Abstract
Learning through social observation is critical for humans. The present study investigates the neural processes underlying the acquisition of placebo effects through observational learning. We created a new functional magnetic resonance imaging (fMRI) paradigm where participants (n = 38, healthy, both sexes) observed a demonstrator experiencing pain relief by a placebo treatment cream and experiencing pain without a treatment (control cream), and subsequently performed the same procedure themselves.
Participants demonstrated placebo hypoalgesia while they performed the procedure themselves, confirming that observational learning can lead to placebo effects. During the observational learning phase, fMRI analysis showed a modulation of the amygdalae, periaqueductal grey, temporoparietal junctions (TPJ), and dorsolateral prefrontal cortex (DLPFC). Connectivity between the DLPFC and TPJ during the observational learning task was modulated by the placebo treatment and predicted subsequent placebo effects. Mediation analysis further confirmed that the DLPFC-TPJ connectivity formally mediated the effect of the observed treatment condition on subsequent placebo effects. Additionally, pre-recorded resting state connectivity between the DLPFC and TPJ also predicted observationally-learned placebo effects. Our findings provide an understanding of the neural processes during the acquisition of placebo effects through observation and indicate a critical role for DLPFC-TPJ integration processes during observational learning of therapeutic outcomes.
Keywords: Social learning, Pain, Placebo analgesia, fMRI, Observational learning
1. Introduction
Humans are able to acquire expectations, views and beliefs about their environment through social observation, rather than direct experience (Bandura, 1971; Koban et al., 2017). Despite the importance of observational learning processes for human behaviors, most neuroscientific research has been performed on direct experience learning (e.g., conditioning). However, it is critical to study observational learning processes, as most human behaviors are learned through social interaction (Bandura, 1971).
In the phenomenon known as placebo hypoalgesia, an individual experiences pain relief induced by treatment outcome expectancies that represent the expected beneficial value of the treatment, even if the treatment contains no active pharmacological agent (Büchel et al., 2014; Wager and Atlas, 2015). Studies have shown that individuals experience placebo hypoalgesia after observing pain relief via observation of a live demonstrator (Colloca and Benedetti, 2009) and via a video presentation (Hunter et al., 2014). However, the neural mechanisms of observational learning in placebo hypoalgesia remain unknown. To address a current gap in research, we aimed to investigate the neural processes underlying observational acquisition of placebo hypoalgesia.
Only a few studies have investigated observational learning in humans, primarily in the field of fear learning. These studies suggest that brain regions related to aversive learning, including the amygdala and the periaqueductal grey (PAG), mediate the acquisition of the aversive fear value via observation (Haaker et al., 2017; Olsson et al., 2007), consistent with animal studies (Ozawa et al., 2017). Social neuroscience research further suggests an important role for empathy and mentalizing processes during observational learning. Empathy refers to the process of sharing an emotional experience when another person feels a similar emotion (Preston and de Waal, 2002). Empathy for others’ pain primarily involves the anterior insula and the posterior part of the anterior cingulate cortex (ACC; Lamm et al., 2011; Singer et al., 2004). Mentalizing refers to the process of cognitively inferring mental states and experiences of others and is primarily associated with the temporoparietal junction (TPJ) and the medial prefrontal cortex (mPFC; Frith and Frith, 2006; Schurz et al., 2014). Furthermore, previous studies on placebo hypoalgesia and instructional learning indicate that the dorsolateral prefrontal cortex (DLPFC) is critical for representing and updating the expected treatment outcome value (Koban et al., 2017; Krummenacher et al., 2010; Lui et al., 2010; Yoshida et al., 2013), and subsequently for activating the descending pain modulatory system (Fields, 2004).
Based on these studies, we recently postulated neural processes that are likely to be important for the acquisition of placebo hypoalgesia via observation (Schenk et al., 2017a). We hypothesized that observation of painful stimulations and treatment-induced pain reduction would result in the involvement of brain regions related to aversive learning, empathy, mentalizing and expectancy processing (see results/methods for more details).
