Abstract
PURPOSE:
The recovery pace of absolute lymphocyte count (ALC) is prognostic after hematopoietic stem cell transplantation (SCT). Previous studies have evaluated a wide range of ALC cutoffs and time points to predict outcomes. We aimed to determine the optimal ALC measure for outcome prediction after SCT from bone marrow grafts (BMT).
METHODS:
518 patients who underwent BMT for acute leukemia or myelodysplastic syndrome between 1999 and 2010 were divided into training and test sets to assess the prognostic values of ALC on days 30, 60, 90, 120, 180, as well as, the first post-transplant day on which a patient achieved ALC of 100, 200, 300, 400, 500, and 1000/μL.
RESULTS:
In the training set, the best predictor of overall survival (OS), relapse-free survival (RFS), and non-relapse mortality (NRM) was ALC on day 60. In the whole patient cohort, multivariable analyses demonstrated significantly better OS, RFS, NRM, and lower incidence of graft-versus-host disease among patients with ALC >300/μL on day 60, both including and excluding patients who had developed graft-versus-host disease prior to day 60. Among the patient-, disease-, and transplant-related factors assessed, only busulfan-based conditioning was significantly associated with higher ALC counts on day 60 in both cohorts.
CONCLUSION:
The optimal ALC cutoff to predict outcomes after BMT is ALC of 300/μL on day 60 post-transplant.
Keywords: Bone marrow transplantation, lymphocyte count, immune reconstitution
INTRODUCTION
Relapse, infectious complications, and graft-versus-host-disease (GVHD) are the major reasons for treatment failure after allogeneic hematopoietic stem cell transplantation (SCT). In the last decade, numerous attempts to reduce relapse incidence1 and treatment-related morbidity/mortality associated with transplantation have been made2, 3. However, such interventions are costly and have side effects; therefore, they may be better suited for patients at high risk for treatment failure. One way high risk patients could be identified is by evaluating patients for a delay in immune reconstitution post-transplant, as it is an important cause of morbidity and mortality. Yet, most methods to assess immune recovery are complex, require special knowledge and are not part of clinical practice. Consequently, there is considerable need for a simple and reliable prognostic marker which will evaluate the recovery of immune function as a whole and can be widely used to identify the patients at high risk for treatment failure.
Immune reconstitution after SCT is a stepwise process where the innate immune system starts to recover before the adaptive system4. NK cells recover during the first weeks of transplant constituting the major part of the lymphocyte count early after transplant5. While thymus-independent donor memory T cells start expanding immediately after SCT, thymus-dependent development of new T cells from progenitors may take 1–2 years6. In addition, B cells are low in number at least during the first 2 months post-transplant7 and reconstitution of the B compartment may take up to 2 years8.
Patient age, in vivo or ex vivo T cell depletion, and donor type may affect immune reconstitution early after SCT9, 10. However, the most important factor affecting reconstitution is thought to be the type of the graft source11. Peripheral blood (PB) grafts contain approximately one log more lymphocytes compared to bone marrow (BM) grafts12. Consequently, absolute lymphocyte counts (ALC) after SCT are higher with PB compared to BM grafts13, 14 and various T cell subsets, i.e. CD45RA+ naïve, reconstitute faster after SCT from PB grafts11.
The lymphocytes reconstituting the recipient’s immune system are crucial in preventing infectious complications and disease relapse, latter through graft-versus-tumor effect. ALC after SCT may be a surrogate marker for immune reconstitution and a predictor of these complications. Various studies have shown that a delayed recovery of lymphocytes after SCT increased non-relapse mortality (NRM) and relapse incidence (RI), shortening survival13–27. However, most of these studies included cohorts with few patients, proposed a wide range of arbitrary time points and thresholds with conflicting findings on relapse and survival, and incorporated SCTs from different graft sources (Table 1).
Table 1 -.
Studies published to date assessing the association of post-transplantation absolute lymphocyte counts (ALC) with clinical outcomes
| Study (year) | Patient characteristics | ALC timepoint and cutoffs assessed and rationale for their selection | OS | RFS | NRM | RI | aGVHD | cGVHD |
|---|---|---|---|---|---|---|---|---|
| Rigoni L, 201515 | 100 chemo-responsive AML/ALL/MDS pts. All sources and donors. 78% MA, 22% RIC | Timepoints and cutoff arbitrarily chosen | OS longer in high ALC group. HR: | HR: | ||||
| 300@d21 (30%) | 1.3 (0.7–2.6) | 1.2(0.6–2.6) | 25% vs. 26% (NS) | 76% vs. 52% (NS) | 33% vs. 36% (NS) | |||
| 300@d30 (18%) | 2.2 (1.0–4.7) | 2.0 (0.9–4.4) | 12% vs. 29% (NS) | 94% vs. 50% (0.003) | 46% vs. 34% (NS) | |||
| Kim HT, 201513 | 1109 pts. All diseases. UCB and haplo excluded. 48% MA, 52% RIC | Timepoints arbitrarily, cutoff b/o RFS curves | At 5 yr: | At 5 yr: | At 5 yr: | pts with <200 at any time point (14% of all pts) vs. >200 at m1, m2 and m3: 40% vs. 43% (NS) | ||
| 200@m1 (8%) | 30% vs. 45% (<0.001) | 19% vs. 38% (<0.001) | 33% vs. 20% (0.002) | |||||
| 200@m2 (6%) | 28% vs. 49% (<0.001) | 25% vs. 41% (<0.001) | 44% vs. 19% (<0.001) | |||||
