was determined to be the best model. Pneumocystis sequences from our study are reported in bold. Pneumocystis murina is used as an outgroup. Bootstrap values (>90) are shown at the internal nodes. This Figure is reproduced in color in the online version of Medical Mycology.
Figure 3.
Phylogenetic maximum likelihood tree of Pneumocystis sequences of mitochondrial large subunit of ribosomal RNA (mtLSU rRNA). Hasegawa-Kishino-Yano and G distribution was determined as best model. Pneumocystis sequences from our study are reported in bold. Pneumocystis wakefieldiae and P. carinii are used as outgroups. Bootstrap values (>90) are shown at the internal nodes. This Figure is reproduced in color in the online version of Medical Mycology.
Discussion
The molecular findings of this study support earlier cytological and histological observations suggesting that cats can harbor Pneumocystis in their lower respiratory tract. Our results suggest that young cats (< 1 year) are more likely to be infected by Pneumocystis than older cats, even though naturally occurring Pneumocystis pneumonia has yet to be reported in feline patients of any age. The presence of Pneumocystis, determined by the visualization of cysts and/or trophic forms in microscopic preparations of feline lung,17–19 are consistent with the present work, where Pneumocystis gene targets were amplified from a finite proportion of lung specimens from normal cats that died following vehicular trauma, chronic kidney disease or systemic viral illness.
Cats investigated subsequent to those early reports were drawn mostly from urban areas (comparable to the “cat colonies” in our study), with lung appearing normal on macroscopic examination, with no inflammatory changes consistent with Pneumocystis pneumonia evident macroscopically or microscopically despite trophic forms and cysts being present in some alveolar spaces.19Pneumocystis prevalence in naturally colonized cats ranged from 0 to 13% based on conventional light or electron microscopy,13,17–19,28 which is in reasonable agreement with the 29% prevalence based on PCR data in our study. A higher observed prevalence using sensitive DNA detection tools is to be expected compared to cytological and histological methods. Unlike some of the original work, we were unable to find evidence of Pneumocystis morphotypes in histological preparations, most probably due to the poor storage of animal carcasses (freeze thaw artifact, autolysis, etc.).
The absence of Pneumocystis morphotypes in histological sections might also be influenced by a low absolute presence of cysts and trophic forms. Pneumocystis cysts are easily visualized using GMS, but trophozoites remain unstained with silver impregnation methods. Thus, trophozoites are difficult or impossible to visualize in histological sections from modestly colonized lung, unless in situ hybridization (ISH) is used, as has been recently described for pig lungs.29
In histology preparations of Pneumocystis PCR-positive cats, we also did not detect lung damage referable to Pneumocystis infection. The same observations have been made in human autopsy studies, in which Pneumocystis jirovecii can be detected without observation of obvious pulmonary lesions in immunocompetent infants.2,30 This might be a reflection of our study representing a single time-point analysis, whereas a sequential analysis using detailed microscopic morphometry to follow the course of Pneumocystis infection, including younger kittens during the primary stage of infection, would permit detection of Pneumocystis-associated pathology. Indeed, a recent rodent model study documented that subclinical primary Pneumocystis infection can induce progressive pathologic changes characteristic of airway disease in immunocompetent hosts, such as increased mucus production and thickening of the airway epithelium.31
To the best of our knowledge, this is the first molecular genetic investigation that describes Pneumocystis in cats or other felids using sequence-based PCR methodology. In 1999, Cho and colleagues20 established that there were at least three distinct karyotype groups of Pneumocystis among mammals in Korea. Furthermore, it was shown that chromosomal band size for cat isolates resembled dog isolates but were larger than rat isolates (730 bp for cat/dog vs 300–700 bp for rat). The chromosomal band in cats and dogs was therefore the first band of Pneumocystis over 700 bp to be described.20
Finding two distinct Pneumocystis lineages in cats is consistent with the heterogeneity seen in other animal species, where co-infection with different Pneumocystis ST (or species) in each mammalian host is well described, especially in rodents.32 Only one ST lineage was found in any given cat. The two novel Pneumocystis lineages represented by our phylogenetic trees do not correspond to known Pneumocystis species or ST. In terms of genetic relatedness, Pneumocystis from rodents remain distantly related, while the two new feline Pneumocystis lineages appear to be closer to those of the Artyodactila (even-toed ungulates) and Carnivora groups, respectively.