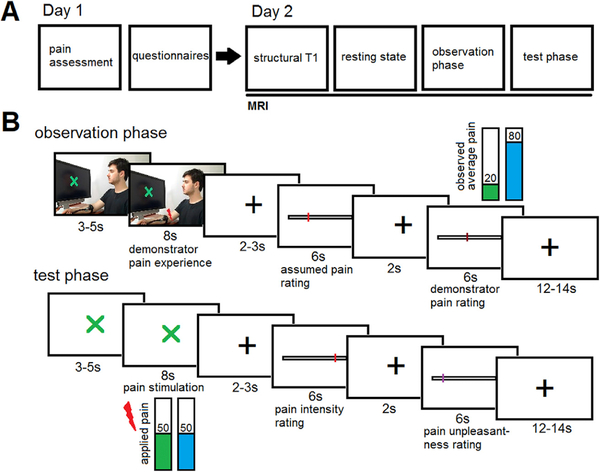
The current study employs a 2-day study design to investigate the neural processes of acquiring placebo hypoalgesia via observational learning (Fig. 1A). On day 1, participants provided informed consent and underwent individual pain assessment. On day 2, participants completed functional magnetic resonance imaging (fMRI), including a resting state acquisition phase, an observational learning phase, and a placebo test phase. During the observation phase, participants observed video clips of a demonstrator receiving heat pain stimulation on a forearm locations with a placebo treatment (placebo condition) and a forearm location with a control cream (control condition; Fig. 1B). During the control condition, the demonstrator showed a painful facial expression and provided a high pain rating while during the placebo condition, the demonstrator showed a neutral facial expression and provided a low pain rating. The comparison of the control and placebo conditions allowed us to investigate the neural processes associated with placebo hypoalgesia acquisition through observational learning. During the subsequent placebo test phase, the same inert creams were applied to the forearm of the participants and they received equally painful heat stimulations on both forearm locations. This allowed us to test for placebo hypoalgesia acquired via observational learning.
Fig. 1. Experimental Design.
(A) Experimental Procedure. On day 1, participants were tested for their pain sensitivity to determine the pain level to be used during the fMRI experiment. Afterwards, participants completed psychological questionnaires. On day 2, participants received information about the MRI procedure and underwent a structural MRI, a resting state fMRI, and a task-based fMRI including the observation phase (placebo and control run) and the test phase (placebo and control run). (B) Trial Design. During the observation phase, participants observed short video clips of a demonstrator receiving pain. The demonstrator was looking at a computer screen with a visual cue (indicating placebo or control), had two colored creams applied to his forearm, and a thermode placed on one of the locations (depending on condition). A red lightning bolt indicated the start of the painful stimulation. The demonstrator showed a relaxed facial expression during the placebo condition and a painful facial expression during the control condition. Afterwards, participants rated their perception of the pain experienced by the demonstrator before being shown the actual pain rating of the demonstrator. The demonstrator rated the pain low during the placebo run and high during the control run. During the test phase, a visual cue indicated the placebo or the control run. Participants had the same creams applied on their forearm and received painful thermal stimuli. After each stimulation, participants rated their experienced pain intensity and pain unpleasantness. The demonstrator granted permission to use his image.
2. Material and methods
2.1. Volunteers
Forty-two healthy volunteers participated in this study, of which four were excluded due to misapprehension of the instructions, technical difficulties, or anxiety in the scanner. Thirty-eight participants (28.1 8.5 (19–55) years, 23 female) were included in the data analysis. Participants were recruited using flyers and internet advertisements. Participants were confirmed to be healthy with approximately 20-min-long phone, as well as in-person interviews. Exclusion criteria included cardiovascular, neurological, pulmonary, kidney, liver, or degenerative neuromuscular diseases; cancer; chronic or current pain; severe psychiatric conditions; alcohol/drug dependence or abuse (past 3 months); pregnancy or breast feeding; color-blindness; impaired hearing; left-handedness; allergies to creams or food coloring; as well as any conditions that might affect MRI safety. Participants performed a drug test before starting the experimental procedures and the experiment was discontinued if the result was positive. Female participants performed a pregnancy test before the MRI. All participants gave written consent and the study was approved by the Internal Review Board at the University of Maryland, Baltimore.
2.2. Experimental paradigm
The study was designed to investigate the neural processes of observational learning, as well as observationally-induced placebo hypoalgesia. The experiment consisted of two experimental sessions (day 1 and day 2). Day 2 was a maximum of 7 days after day 1 (mean: 2.9 days 2.2 (SD)). fMRI measurements were performed on day 2. Day 1 was performed at the University of Maryland, School of Nursing behavioral labs. Day 2 was performed at the University of Maryland, School of Medicine, Core for Translational Research in Imaging at Maryland (CTRIM).