| 200@m3 (6%) | 27% vs. 53% (<0.001) | 22% vs. 45% (<0.001) | 41% vs. 18% (<0.001) | |||||
| Yamamo to, 201416 | 206 AML/ALL/MDS pts. MA and RIC. All sources and donors | Timepoint d100 selected to exclude aGVHD effect. Cutoff b/o OS curves | OS longer in high ALC group. HR: | NRM lower in high ALC group. HR: | ||||
| 500@d100 (18%) | 2.4 (1.3–4.5) | 2.8 (1.1–6.8) | 1.4 (0.7–3.0) | |||||
| Michelis FV, 201417 | 191 AML pts in CR. MRD or MUD. PB only. MA and RIC | Cutoff arbitrarily chosen. Timepoint b/o the median # of days to achieve ALC500 | RI lower in high ALC group | |||||
| 500@d28 (42%) | NS in MVA | NS in MVA | 0.49 (0.26–0.92) | |||||
| Han DK, 201318 | 69 children with heme malignancies. 64 MA, 5 RIC. All sources and donors | Cutoff b/o prelim analyses between ALC200, 300, 400, 500 | At 5 yr: | At 5 yr | At 5 yr: | GII-IV incidence: | Extensive | |
| 500@d21 (41%) | 62% vs. 67% (NS) | 19% vs. 16% (NS) | 20% vs. 22% (NS) | 14% vs. 15% (NS) | ||||
| 500@d30 (28%) | 53% vs. 71% (0.043) (NS on MVA) | 34% vs. 11% (0.019 | 20% vs. 22% (NS) | 29% vs. 17% (NS) | 11% vs. 16% (NS) | |||
| DeCook LJ, 201219 | 118 pts with heme malignancies. RIC with Flu/Mel. PB and BM. All donors | Rationale not provided | UVar OS analyses: On MVar only d100 was sig (0.049) | |||||
| 300@d15 (57%) | 0.25 | |||||||
| 300@d30 (6%) | <0.001 | |||||||
| 300@d60 (11%) | <0.001 | |||||||
| 300@d100 (18%) | <0.001 | |||||||
| Le Blanc K, 200920 | 102 pts AML-CML-MDS only, MA only, MUD only, PB and BM | MVarA performed with ALC on day 30 as a continuous variable. d30 chosen b/o previous studies | NS on MVarA | sig increases with ALC (0.04) | sig decreases with ALC (<0.05) | |||
| Afzal S, 200921 | 71 children with AML in CR. All sources. | Rationale not provided | At 3 yr: | At 3 yr: | G III-IV: | Extensive: | ||
| 300@d21 | 14% vs. 27% (NS) | 25% vs. 20% (NS) | 16% vs. 12% (NS) | 18% vs. 13% (NS) | ||||
| 300@d30 | 21% vs. 13% (NS) | 21% vs. 26% (NS) | 18% vs. 16% (NS) | 21% vs. 11% (NS) | ||||
| Ishaqi MK, 200822 | 132 children with ALL in CR. All sources. MA only | Rationale not provided | At 3 yr: | At 3 yr: | At 3 yr: | G III-IV: | Extensive: | |
| 300@d21 | 42% vs. 66% (0.02) | 17% vs. 19% (NS) | 40% vs. 10% (0.002) | 32%−24% (NS) | 13% vs. 12% (NS) | |||
| 300@d30 | 30% vs. 57% (<0.001) | 25% vs. 14% (NS) | 46% vs. 26% (0.01) | 31% vs. 29% | 19% vs. 9% (NS) | |||
| Savani BN, 200723 | 160 pts with leukemia after TCD from MRD with MA. BM and PB grafts | d30 chosen as a marker for NK cells.Cutoff b/o median ALC on d30 | OS longer in high ALC group | NRM lower in high ALC group | Worsening effect of low ALC seen only in AML/MDS but not in ALL pts | Low ALC a/w more aGVHD.G II-IV: | High ALC a/w more cGVHD | |
| 450@d30 | 2.7 (1.03–5.1) | 3.6 (1.2–10.6) | 3(1.5–6) | 1.8 (1.1–2.9) | 0.55 (0.34–0.87) | |||
| Kim DH, 200414 | 82 pts with heme malignancies. MA and RIC. BM and PB grafts. All donors | d21 chosen because it was an early timemark. Rationale for ALC cutoff not provided | OS longer in high ALC group | RFS longer in high ALC group | At 1 yr: | RI lower in high ALC group | ||
| 350@d21 | 2.7 (1.2–6.0) | 2.8 (1.4–5.8) | 52% vs. 31% (NS) | 2.5 (1.1–6.2) | ||||
| Kumar S, 200324 | 43 pts with ALL; MA only; BM only | Assessed measures: ALC@d21 => 150, 175, 200, 225 ALC@d30 => 150, 175, 200, 225 |
RFS longer in high ALC group | RI lower in high ALC group | ||||
| 175@d21 | p = 0.0028 | 4.5 (1.2–16.6) | NS | NS | ||||
| Chakrabarti S, 200325 | 29 pts with heme malignancies. TCD BM or PB followed by TCAB. UCB and haplo excluded | Timepoint chosen arbitrarily. Cutoff b/o median ALC on d30 | OS longer in high ALC group | |||||
| 350@d30 | 18.5 (1.3–256) | NS | ||||||
| Kumar S, 200126 | 87 AML pts. BM only. Syngeneics and haplos excluded | Assessed measures: ALC@d21: 100, 150, 200 ALC@d30: 125, 150, 175, 200, 225 | OS longer in high ALC group | RFS longer in high ALC group | RI lower in high ALC group | |||
| 150@d30 with highest sig in RI | 0.0047 UVar | 0.0079 UVar | 8.2 (2.2–30.1) | |||||
| Powles R, 199827 | 201 AML pts. BM only. MA only. MRD only | d27,28,29,30 timepoints and ALC 100, 200, 300 cutoffs assessed | At 1 yr: | |||||
| 200@d29 | 25% vs. 65% (0.003) | 2.9 (0.9–9.1) |
Where percentages are given for OS, RFS, NRM, RI, and GVHD incidences, the first and second percentages indicate the survival/incidence in low and high absolute lymphocyte count groups, respectively
aGVHD: acute graft-versus-host disease, ALC: Absolute lymphocyte count, ALL: Acute lymphoid leukemia, AML: Acute myeloid leukemia, ATG: Anti-thymocyte globulin, BM: Bone marrow, b/o: based on, cGVHD: chronic graft-versus-host disease, CR: Complete remission, d: day, haplo: Haploidentical donor, HR: Hazard ratio, m: month, MA: Myeloablative, MDS: Myelodysplastic syndrome, MRD: Matched-related donor, MUD: Matched unrelated donor, NRM: Non-relapse mortality, NS: Not significant, OS: Overall survival PB: Peripheral blood, pts: patients, RFS: Relapse-free survival, RI: Relapse incidence, RIC: Reduced intensity conditioning, sig: significant, TBI: Total body irradiation, TCAB: T cell add-back, TCD: T cell depleted, UCB: Umbilical cord blood, UV: Univariate, yr: year
Here, we aimed to identify the optimal post-transplant ALC time point/cutoff that would best predict clinical outcomes in the early post-SCT period. This could be used to globally assess the recovery of immune function and to possibly identify the high-risk patients for intervention.