In phylogenetic studies of mammals, the Rodentia order is often suggested to be an outgroup, compared with lagomorph (rabbit), primate, artiodactyl and carnivore orders.33 We cannot exclude that additional Pneumocystis lineages might eventually be characterized from feline lung. One difficulty in studying this organism is the poor availability of Pneumocystis sequences in well-curated public databases for use in phylogenetic analyses. For example, no Pneumocystis sequences from cats or other Felidae were present in Genbank. It would be of great interest to extend the present work to pulmonary tissue specimens from domestic cats to the rest of the Felidae, perhaps using archived formalin-fixed paraffin embedded tissues from lions, tigers, cheetahs, and so forth, as such animals living in zoological collections are typically subjected to terminal necropsy after death or euthanasia.
In addition, whole genome sequencing studies on Pneumocystis from different mammalian species, facilitated by the description of Pneumocystis metabolic pathways by Ma et al. (2016),6 would help provide additional insights into key differences between Pneumocystis isolated from disparate mammalian species. This might provide insights into why species specificity is such a pronounced feature of this genus.
We cannot exclude that one of the lineages found in cats (lineage 1 or 2) are not exclusively feline-associated, but shared among several host species (i.e., weaker host specificity), as has been described for wild rodents.26,32 In nature, this proposition is teleologically unattractive, as cats for most of their evolutionary history experience a mostly solitary life, functioning as territorial predators. Thus, the natural route for transmission of Pneumocystis is most likely queen (mother) to kitten, and this would impact on the evolution of Pneumocystis in cats. On the other hand, it seems plausible that Pneumocystis from rodents (rats and mice), the natural prey of cats, might “cross over” during predation, although our data and the generally accepted species specificity of Pneumocystis argue against such a proposition. This is of course quite different from the situation when cats live in domesticated or semidomesticated life situations in close association with human society, where cat-to-cat transmission within households, or in areas where cats congregate outside (e.g., rubbish tips, cat shelters, and free-roaming colonies) is also possible.
In mammals, most Pneumocystis infections seem to consist of benign colonization, perhaps occasionally extending to subclinical disease as a co-pathogen to bacterial or viral primary respiratory pathogens. This type of scenario occurs for example in rats, where Sendai virus, sialodacryoadenitis virus, and pneumonia virus of rats potentially act in concert with mycoplasmas or Pneumocystis spp. to cause disease.34,35 Symptomatic PCP has been shown to occur in cats under artificial experimental conditions, usually using enormous doses of corticosteroids given repeatedly to suppress immune responses. However, clinical PCP, analogous to what is described for man, dog and horse,19,36,37 is yet to be described for any cat despite widespread use of immunosuppressive drug regimens (corticosteroids, chlorambucil, cyclosporine), renal transplantation, and comorbid viral infections such as pox, FeLV, FIV, FIP Felis catus gamma herpesvirus 1. Our failure to detect any PCP PCR-positive BAL fluid specimens from cats with a wide range of lower respiratory conditions suggests Pneumocystis is not likely to be an important player in infectious diseases of the airways and lung parenchyma of cats.
In humans, the occurrence of clinical PCP with fulminant multiplication of the agent is clearly linked with immunosuppression (e.g., long-standing HIV infection with CD4-cytopenia),; however, this association is less evident in various animal species.28,38 In the dog and horse, clinical PCP is generally seen in young animals and related to inherited immunodeficiency states, for example, combined immunodeficiency in Arabian horses37 and PCP in Cavalier King Charles Spaniels and miniature dachshunds, and their hybrids.21,36,39,40
Rare spontaneous cases of Pneumocystis pneumonia (PCP) in pigs (adult and piglets) are mostly linked with immunosuppression from adverse environmental conditions (stressful farming conditions, overcrowding, high ambient ammonia concentrations, perhaps complicated by suboptimal nutrition) and/or genetic predispositions.41–43 In pigs, Pneumocystis is generally eliminated from the lungs or constrained to small quiescent foci unless such immunosuppressive conditions are present.43,44
The concurrent presence of other opportunistic pathogens such as Bordetella bronchiseptica in the airways and demodicosis in the skin of dogs,40 and porcine circovirus type 2 (PCV-2) and Mycoplasma hyopneumoniae in pigs29 with Pneumocystis has been well established. The same is true also for macrobats, where, for example, Histoplasma capsulatum coexists with Pneumocystis spp. in many individuals.45 In contrast, in pigs there seems to be a general tendency that concurrent viral and bacterial infections are less frequently observed in Pneumocystis-positive cases than in Pneumocystis-negative individuals.29 A negative association between the presence of P. jirovecii and bacterial colonization was also shown in patients with idiopathic interstitial pneumonia.46 Thus, which factor(s) trigger multiplication of trophic forms and whether a simple infection with Pneumocystis (without the presence of other infectious agents) can induce lung injury and dysfunction appears to be unique in different host-environment-pathogen systems.