On day 1, participants were informed about the research and signed consent forms. Participants were told that we want to compare the neural processing of observing someone in pain (with and without pain treatment) with the neural processing of directly experienced pain (with and without pain treatment). A drug test was conducted and the volunteer was dis-qualified if the drug test was positive.
The experiment started with the collection of basic vital information (e.g., height, weight, blood pressure). Afterwards, a heat pain sensitivity assessment was performed according to the methods of limits (Fruhstorfer et al., 1976). Heat pain stimuli were applied using a thermode (PATHWAY System, Medoc, Ramat Yishai, Israel). Participants were then instructed on how to use the rating device (Celeritas Fiber Optic Response System, Psychology Software Tools Inc, Sharpsburg, PA, USA) and the visual analogue scale (VAS) used to collect pain ratings (ranging from 0 = no pain to 100 = maximum tolerable pain). Then, participants rated the pain intensity of a series of painful heat stimulations (8 stimuli, 6s each) to confirm the pain sensitivity assessment, and completed an implicit association test and several questionnaires.
On day 2, participants were informed about the experimental task in the MRI. Female participants performed a pregnancy test. The placebo treatment consisted of two creams that were applied to the forearm of each participant. The creams consisted of colored skin lotion (green and blue). Participants were told that they will receive the same creams as the person they will be observing, but were not told which cream would be the treatment nor about the expected efficiency of the cream. The color that was associated with treatment and the location of application (top vs bottom on volar forearm) was randomized across participants. Participants also filled out questionnaires about mood and anxiety (MDMQ and STAI-state).
Participants were then placed in the MRI scanner and an additional short pain assessment was performed. This pain assessment was performed to account for contextual effects of the MRI and allowed participants to adapt to the scanner environment and pain stimuli. Visibility of the whole screen was tested with 4 cross signs that were placed in the corners of the screen and participants were asked to report the number of cross signs. The MRI measurement started with a structural T1 scan (6min), followed by an eyes-open resting state scan (8min, white fixation cross in the middle of the screen).
The study paradigm started with the observation phase, consisting of two runs of 12 trials each (treatment and control run). The order of the treatment and control run were randomized across participants. During each trial, participants observed a short video clip of a demonstrator (same demonstrator for all participants, 11–13s in total). The demonstrator was sitting in front of a computer screen and had two creams (green and blue) and a thermode placed on his forearm. A visual cue on the computer screen (green or blue) indicated which location on the forearm (green or blue cream) was going to receive pain during this run. After a variable delay (3–5s), a red lightning bolt appeared, indicating that the demonstrator was receiving pain (8s). During this time, the demonstrator showed a painful facial expression during the control run and a neutral facial expression during the treatment run. After a delay (white cross, 2–3s), participants were asked to rate how much pain they thought the demonstrator experienced on a VAS from 0 = no pain to 100 = maximum tolerable pain (6s). After another delay (white cross, 2s), a VAS from 0 = no pain to 100 = maximum tolerable pain was presented showing the pain rating of the demonstrator. The demonstrator rated the pain VAS 70–90 in the control condition and VAS 10–30 in the placebo condition. This was followed by a variable inter-trial interval (12–14s).
After the observation phase, participants answered four questions regarding their expected pain and anxiety for both conditions. The test phase also consisted of two runs (placebo and control run) with 12 trials each. The test phase had a similar trial design as the observation phase to facilitate the transfer of the acquired expectations into the test phase. For each run, the thermode was moved to the location with the cream that corresponded to the current color. During each trial, a visual cue (green or blue cross) indicated that pain stimulation on the forearm location with either the green or blue cream will begin shortly (3–5s), followed by the pain stimulation (1s ramp up, 6s stimulation, 1s ramp down). The applied pain intensity corresponded to a previously calibrated VAS 50/ 100. After a delay (white cross, 2–3s), participants rated their pain intensity on a VAS from 0 = no pain to 100 = maximum tolerable pain (6s). After another delay (white cross, 2s), participants rated their pain unpleasantness on a VAS from 0 = no unpleasantness to 100 = maximum tolerable unpleasantness (6s). The trials ended with a variable inter-trial interval (12–14s).
Afterwards, participants were taken out of the scanner and completed a few additional questionnaires. At the end of the experimental session, participants were debriefed about the use of deceptive information and offered to withdraw their data. None of the participants withheld their approval. Participants were compensated for their time with $100.