PATIENTS and METHODS
Patients
Included in this study were all patients older than 18 years with acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), and myelodysplastic syndrome (MDS) who underwent a SCT with a BM graft (BMT) between 1999 and 2010 identified through the departmental registry. Demographics, disease characteristics, treatment, GVHD, cytomegalovirus (CMV), and survival data were retrieved from the departmental database and patient charts. ALC on days 30, 60, 90, 120, 180, as well as the first post-transplant day on which a patient achieved ALC of 100, 200, 300, 400, 500, and 1000/μL were collected from the institutional laboratory information system through a computer algorithm developed specifically for this study to minimize human error.
Patients were managed clinically according to institutional guidelines including infection prophylaxis for Pneumocystis carinii, herpes viruses, and fungus. CMV reactivation was monitored by CMV pp65 antigenemia assay or CMV PCR from peripheral blood. Preemptive therapy was instituted in patients with documented CMV viremia. Patients received G-CSF beginning at day +7 after transplantation. GVHD was diagnosed clinically, confirmed pathologically whenever possible, and classified according to standard criteria28. GVHD diagnosed after day 100 post-transplant was classified as chronic GVHD. Only patients who engrafted were evaluable for GVHD assessment. Donor-recipient human leukocyte antigen (HLA) matching was established by DNA sequence-specific oligonucleotide typing for HLA-A, -B, -Cw, -DQB1, and - DRB1 loci. Donors were HLA matched related, unrelated or haploidentical.
Definitions
A haploidentical donor was defined as a related donor with ≥2 HLA allele mismatches in the same haplotype. Complete remission was defined as ≤5% blasts in bone marrow, absence of blasts in peripheral blood, platelet count ≥100K/L, and absolute neutrophil count ≥1000/L. Overall survival (OS) and relapse-free survival (RFS) were defined as the time from BMT until death from any cause, and disease relapse or death, respectively. NRM was defined as death in a patient without leukemia relapse. Other time-to-event measures (relapse, CMV reactivation, acute and chronic GVHD) were computed from date of BMT to date of event.
Statistical Methods
To determine the optimal ALC threshold, the dataset was first divided into a training set (70% of the data) and test set (remaining 30%) by random assignment. The application Cutoff Finder29 was used to find the optimal cutoff point of each ALC measure for OS, RFS, NRM, and relapse on the training set (based on a univariate Cox proportional hazards regression model). The determined cutoff value was then used to dichotomize patients in the test set and a univariate Cox proportional hazards regression model was used to determine the association between the outcome measure and the dichotomized group. To determine the robustness of the estimates, 1,000 bootstrap samples from the test set were created. A Cox proportional hazards regression model was performed on each bootstrapped sample and the mean and 95% confidence interval of the distribution of hazard ratios were computed. Lastly, the percentage of the bootstrapped samples with p-values less than 0.05 (from the Cox model) was computed (power).
To assess the factors affecting ALC, the whole cohort was grouped by the determined optimal ALC cutoff value and assessed using Pearson’s chi-square test (categorical measures) and Wilcoxon rank sum test (age at SCT). OS estimates were determined using the Kaplan-Meier method and difference between ALC groups was assessed using the log-rank test. Associations between measures of interest and OS/RFS were assessed in the whole patient cohort using Cox proportional hazards regression models. The cumulative incidence of relapse (RI), NRM, GVHD, and CMV was determined using the competing risks method. The competing risk included for relapse was death before progression and the competing risk included for NRM was relapse. For GVHD and CMV, the competing risks included were relapse and death. For all outcomes, patients who experienced the event before the determined ALC cutoff day were excluded from that outcome analyses and patients who did not experience the event were censored.
All statistical analyses were performed using SAS 9.3 for Windows (SAS Institute Inc., Cary, NC). All statistical tests used a significance level of 5%. No adjustments for multiple testing were made.
RESULTS
Among 518 patients included in the study, median ALC on days 30, 60, 90, 120, and 180 were 375/μL, 540/μL, 610/μL, 685/μL, and 835/μL, respectively. The optimal ALC cutoff values with the highest power for OS, RFS, relapse, and NRM prediction are presented in Table 2 (the measures that were not found to be significantly associated with outcomes are not shown). The distribution of hazard ratios for OS according to different cutoff levels of ALC on day 60 is demonstrated in Figure 1. Of those, the measures with the best prediction of OS and RFS were days 60, 120, and 180. Only ALC measures at days 30 and 180 were associated with time-to-relapse in the training set. However, neither was found to be significant in the test set. Consistent with OS and RFS, the ALC on day 60 produced the best results for NRM with a power > 99% albeit at a different ALC cutoff. The time to achieve an ALC of 100, 200, 300, 400, 500 or 1000/μL were not found to significantly affect clinical outcomes. ALC on day 60 was chosen as the optimal threshold over days 30, 120, and 180 because: 1) Day 60 measure had the highest power to detect NRM; 2) Day 30 had lower power to predict OS and its association with relapse was not confirmed in the test set; 3) Compared to days 120 and 180, i) the hazard ratios from the training and test sets as well as the bootstrapped samples were more consistent at day 60 and ii) earlier prediction could be clinically more useful.
Table 2 -.