In the 1980s, cats affected by FeLV, an oncogenic feline retrovirus capable of causing severe acquired immunodeficiency, were studied as a potential animal model for the development of opportunistic infection in AIDS patients. In one study of FeLV-infected cats, acute bronchopneumonia, leukemic infiltrates, interstitial pneumonitis, and adenomatosis were all observed13 (Table 1), but despite a concerted search, there was no microscopic evidence for Pneumocystis infection. These findings suggested that Pneumocystis is not commonly a complication of FeLV infection, nor a cause of lethal interstitial pneumonia in cats13 (Table 1). Along similar lines, FIV is a very well-studied lentivirus of cats, and despite great scrutiny in both experimental and naturally occurring settings, no association has ever been made between development of PCP and long-standing FIV infection in cats with greatly reduced numbers of circulating CD4 cells.14
Results of the present study support the notion that cats can be asymptomatically infected by Pneumocystis spp. Such cats are likely to be colonized or subclinically infected but not in association with substantial tissue destruction or injury, and thus with no discernible lung dysfunction.47 None of the cats investigated in the current study were thought to have pathological pulmonary changes referable to pneumocystosis. As in humans, young cats seem more likely to be Pneumocystis-positive, but no other factors (sex, lifestyle, cause of death, etc.) in this study were associated with colonized cats. In experimental animal model studies, cats have been shown to be less susceptible to Pneumocystis infection when compared with rodents28 (Table 1). Indeed, corticosteroid treated cats generally developed only a mild Pneumocystis infection, in marked contradistinction with the heavy symptomatic PCP that developed in corticosteroid treated rats,28 suggesting that cats are less susceptible to Pneumocystis disease than other mammals. Without evidence of Pneumocystis in a specimen (BAL or lung biopsy) from a cat with respiratory distress and radiological findings consistent with PCP, we cannot confirm or deny any role for Pneumocystis in the pathogenesis of feline lung disease. In addition, modern metagenomics tools open the possibility to hypothesize that the presence/absence of Pneumocystis might be affected not only by the microbiota of lungs, but also of intestinal microbial community interfering in metabolic pathways,46,48,49 a field of research yet to be well explored in veterinary patients.
Supplementary Material
Acknowledgements
We thank Giorgia Monetti and Erika Melchiotti for technical support. The work was partially supported by the Italian Ministry of Health (project RC IZSVe 01/2015). R.M. is supported by the Valentine Charlton Bequest of the Centre of Veterinary Education at the University of Sydney.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Aliouat-Denis CM, Chabé M, Demanche C et al.. Pneumocystis species, co-evolution and pathogenic power. Infect Genet Evol. 2008; 8: 708–726. [DOI] [PubMed] [Google Scholar]
- 2. Vargas SL, Hughes WT, Santolaya ME et al.. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis. 2001; 32: 855–861. [DOI] [PubMed] [Google Scholar]
- 3. Rojas P, Friaza V, García E et al.. Early acquisition of Pneumocystis jirovecii colonization and potential association with respiratory distress syndrome in preterm newborn infants. Clin Infect Dis. 2017; 65: 976–981. [DOI] [PubMed] [Google Scholar]
- 4. Alanio A, Bretagne S.. Pneumocystis jirovecii detection in asymptomatic patients: what does its natural history tell us? F1000Research. 2017; 6: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012; 25: 297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma L, Chen Z, Huang DW et al.. Genome analysis of three Pneumocystis species reveals adaptation mechanisms to life exclusively in mammalian hosts. Nat Commun. 2016; 7: 10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma L, Cissé OH, Kovacs JA. A molecular window into the biology and epidemiology of pneumocystis spp. Clin Microbiol Rev. 2018; 31: e00009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chabé M, Dei-Cas E, Creusy C et al.. Immunocompetent hosts as a reservoir of Pneumocystis organisms: histological and rt-PCR data demonstrate active replication. Eur J Clin Microbiol Infect Dis. 2004; 23: 89–97. [DOI] [PubMed] [Google Scholar]