2.3. Data acquisition
The control of the experimental timing, stimulus presentation and behavioral data collection was carried out using Eprime v2 (Psychology Software Tools, Sharpsburg, PA, USA). Participants operated a Celeritas Fiber Optic Response System in order to provide responses (Psychology Software Tools, Sharpsburg, USA). fMRI data were acquired on a 3 T system (Magnetom TIM Trio, Siemens, Erlangen, Germany) equipped with a 32-channel head coil. To measure BOLD responses, a T2*weighted 2D multiband gradient echo planar imaging sequence was used (TR: 1.52s; TE: 30 ms; flip angle: 75°; field of view: 224 mm2; multiband acceleration: 4). Each volume consisted of 72 transversal slices with a voxel size of 2 × 2 × 2mm3. High resolution anatomical T1-scans were acquired using an MPRAGE sequence with a voxel size of 1 × 1 × 1mm3.
2.4. Behavioral data analysis
Behavioral data analysis was performed using Statistical Package for the Social Sciences (SPSS) 24 (IBM, Armonk, USA). Data were analyzed using repeated ANOVAs with condition and trial as predictors and pain ratings as dependent variables. For the analysis of potential covariates (see supplementary information), we used Pearson’s correlation, one-way ANOVAs and two sample t-tests. All effects were considered significant at P < 0.05 (one or two tailed, depending on a-priori hypothesis).
2.5. fMRI data analysis
fMRI data preprocessing and statistical analyses were performed using SPM12 (Wellcome Department of Imaging Neuroscience, London, UK) and CONN (https://www.nitrc.org/projects/conn) (Whitfield-Gabrieli and Nieto-Castanon, 2012). Data preprocessing consisted of slice timing, motion correction (realignment and unwarp) and co-registration of the T1 anatomical scan to the functional images. Afterwards, the contrast images were spatially normalized using DARTEL of the T1 anatomical scan (based on the CAT12 template, http://dbm.neuro.uni-jena.de/cat/) and smoothed using an 8-mm (FWHM) isotropic Gaussian kernel. Then, images were entered into CONN for denoising. Six movement regressors, six temporal derivatives of movement, five CSF regressors, five white matter regressors and a high pass filter (0.008 Hz) were used to denoise the imaging data (Behzadi et al., 2007).
Afterwards, we performed a first level analysis using a general linear model in SPM12. Each regressor was modeled by boxcar functions convolved with a canonical hemodynamic response function (HRF). For each condition during the observation phase, we included regressors for cue observation, demonstrator pain observation, participant’s pain expectancy rating, and demonstrator pain report observation. For each condition during the test phase, we included regressors for cue, pain stimulation, pain intensity rating, and pain unpleasantness rating. T-contrasts of interest were then calculated. Collinearity between regressors was confirmed to be low for both phases using SPM12 (average: cos(θ) <0.06 [0.05 – 0.08]). One participant was excluded from the analysis of the test phase because of excessive movement (>5 movements of >1 mm across both test runs).
To maximize power, we employed a ROI approach based on previous meta-analysis or relevant papers. Observation of pain stimulation: left and right amygdala (6 mm radius; (Haaker et al., 2017)), PAG (6 mm; (Fairhurst et al., 2012), based on (Haaker et al., 2017)), left and right TPJ (10 mm; (Igelström et al., 2015)), mPFC (10 mm; (Molenberghs et al., 2016; Schurz et al., 2014)), left and right anterior insula (8 mm; (Bzdok et al., 2012; Lamm et al., 2011)), posterior ACC (10 mm; (Bzdok et al., 2012; Lamm et al., 2011)). Observation of pain rating: left and right DLPFC (10 mm; (Atlas and Wager, 2014)), rostral ACC (10 mm; (Atlas and Wager, 2014)). Test phase (pain stimulation): left and right DLPFC (10 mm; (Atlas and Wager, 2014)), rostral ACC (10 mm; (Atlas and Wager, 2014)), PAG (6 mm; (Atlas and Wager, 2014)), left and right posterior insula (8 mm; (Atlas and Wager, 2014)) and middle cingulate cortex (MCC, 10 mm; (Atlas and Wager, 2014)). The selection of anatomical ROIs was based on two previous meta-analyses on empathy (Bzdok et al., 2012; Lamm et al., 2011), mentalizing (Molenberghs et al., 2016; Schurz et al., 2014) and a meta-analysis on placebo hypoalgesia (Atlas and Wager, 2014). Three ROIs were based on relevant papers. Namely, amygdala and PAG ROIs for the observation phase were taken from a recent article investigating aversive observational learning (Haaker et al., 2017), as no relevant meta-analysis was available. In order to get a ROI in the posterior part of the TPJ, we used a recent study investigating TPJ subdivisions and functions (Igelström et al., 2015, see also Igelström et al., 2016), as we considered the provided coordinates from meta-analyses in this region as too unspecific. If multiple activation peaks were available (e.g. from two meta-analyses), the centroid of the reported activation peaks was used as ROI center. For medial ROIs, one ROI with x = 0 was used (PAG, mPFC, posterior ACC, rostral ACC, MCC). The ROI images were masked with the averaged grey matter image at a threshold of 0.75 to exclude the influence of white matter and CSF areas of no interest and increase sensitivity for grey matter voxels. The remaining voxels within an ROI were averaged and t-tests were used to test for significance between the ROI means of the placebo and the control condition. The resulting p-values of each investigated cognitive process (observation of pain stimulation, observation of pain rating, pain stimulation) were Bonferroni corrected for the number of ROIs for the process under consideration. In the results section, T- and P-values are provided for the ROI average (corrected for number of ROIs) as well as T-values and coordinates for the peak voxel within a significant ROI.