Determination of optimal threshold for absolute lymphocyte count (ALC) for prediction of clinical outcomes
| Measure | ALC Cutoff | Training Set | Test Set | Bootstrap (Test Set) | |
|---|---|---|---|---|---|
| (/μL) | HR (95% CI) | HR (95% CI) | HR mean (95% CI) | Power | |
| Overall survival | |||||
| ALC @ day 30 | 250 | 0.59 (0.45–0.78) | 0.57 (0.37–0.89) | 0.58 (0.34–0.90) | 0.69 |
| ALC @ day 60 | 300 | 0.42 (0.32–0.56) | 0.43 (0.27–0.67) | 0.43 (0.25–0.71) | 0.93 |
| ALC @ day 90 | 500 | 0.53 (0.39–0.71) | 0.59 (0.37–0.93) | 0.59 (0.35–0.91) | 0.64 |
| ALC @ day 120 | 420 | 0.50 (0.35–0.72) | 0.36 (0.21–0.63) | 0.38 (0.20–0.65) | 0.93 |
| ALC @ day 180 | 500 | 0.46 (0.30–0.72) | 0.26 (0.11–0.59) | 0.27 (0.01–0.63) | 0.88 |
| Relapse-free survival | |||||
| ALC @ day 30 | 250 | 0.61 (0.47–0.79) | 0.57 (0.37–0.88) | 0.58 (0.35–0.87) | 0.72 |
| ALC @ day 60 | 280 | 0.49 (0.37–0.66) | 0.51 (0.33–0.79) | 0.52 (0.30–0.83) | 0.85 |
| ALC @ day 90 | 500 | 0.57 (0.43–0.76) | 0.57 (0.37–0.89) | 0.57 (0.34–0.86) | 0.73 |
| ALC @ day 120 | 420 | 0.53 (0.37–0.75) | 0.37 (0.22–0.64) | 0.38 (0.21–0.64) | 0.94 |
| ALC @ day 180 | 500 | 0.47 (0.31–0.72) | 0.22 (0.09–0.50) | 0.23 (0.08–0.51) | 0.93 |
| Relapse | |||||
| ALC @ day 30 | 220 | 0.67 (0.47–0.94) | 0.81 (0.43–1.54) | 0.87 (0.45–1.55) | 0.11 |
| ALC @ day 180 | 750 | 0.55 (0.33–0.93) | 0.67 (0.28–1.62) | 0.76 (0.27–1.69) | 0.16 |
| Non-relapse mortality | |||||
| ALC @ day 30 | 250 | 0.50 (0.33–0.76) | 0.27 (0.14–0.51) | 0.28 (0.13–0.50) | 0.98 |
| ALC @ day 60 | 450 | 0.17 (0.10–0.29) | 0.18 (0.09–0.38) | 0.19 (0.07–0.36) | >0.99 |
| ALC @ day 90 | 500 | 0.28 (0.17–0.46) | 0.25 (0.12–0.53) | 0.26 (0.10–0.50) | 0.96 |
| ALC @ day 120 | 415 | 0.38 (0.22–0.66) | 0.18 (0.07–0.49) | 0.20 (0.05–0.50) | 0.94 |
| ALC @ day 180 | 500 | 0.35 (0.18–0.70) | 0.07 (0.01–0.40) | * | |
HR: Hazard ratio
Many of the bootstrapped samples for ALC on day 180 did not have non-relapse mortality events in one of the ALC groups, therefore, the results for this category were deemed questionable and not reported
Figure 1 -.

Distribution of Hazard ratios for overall survival according to different cutoff levels of absolute lymphocyte count on post-transplantation day 60
In the whole patient cohort, 102 of 134 (76%) patients with ALC ≤ 300/μL and 173 of 353 (49%) patients with ALC >300/μL on day 60 died. The identified primary causes of death are presented in Table 3. While 14% and 17% of patients with ALC ≤ 300/μL on day 60 died from acute and chronic GVHD, 1% and 5% of patients with ALC >300/μL died from the same causes. A significantly increased risk in OS and RFS in addition to increased NRM and decreased RI was seen in the univariate analyses in patients with ALC ≤ 300/μL compared to those with >300/μL on day 60 (Figure 2). These results were maintained after controlling for clinical factors in multivariable analyses (Table 4). Patients with ALC >300/μL experienced significantly less acute GVHD (aGVHD). In addition, there was a significant association between ALC group and aGVHD grade II-IV (HR [95% CI]:0.30 [0.14 – 0.68]; p=0.004) but not with aGVHD grade III-IV. There was no significant association between ALC and chronic GVHD (cGVHD) and CMV incidence in univariate or multivariable analyses. While the remission status at the time of BMT and busulfan-based conditioning regimen were the only other significant measures associated with OS and RFS; age, donor HLA-match, and use of anti-thymocyte globulin (ATG) or alemtuzumab were other factors affecting NRM. To assess the potential confounding effect of corticosteroid treatment for aGVHD, we repeated the multivariable outcome analysis after excluding patients who had developed aGVHD prior to day 60. These verified the significant improvement of OS, RFS, and NRM with higher ALC, while RI was no longer associated with ALC.
Table 3 -.
Primary causes of death according to absolute lymphocyte counts on post-transplant day 60 (ALC60)