- 9. Demanche C, Berthelemy M, Petit T et al.. Phylogeny of Pneumocystis carinii from 18 primate species confirms host specificity and suggests coevolution. J Clin Microbiol. 2001; 39: 2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017; 17: e334–e343. [DOI] [PubMed] [Google Scholar]
- 11. Phipps LM, Chen SC-A, Kable K et al.. Nosocomial Pneumocystis jirovecii pneumonia: lessons from a cluster in kidney transplant recipients. Transplantation. 2011; 92: 1327–1334. [DOI] [PubMed] [Google Scholar]
- 12. Chapman JR, Marriott DJ, Chen SC-A, MacDonald PS. Post-transplant Pneumocystis jirovecii pneumonia a re-emerged public health problem? Kidney Int. 2013; 84: 240–243. [DOI] [PubMed] [Google Scholar]
- 13. Hagler DN, Kim CK, Walzer PD. Feline leukemia virus and Pneumocystis carinii infection. J Parasitol. 1987; 73: 1284–1286. [PubMed] [Google Scholar]
- 14. Miller C, Abdo Z, Ericsson A, Elder J, VandeWoude S. Applications of the FIV model to study HIV pathogenesis. Viruses. 2018; 10: E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kadar E, Sykes JE, Kass PH, Bernsteen L, Gregory CR, Kyles AE. Evaluation of the prevalence of infections in cats after renal transplantation: 169 cases (1987–2003). J Am Vet Med Assoc. 2005; 227: 948–953. [DOI] [PubMed] [Google Scholar]
- 16. Shiota T, Shimada Y, Kurimoto H, Oikawa H. Pneumocystis carinii infection in corticosteroid-treated cats. J Parasitol. 1990; 76: 441–445. [PubMed] [Google Scholar]
- 17. Davalos Mata A. Latent infection produced by Pneumocystis carinii in domestic animals in Mexico. Salud Publica Mex.1963; 5: 975–977. [PubMed] [Google Scholar]
- 18. Zavala J, Rosado R. Pneumocystis carinii in domestic animals of the city of Mexico, Yucatan. Salud Publica Mex. 1972; 14: 103–106. [PubMed] [Google Scholar]
- 19. Settnes OP, Hasselager E. Occurrence of Pneumocystis carinii Delanoë & Delanoë, 1912 in dogs and cats in Denmark. Nord Vet Med. 1984; 36: 179–181. [PubMed] [Google Scholar]
- 20. Cho SR, Park YG, Moon HN, Lee SH, Hong ST. Karyotypes of Pneumocystis carinii derived from several mammals. Korean J Parasitol. 1999; 37: 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danesi P, Ravagnan S, Johnson LR et al.. Molecular diagnosis of Pneumocystis pneumonia in dogs. Med Mycol. 2017; 55: 828–842. [DOI] [PubMed] [Google Scholar]
- 22. Wakefield AE. DNA sequences identical to Pneumocystis carinii f. sp. carinii and Pneumocystis carinii f. sp. hominis in samples of air spora. J Clin Microbiol. 1996; 34: 1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dieffenbach CW, Lowe TM, Dveksler GS. General concepts for PCR primer design. PCR Methods Appl. 1993; 3: S30–S37. [DOI] [PubMed] [Google Scholar]
- 24. Madden TL, Tatusov RL, Zhang J. Applications of network BLAST server. Methods Enzymol. 1996; 266: 131–141. [DOI] [PubMed] [Google Scholar]
- 25. Ferreira EC, Gontijo CM, Cruz I, Melo MN, Silva AM. Alternative PCR protocol using a single primer set for assessing DNA quality in several tissues from a large variety of mammalian species living in areas endemic for leishmaniasis. Mem Inst Oswaldo Cruz. 2010; 105: 895–898. [DOI] [PubMed] [Google Scholar]
- 26. Danesi P, da Rold G, Rizzoli A et al.. Barcoding markers for Pneumocystis species in wildlife. Fungal Biol. 2016; 120: 191–206. [DOI] [PubMed] [Google Scholar]
- 27. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 2013; 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hong ST, Park KH, Lee SH. Susceptibility of various animals to Pneumocystis carinii infection. Kisaengchunghak Chapchi. 1992; 30: 277–281. [DOI] [PubMed] [Google Scholar]
- 29. Binanti D, Mostegl MM, Weissenbacher-Lang C, Nedorost N, Weissenböck H. Detection of Pneumocystis infections by in situ hybridization in lung samples of Austrian pigs with interstitial pneumonia. Med Mycol. 2014; 52: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vargas SL, Ponce CA, Hughes WT et al.. Association of primary Pneumocystis carinii Infection and sudden infant death syndrome. Clin Infect Dis. 1999; 29: 1489–1493. [DOI] [PubMed] [Google Scholar]