To test for a modulation of connectivity between the DLPFC and TPJ, we performed a Psycho-Physiological Interaction (PPI) analysis (Friston et al., 1997). We extracted the time series around the peak activation of the right DLPFC within a sphere with 8 mm diameter during the demonstrator pain report. We then calculated the PPI interaction term as the time series multiplied by the psychological predictor (condition). All three regressors were subsequently included in a new first level analysis. After model estimation, t-contrasts of interest were calculated for the PPI interaction regressor, and TPJ modulation was investigated within ROIs of 10 mm radius. To investigate whether the BOLD signals were associated with the placebo effects, ROI data was extracted and correlated (Pearson’s) with the placebo effects. Based on previous results (Golkar et al., 2015; Golkar and Olsson, 2017) and our data (see supplementary information), the placebo effect was adjusted for race.
To formally test whether DLPFC – TPJ connectivity mediates the influence of the treatment cue on the behavioral placebo effect, a mediation analysis using the Multilevel Mediation and Moderation(M3)Toolbox(https://github.com/canlab/MediationToolbox) was performed (Wager et al., 2009, 2008). A 2-path mediation model was employed. The initial variable (X) was set as the placebo and control condition (1 vs −1), the outcome variable was defined as the VAS pain intensity reports per condition, and the mediator was defined as the right DLPFC–TPJ connectivity values per condition. Dummy coded subject regressors were used to account for subject specific effects (one regressor for each subject, with 1 = subject and 0 = all other subjects). A Bootstrap procedure with 10,000 samples was used for significance testing (Efron and Tibshirani, 1994).
Preprocessing of resting state data was done similarly to the task-based data and data was analyzed using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012). Six movement regressors, six temporal derivatives of movement, five CSF regressors, five white matter regressors, 2 rest regressors and a band pass filter (0.008–0.09 Hz) were used to denoise the imaging data (Behzadi et al., 2007). First-level ROI-ROI correlations were performed and then z-values were extracted for second-level correlation analysis in Matlab. ROIs were based on the observation phase (right DLPFC and right TPJ) with a radius of 10 mm. For illustration purposes, all statistical maps used a significance threshold of p < 0.005 uncorrected and were overlaid on the mean structural image of all participants. All activations are reported using x, y, z coordinates in MNI (Montreal Neurological Institute) standard space. Data and analysis code for SPM will be made available upon request.