| Cause of death | ALC60 ≤300/μL (%) | ALC60 >300/μL |
|---|---|---|
| (N=102) | (N=173) | |
| Recurrence/persistence of disease | 41 (40) | 126 (73) |
| Chronic GVHD | 23 (23) | 18 (10) |
| Acute GVHD | 19 (19) | 5 (3) |
| Infection | 9 (9) | 6 (3) |
| Organ failure | 4 (4) | 7 (4) |
| Graft rejection | 1 (1) | 4 (2) |
| Secondary malignancy | 1 (1) | 1 (1) |
| Hemorrhage | 2 (2) | 1 (1) |
| Other | 2 (2) | 5 (3) |
Figure 2 -.
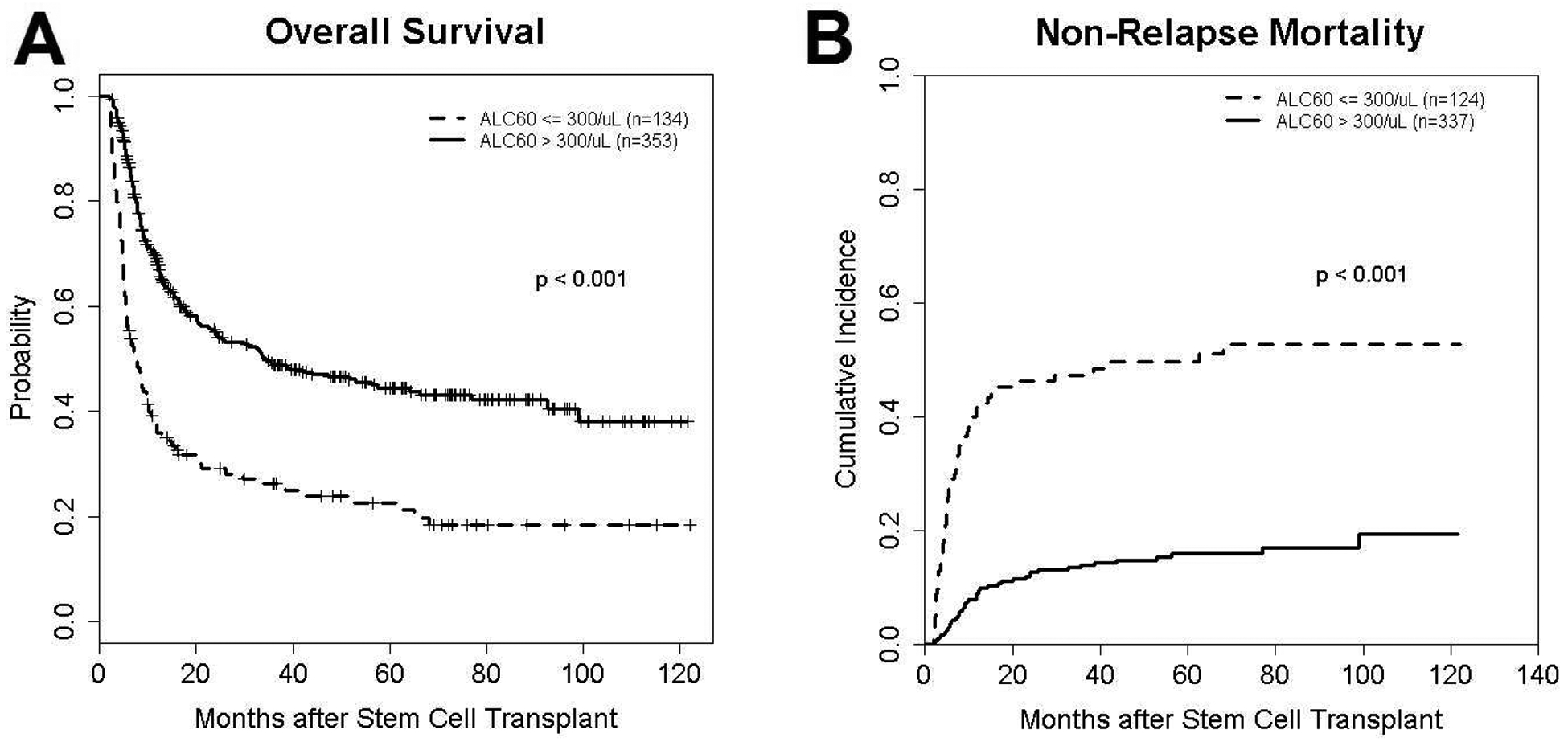
Kaplan-Meier curve of overall survival (A) and cumulative incidence curve of non-relapse mortality (B) in high (>300/μL on day 60) and low ALC (≤300/μL) groups in the whole patient set
Table 4 -.
Multivariable analyses assessing the association between various patient-, disease-, and transplant-related factors and clinical outcomes
| Measure | OS | RFS | RI | NRM | aGVHD* | cGVHD | CMV |
|---|---|---|---|---|---|---|---|
| Age (years) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 0.99 (0.97, 1.00) | 1.02 (1.00, 1.04) | 0.99 (0.96, 1.02) | 1.00 (0.98, 1.01) | 0.99 (0.96, 1.02) |
| Diagnosis (AML/MDS vs. ALL) | 0.84 (0.54, 1.32) | 0.93 (0.60, 1.45) | 0.83 (0.47, 1.44) | 1.49 (0.65, 3.40) | 0.78 (0.23, 2.72) | 1.41 (0.82, 2.44) | 0.59 (0.16, 2.18) |
| Matched donor (yes vs. no) | 0.84 (0.61, 1.15) | 0.80 (0.59, 1.10) | 1.03 (0.69, 1.54) | 0.59 (0.37, 0.95) | 0.48 (0.19, 1.19) | 0.86 (0.62, 1.21) | 0.40 (0.17, 0.96) |
| Related donor (yes vs. no) | 0.79 (0.54, 1.15) | 0.72 (0.49, 1.05) | 0.95 (0.56, 1.61) | 0.60 (0.34, 1.07) | 0.49 (0.17, 1.45) | 0.74 (0.44, 1.24) | 1.18 (0.39, 3.60) |
| CR at BMT (yes vs. no) | 0.38 (0.29, 0.50) | 0.42 (0.32, 0.55) | 0.37 (0.26, 0.52) | 0.91 (0.59, 1.39) | 0.87 (0.41, 1.86) | 0.97 (0.72, 1.30) | 0.55 (0.25, 1.22) |
| Preparatory regimen (TBI vs. Other) | 0.81 (0.49, 1.33) | 0.89 (0.54, 1.46) | 1.15 (0.59, 2.24) | 0.95 (0.40, 2.26) | 1.55 (0.26, 9.22) | 1.03 (0.55, 1.92) | 0.09 (0.00, 1.84) |
| Preparatory regimen (Busulfan vs. Other) | 0.71 (0.54, 0.94) | 0.69 (0.52, 0.91) | 0.84 (0.59, 1.19) | 0.68 (0.43, 1.08) | 3.45 (1.03, 11.56) | 0.87 (0.62, 1.23) | 0.91 (0.39, 2.16) |
| ATG or alemtuzumab (yes vs. no) | 0.79 (0.55, 1.14) | 0.91 (0.63, 1.32) | 1.40 (0.84, 2.33) | 0.54 (0.31, 0.95) | 0.46 (0.15, 1.42) | 0.84 (0.51, 1.38) | 1.74 (0.49, 6.18) |
| Post-BMT Cyclophos (yes vs. no) | 0.82 (0.49, 1.39) | 1.01 (0.61, 1.65) | 1.27 (0.69, 2.32) | 0.56 (0.23, 1.37) | 2.01 (0.56, 7.21) | 0.71 (0.38, 1.32) | 1.34 (0.36, 4.98) |
| ALC60 group (>300 vs. ≤300/μL) | 0.46 (0.36, 0.60) | 0.56 (0.43, 0.72) | 1.68 (1.12, 2.51) | 0.21 (0.14, 0.31) | 0.37 (0.15, 0.92) | 0.79 (0.58, 1.08) | 1.07 (0.46, 2.50) |
aGVHD: acute graft-versus-host disease, ALC60: Absolute lymphocyte count on post-BMT day 60, ALL: Acute lymphoid leukemia, AML: Acute myeloid leukemia, ATG: Anti-thymocyte globulin, BMT: Bone marrow transplantation, cGVHD: chronic graft-versus-host diseae, CMV: Cytomegalovirus, CR: Complete remission, MDS: Myelodysplastic syndrome, NRM: Non-relapse mortality, OS: Oveall survival, RFS: Relapse-free survival, RI: Relapse incidence, TBI: Total body irradiation
aGVHD developed prior to day 60 were excluded from the analyses
Patient age, diagnosis, donor type, remission status at the time of BMT, ATG/alemtuzumab use, post-BMT cyclophosphamide use, graft total nucleated, CD34+, and CD3+ cell counts were not associated with whether a patient had ALC above or below 300/μL on day 60 (Table 5). In a separate analysis, ATG/alemtuzumab was not found to be associated with ALC recovery on day 30, either. TBI-based conditioning was significantly associated with lower ALC on day 60, however, this did not remain significant when patients who developed aGVHD before day 60 were excluded from analysis (p=0.138). Busulfan based conditioning was significantly associated with higher ALC counts on day 60 both including and excluding patients developing aGVHD prior to day 60.