- 31. Iturra PA, Rojas DA, Pérez FJ et al.. Progression of type 2 helper T Cell–type inflammation and airway remodeling in a rodent model of naturally acquired subclinical primary pneumocystis infection. Am J Pathol. 2018; 188: 417–431. [DOI] [PubMed] [Google Scholar]
- 32. Latinne A, Bezé F, Delhaes L et al.. Genetic diversity and evolution of Pneumocystis fungi infecting wild Southeast Asian murid rodents. Parasitology. 2018; 145: 885–900. [DOI] [PubMed] [Google Scholar]
- 33. Li WH, Gouy M, Sharp PM, O′hUigin C, Yang YW. Molecular phylogeny of Rodentia, Lagomorpha, Primates, Artiodactyla, and Carnivora and molecular clocks. Proc Natl Acad Sci U S A. 1990; 87: 6703–6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albers TM, Simon MA, Clifford CB. Histopathology of naturally transmitted “rat respiratory virus”: progression of lesions and proposed diagnostic criteria. Vet Pathol. 2009; 46: 992–999. [DOI] [PubMed] [Google Scholar]
- 35. Easterbrook JD, Kaplan JB, Glass GE, Watson J, Klein SL. A survey of rodent-borne pathogens carried by wild-caught Norway rats: a potential threat to laboratory rodent colonies. Lab Anim. 2008; 42: 92–98. [DOI] [PubMed] [Google Scholar]
- 36. Lobetti RG, Leisewitz AL, Spencer JA. Pneumocystis carinii in the miniature dachshund: case report and literature review. J Small Anim Pract. 1996; 37: 280–285. [DOI] [PubMed] [Google Scholar]
- 37. Ainsworth DM, Weldon AD, Beck KA, Rowland PH. Recognition of Pneumocystis carinii in foals with respiratory distress. Equine Vet J. 1993; 25: 103–108. [DOI] [PubMed] [Google Scholar]
- 38. Kondo H, Taguchi M, Abe N, Nogami Y, Yoshioka H, Ito M. Pathological changes in epidemic porcine Pneumocystis carinii pneumonia. J Comp Pathol. 1993; 108: 261–268. [DOI] [PubMed] [Google Scholar]
- 39. Lobetti R. Common variable immunodeficiency in miniature dachshunds affected with Pneumocystis carinii pneumonia. J Vet Diagn Invest. 2000; 12: 39–45. [DOI] [PubMed] [Google Scholar]
- 40. Furuta T, Nogami S, Kojima S, Fujita M, Kamata H, Kuwabara M et al.. Spontaneous Pneumocystis carinii infection in the dog with naturally acquired generalised demodicosis. Vet Rec. 1994; 134: 423–424. [DOI] [PubMed] [Google Scholar]
- 41. Esgalhado R, Esteves F, Antunes F, Matos O. Study of the epidemiology of Pneumocystis carinii f. sp. suis in abattoir swine in Portugal. Med Mycol. 2013; 51: 66–71. [DOI] [PubMed] [Google Scholar]
- 42. Kureljušić B, Weissenbacher-Lang C, Nedorost N, Stixenberger D, Weissenböck H. Association between Pneumocystis spp. and co-infections with Bordetella bronchiseptica, Mycoplasma hyopneumoniae and Pasteurella multocida in Austrian pigs with pneumonia. Vet J. 2016; 207: 177–179. [DOI] [PubMed] [Google Scholar]
- 43. Weissenbacher-Lang C, Nedorost N, Knecht C, Hennig-Pauka I, Weissenböck H. Establishment of a quantitative real-time PCR for the detection of Pneumocystis carinii f. sp. suis in bronchoalveolar lavage samples from pigs. J Vet Diagn Invest. 2016; 28: 257–262. [DOI] [PubMed] [Google Scholar]
- 44. Weissenbacher-Lang C, Nedorost N, Knecht C et al.. Comparison of Pneumocystis nucleic acid and antibody profiles and their associations with other respiratory pathogens in two Austrian pig herds. PLoS One. 2017; 12: e0185387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. González-González AE, Aliouat-Denis CM, Ramírez-Bárcenas JA et al.. Histoplasma capsulatum and Pneumocystis spp. co-infection in wild bats from Argentina, French Guyana, and Mexico. BMC Microbiol. 2014; 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Friaza V, de la Horra C, Rodríguez-Domínguez MJ et al.. Metagenomic analysis of bronchoalveolar lavage samples from patients with idiopathic interstitial pneumonia and its antagonic relation with Pneumocystis jirovecii colonization. J Microbiol Methods. 2010; 82: 98–101. [DOI] [PubMed] [Google Scholar]
- 47. Casadevall A, Pirofski LA. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect Immun. 2000; 68: 6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Samuelson DR, Charles TP, De La Rua NM et al.. Analysis of the intestinal microbial community and inferred functional capacities during the host response to Pneumocystis pneumonia. Exp Lung Res. 2016; 42: 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kehrmann J, Veckollari B, Schmidt D et al.. The lung microbiome in patients with pneumocystosis. BMC Pulm Med. 2017; 17: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.