Table 5 -.
Comparison of clinical characteristics according to absolute lymphocyte count on post-bone marrow transplantation (BMT) day 60 (ALC60)
| ALC60 ≤300/μL | ALC60 >300/μL | ||
|---|---|---|---|
| Measure | (N=134) | (N=353) | p-value |
| Age (years), median (range) | 46 (18–71) | 47 (18–71) | 0.75 |
| Diagnosis, n (%) | |||
| AML/MDS | 105 (78) | 292 (83) | 0.27 |
| ALL | 29 (22) | 61 (17) | |
| Donor type, n (%) | |||
| Matched unrelated | 97 (72) | 230 (65) | 0.11 |
| Mismatch unrelated | 13 (10) | 45 (13) | |
| Haploidentical | 17 (13) | 37 (10) | |
| Matched related | 7 (5) | 41 (12) | |
| Matched donor, n (%) | |||
| Yes | 104 (78) | 271 (77) | 0.84 |
| No | 30 (22) | 82 (23) | |
| Related donor, n (%) | |||
| Yes | 24 (18) | 78 (22) | 0.31 |
| No | 110 (82) | 275 (78) | |
| CR at BMT, n (%) | |||
| Yes | 63 (47) | 185 (52) | 0.29 |
| No | 71 (53) | 168 (48) | |
| TBI-based conditioning, n (%) | |||
| Yes | 26 (19) | 39 (11) | 0.0155 |
| No | 108 (81) | 314 (89) | |
| Busulfan-based conditioning, n (%) | |||
| Yes | 63 (47) | 218 (62) | 0.0033 |
| No | 71 (53) | 135 (38) | |
| Conditioning intensity, n (%) | |||
| Myeloablative | 97 (72) | 283 (80) | 0.06 |
| Reduced-intensity | 37 (28) | 70 (20) | |
| ATG or alemtuzumab, n (%) | |||
| Yes | 96 (72) | 254 (72) | 0.95 |
| No | 38 (28) | 99 (28) | |
| Post-BMT cyclophosphamide, n (%) | |||
| Yes | 12 (9) | 31 (9) | 0.95 |
| No | 122 (91) | 322 (91) | |
| Graft total nucleated cell count | |||
| continuous, median(range) | 2.42 (0.03 – 6.26) | 2.67 (0.15 – 12.37) | 0.13 |
| ≤ 2.59*, n (%) | 73 (54) | 171 (48) | 0.23 |
| > 2.59, n (%) | 61 (46) | 182 (52) | |
| Graft CD34+ cell count | |||
| continuous, median(range) | 2.99 (0 – 9.57) | 3.12 (0 – 12.67) | 0.24 |
| ≤ 3.03*, n (%) | 73 (54) | 171 (48) | 0.23 |
| > 3.03, n (%) | 61 (46) | 182 (52) | |
| Graft CD3+ cell count | |||
| continuous, median(range) | 18.79 (0 – 69.02) | 21.16 (0 – 83.13) | 0.20 |
| ≤ 20.43*, n (%) | 75 (56) | 166 (48) | 0.10 |
| > 20.43, n (%) | 59 (44) | 182 (52) | |
ALL: Acute lymphoid leukemia, AML: Acute myeloid leukemia, ATG: Anti-thymocyte globulin, CR: Complete remission, MDS: Myelodysplastic syndrome, TBI: Total body irradiation
DISCUSSION
The advent of post-SCT early interventions tackling relapse and NRM before they occur necessitates a practical and reliable prognostic marker to select high-risk patients for these costly procedures. ALC recovery pace may be such a marker as it has been shown to be associated with improved clinical outcomes. However, studies to date could not determine the optimal ALC threshold because of 1) small cohort size, 2) heterogeneity of the graft sources and the diseases in their cohorts, and 3) lack of a robust statistical methodology. In this study, we confirmed the positive impact of early lymphocyte recovery on survival and NRM after BMT, and determined the optimal ALC threshold for outcome prediction to be 300/μL on post-BMT day 60.
To our knowledge, among the studies assessing post-SCT ALC recovery, ours has the largest cohort that includes SCTs solely from a single graft source. We believe it was essential to include only one graft source because optimal prognostic ALC thresholds could vary between different graft types. The lymphocyte repopulation kinetics is significantly different between the PB and BM grafts11 likely due to the one log difference in their lymphocyte contents12. Accordingly, while Michelis et al. found that 58% of patients who underwent SCT from PB grafts achieved ALC of 500/μL by day 30, in our study median ALC on day 30 was only 375/μL. We chose to study SCTs from BM grafts which have a slower lymphocyte recovery pace while a follow-up study using PB grafts is planned.
Our study is also the first to methodologically analyze broad ALC measures to determine the most prognostic measure. After finding the optimum cutoff ALC level for each post-SCT day and verifying their prognostic significance in a separate test set, we also analyzed the time to achieve specific ALC levels but these were not found to be prognostic. While we studied ALC on various days from 30 to 180, most of the previous studies had used ALC on days 21–3014, 15, 17, 18, 20–22, 24–26 indirectly assessing NK cell recovery as NK cells are the dominant lymphocyte subset 3–4 weeks after SCT30, 31. Among the few studies assessing the impact of ALC after day 3013, 16, 19, only Kim et al. used a methodology - restricted cubic spline smoothing method- to assess different ALC cutoff levels13. However, instead of individually calculating HRs for each different ALC cutoff level, Kim et al. performed one analysis in which ALCs on day 30 were stratified into five comparison (0–200, 200–300, 300–400, 400–500, >2600) and one reference arm (500–2600). ALC of 200 was chosen to assess outcomes since it was significantly higher than the reference group. This cutoff level was also used for days 60 and 90. Given that potential cutoff levels for days 60 and 90 were not assessed, the optimal ALC cutoff level with the highest power for prognostication may not have been identified in that study.
We found the optimal time point to assess ALC to be day 60. The power to predict OS, RFS, and NRM was significantly higher for day 60 than for day 30 which most of previous studies used as the time point to assess ALC recovery. However, our study cohort comprised SCTs solely from BM grafts whereas others included both PB and BM sources (Table 1). It is possible that the optimal time point for PB grafts would be earlier than day 60 due to faster recovery of lymphocytes after SCT with PB grafts. Similar to our study, the few studies assessing extended days found higher ALC on days 60, 90 and 100 were also associated with improved survival and NRM13, 16, 19.
While further studies are needed, the improved survival and NRM with faster ALC recovery is likely related to a lower incidence of GVHD and infectious complications, as previously observed by us32. However, we did not detect any significant difference in CMV reactivation incidence between the low and high ALC groups although previous studies had shown an inverse relationship between lymphocyte recovery pace and infection rates14, 33. Moreover, in the present study we had ruled out the confounding effect of corticosteroids used in the treatment of aGVHD by demonstrating the same outcome results after excluding the patients who had developed aGVHD prior to ALC measurement time point of day 60. Another explanation could be a lower incidence of GVHD with faster ALC recovery. Similar to Rigoni et al.’s report of a significantly lower aGVHD incidence in patients with ALC >300 on day 3015, we also observed a lower incidence of aGVHD in patients with ALC > 300 on day 60. Moreover, higher ALC at the time of aGVHD diagnosis was previously shown to be associated with better prognosis34 and may have played a partial role in improved survival and NRM in our study.
Various patient/donor, disease, graft, and transplant characteristics were reported to be associated with immune recovery pace after SCT. Klyuchnikov et al. summarized these findings in their paper35. In our cohort, busulfan- and TBI-based conditioning were the only two clinical characteristics associated with ALC recovery, although TBI-based conditioning was not significantly associated with ALC recovery when patients who experienced aGVHD prior to day 60 were excluded from the analysis. Previous studies had suggested that the graft source was the most important factor affecting ALC recovery – faster after SCTs from PB compared to those from BM and umbilical cord blood11, 36, 37 Hence, we opted to include SCTs from a single graft source in our cohort. ATG was previously reported to slow CD4+ T cell recovery but improve B cell and NK cell recovery38. We did not observe slow ALC recovery in patients treated with ATG or alemtuzumab. While there are conflicting reports on the impact of patient age and donor type on the recovery of ALC and certain lymphocyte subsets9–11, 35, 39, we did not observe such impact on ALC recovery.
Our study is limited by its retrospective nature. We attempted to limit human error in data collection by retrieving ALC electronically from the laboratory information system with the help of a computer algorithm. Second, this is a single center study and results may not apply to other centers with different standards, algorithms, and patient population. Eighty percent of the SCTs in our cohort were from unrelated donors, and 53% of the remaining SCTs were from haploidentical donors. The ideal ALC cutoff may differ in centers primarily using bone marrow grafts for related donors. Third, although we chose to assess ALC as a prognostic marker to identify high-risk patient groups, there may be more powerful assays such as certain lymphocyte subset counts. For instance, NK cell count may correlate more with RI and CD4+ T cell count may be a better predictor for infectious complications. Fourth, while ours and several other studies demonstrated association between ALC recovery and NRM, this does not prove causality. The studies to date show prognostic value of ALC but this may not confer to a predictive value for an early prevention method. Finally, our results are limited to BMTs and should not be employed for SCT from PB grafts as the optimal cutoff is very likely to be on an earlier timepoint than day 60. A separate study is needed for those patients.
In conclusion, we determined the optimal ALC cutoff to predict outcomes after BMT to be ALC of 300/μL on post-transplant day 60. This was significantly associated with survival and NRM. We believe patients with ALC lower than 300 on day 60 should be targeted for morbidity prevention. Further studies are needed to determine a cutoff for SCT from PB grafts and to verify our findings in multi-center cohorts.
Highlights.
Day 60 absolute lymphocyte count (ALC) of 300/μL is the optimum prognostic threshold
Patients with ALC >300 on day 60 have better OS, RFS, NRM, and less GVHD
Conditioning regimen may influence lymphocyte recovery after marrow transplantation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: Authors have no pertinent financial relationships to disclose.
Conflict of Interest: Authors declare no conflicts of interest.
References
- 1.de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. The lancet oncology. 2009;10:489–500. [DOI] [PubMed] [Google Scholar]
- 3.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. The New England journal of medicine. 2011;365:1673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30:425–37. [DOI] [PubMed] [Google Scholar]
- 5.Chklovskaia E, Nowbakht P, Nissen C, Gratwohl A, Bargetzi M, Wodnar-Filipowicz A. Reconstitution of dendritic and natural killer-cell subsets after allogeneic stem cell transplantation: effects of endogenous flt3 ligand. Blood. 2004;103:3860–8. [DOI] [PubMed] [Google Scholar]
- 6.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995;16:413–25. [PubMed] [Google Scholar]
- 7.Storek J, Ferrara S, Ku N, Giorgi JV, Champlin RE, Saxon A. B cell reconstitution after human bone marrow transplantation: recapitulation of ontogeny? Bone Marrow Transplant. 1993;12:387–98. [PubMed] [Google Scholar]
- 8.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115:3861–8. [DOI] [PubMed] [Google Scholar]
- 9.Fallen PR, McGreavey L, Madrigal JA, Potter M, Ethell M, Prentice HG, et al. Factors affecting reconstitution of the T cell compartment in allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant. 2003;32:1001–14. [DOI] [PubMed] [Google Scholar]
- 10.Berger M, Figari O, Bruno B, Raiola A, Dominietto A, Fiorone M, et al. Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transplant. 2008;41:55–62. [DOI] [PubMed] [Google Scholar]
- 11.Heining C, Spyridonidis A, Bernhardt E, Schulte-Monting J, Behringer D, Grullich C, et al. Lymphocyte reconstitution following allogeneic hematopoietic stem cell transplantation: a retrospective study including 148 patients. Bone Marrow Transplant. 2007;39:613–22. [DOI] [PubMed] [Google Scholar]
- 12.Ottinger HD, Beelen DW, Scheulen B, Schaefer UW, Grosse-Wilde H. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood. 1996;88:2775–9. [PubMed] [Google Scholar]
- 13.Kim HT, Armand P, Frederick D, Andler E, Cutler C, Koreth J, et al. Absolute Lymphocyte Count Recovery after Allogeneic Hematopoietic Stem Cell Transplantation Predicts Clinical Outcome. Biol Blood Marrow Transplant. 2015;21:873–80. [DOI] [PubMed] [Google Scholar]
- 14.Kim DH, Kim JG, Sohn SK, Sung WJ, Suh JS, Lee KS, et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Br J Haematol. 2004;125:217–24. [DOI] [PubMed] [Google Scholar]
- 15.Rigoni L, Scroferneker ML, Pitombeira BS, Ottoni E, Paz A, Fischer G, et al. Importance of early absolute lymphocyte count after allogeneic stem cell transplantation: a retrospective study. Transplant Proc. 2015;47:511–6. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto W, Ogusa E, Matsumoto K, Maruta A, Ishigatsubo Y, Kanamori H. Lymphocyte recovery on day 100 after allogeneic hematopoietic stem cell transplant predicts non-relapse mortality in patients with acute leukemia or myelodysplastic syndrome. Leuk Lymphoma. 2014;55:1113–8. [DOI] [PubMed] [Google Scholar]
- 17.Michelis FV, Messner HA, Loach D, Uhm J, Gupta V, Lipton JH, et al. Early lymphocyte recovery at 28 d post-transplant is predictive of reduced risk of relapse in patients with acute myeloid leukemia transplanted with peripheral blood stem cell grafts. Eur J Haematol. 2014;93:273–80. [DOI] [PubMed] [Google Scholar]
- 18.Han DK, Baek HJ, Kim SY, Hwang TJ, Kook H. Implication of early lymphocyte recovery after allogeneic hematopoietic stem cell transplantation in children with leukemia. Yonsei medical journal. 2013;54:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decook LJ, Thoma M, Huneke T, Johnson ND, Wiegand RA, Patnaik MM, et al. Impact of lymphocyte and monocyte recovery on the outcomes of allogeneic hematopoietic SCT with fludarabine and melphalan conditioning. Bone Marrow Transplant. 2013;48:708–14. [DOI] [PubMed] [Google Scholar]
- 20.Le Blanc K, Barrett AJ, Schaffer M, Hagglund H, Ljungman P, Ringden O, et al. Lymphocyte recovery is a major determinant of outcome after matched unrelated myeloablative transplantation for myelogenous malignancies. Biol Blood Marrow Transplant. 2009;15:1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afzal S, Ishaqi MK, Dupuis A, Doyle J, Gassas A. Early lymphocyte recovery after allogeneic hematopoietic SCT is associated with significant GVL effect in pediatric ALL but not acute myelogenous leukemia-Update study. Bone Marrow Transplant. 2009;44:799–804. [DOI] [PubMed] [Google Scholar]
- 22.Ishaqi MK, Afzal S, Dupuis A, Doyle J, Gassas A. Early lymphocyte recovery post-allogeneic hematopoietic stem cell transplantation is associated with significant graft-versus-leukemia effect without increase in graft-versus-host disease in pediatric acute lymphoblastic leukemia. Bone Marrow Transplant. 2008;41:245–52. [DOI] [PubMed] [Google Scholar]
- 23.Savani BN, Mielke S, Rezvani K, Montero A, Yong AS, Wish L, et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after T cell-depleted allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Chen MG, Gastineau DA, Gertz MA, Inwards DJ, Lacy MQ, et al. Lymphocyte recovery after allogeneic bone marrow transplantation predicts risk of relapse in acute lymphoblastic leukemia. Leukemia. 2003;17:1865–70. [DOI] [PubMed] [Google Scholar]
- 25.Chakrabarti S, Brown J, Guttridge M, Pamphilon DH, Lankester A, Marks DI. Early lymphocyte recovery is an important determinant of outcome following allogeneic transplantation with CD34+ selected graft and limited T-cell addback. Bone Marrow Transplant. 2003;32:23–30. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Chen MG, Gastineau DA, Gertz MA, Inwards DJ, Lacy MQ, et al. Effect of slow lymphocyte recovery and type of graft-versus-host disease prophylaxis on relapse after allogeneic bone marrow transplantation for acute myelogenous leukemia. Bone Marrow Transplant. 2001;28:951–6. [DOI] [PubMed] [Google Scholar]
- 27.Powles R, Singhal S, Treleaven J, Kulkarni S, Horton C, Mehta J. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation. Blood. 1998;91:3481–6. [PubMed] [Google Scholar]
- 28.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 29.Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PloS one. 2012;7:e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niederwieser D, Gastl G, Rumpold H, Marth C, Kraft D, Huber C. Rapid reappearance of large granular lymphocytes (LGL) with concomitant reconstitution of natural killer (NK) activity after human bone marrow transplantation (BMT). Br J Haematol. 1987;65:301–5. [DOI] [PubMed] [Google Scholar]
- 31.Savani BN, Rezvani K, Mielke S, Montero A, Kurlander R, Carter CS, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107:1688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciurea SO, Mulanovich V, Jiang Y, Bassett R, Rondon G, McMannis J, et al. Lymphocyte Recovery Predicts Outcomes in Cord Blood and T Cell-Depleted Haploidentical Stem Cell Transplantation. Biol Blood Marrow Transplant. 2011;17:1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Servais S, Lengline E, Porcher R, Carmagnat M, Peffault de Latour R, Robin M, et al. Long-term immune reconstitution and infection burden after mismatched hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:507–17. [DOI] [PubMed] [Google Scholar]
- 34.Lee KH, Choi SJ, Lee JH, Lee JS, Kim WK, Lee KB, et al. Prognostic factors identifiable at the time of onset of acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Haematologica. 2005;90:939–48. [PubMed] [Google Scholar]
- 35.Klyuchnikov E, Asenova S, Kern W, Kilinc G, Ayuk F, Wiedemann B, et al. Post-transplant immune reconstitution after unrelated allogeneic stem cell transplant in patients with acute myeloid leukemia. Leuk Lymphoma. 2010;51:1450–63. [DOI] [PubMed] [Google Scholar]
- 36.Kanda J, Chiou LW, Szabolcs P, Sempowski GD, Rizzieri DA, Long GD, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1664–76.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamza NS, Lisgaris M, Yadavalli G, Nadeau L, Fox R, Fu P, et al. Kinetics of myeloid and lymphocyte recovery and infectious complications after unrelated umbilical cord blood versus HLA-matched unrelated donor allogeneic transplantation in adults. Br J Haematol. 2004;124:488–98. [DOI] [PubMed] [Google Scholar]
- 38.Bosch M, Dhadda M, Hoegh-Petersen M, Liu Y, Hagel LM, Podgorny P, et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy. 2012;14:1258–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baron F, Storer B, Maris MB, Storek J, Piette F, Metcalf M, et al. Unrelated donor status and high donor age independently affect immunologic recovery after nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006;12:1176–87. [DOI] [PubMed] [Google Scholar]