3. Results
3.1. Observational learning leads to placebo hypoalgesia
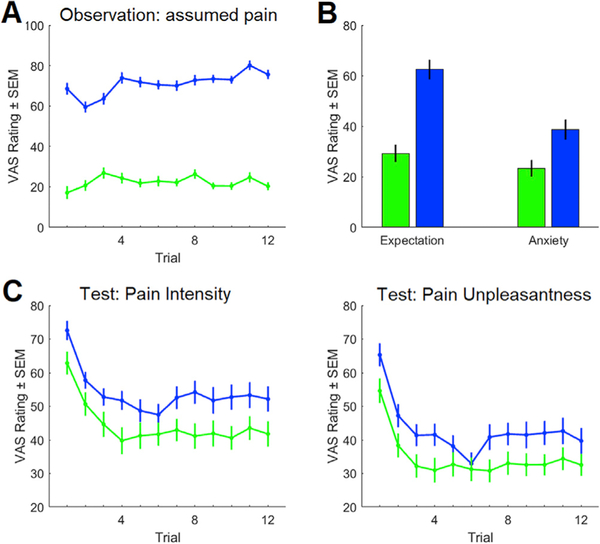
During the observation phase, participants rated the pain they thought the demonstrator experienced during each trial. The average assumed pain was lower in the placebo condition compared to the control condition (visual analogue scale (VAS): 22.3 (1.6) vs 71.0 (1.8) mean (standard error of the mean (SEM)), F(1,37) = 232.8, P < 0.001), indicating that the contents of the video were well-understood (Fig. 2A). At the end of the observation phase, we measured their expectations and anxiety regarding the upcoming pain experience during the test phase. For the placebo condition, participants expected less pain (VAS: 29.2 (3.4) vs 62.5 (4.0), T(37) = 5.3, P < 0.001) and reported less anxiety about the upcoming pain (VAS: 23.3 (3.3) vs 38.6 (4.1), T(37) = 3.2, P = 0.002) as compared to the control condition (Fig. 2B). These results show that the observation was effective in creating an expectation of pain relief in the placebo condition.
Fig. 2. Observational learning leads to placebo hypoalgesia.
(A) During the observation phase, participants rated the demonstrator’s pain significantly lower in the placebo run as compared to the control run. (B) After the observation phase, participants expected that they would feel less pain and were less anxious about the pain for the upcoming placebo run as compared to the control run. (C) During the test phase, participants experienced a lower pain intensity and pain unpleasantness during the placebo run as compared to the control run.
During the placebo test phase, participants were asked to rate their experienced pain intensity as well as their experienced pain unpleasantness. The experienced pain intensity (VAS: 44.4 (3.3) vs 54.0 (2.8), F(1,37) = 28.0, P < 0.001) and pain unpleasantness (VAS: 34.7 (3.1) vs 42.9 (2.9), F(1,37) = 18.7, P < 0.001) were lower in the placebo condition compared to the control condition. We also observed a significant trial effect for pain intensity (F(1,27) = 11.3, P < 0.001) and pain unpleasantness (F(1,27) = 10.2, P < 0.001; Fig. 2C). This data shows that our study participants successfully learned placebo hypoalgesia from the demonstrator. The significant trial results are primarily due to the increased pain perception during the first trials after repositioning the thermal stimulation on the forearm. We did not observe significant interactions between condition and trials. Please see supplementary information for additional behavioral analyses.
3.2. Brain regions associated with observational learning
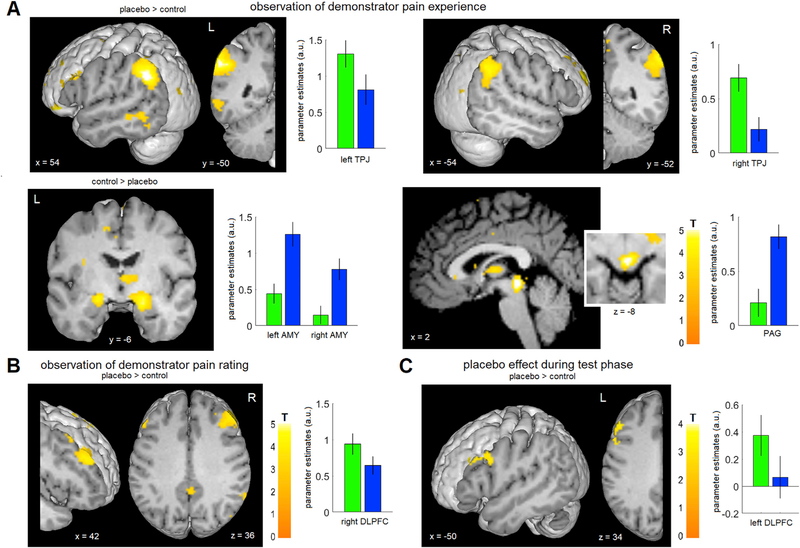
We first investigated blood-oxygen-level dependent (BOLD) responses during the observation of the demonstrator’s pain experience (Fig. 3A, Table 1, Fig. S1). Our hypotheses were based on the following rationale: First, participants have to understand that the pain experience of the demonstrator in the placebo condition is reduced due to the applied treatment cream, on top of understanding that the demonstrator is experiencing pain, which is relevant in both conditions. Based on this we expected a higher mentalizing effort in the placebo condition, and hypothesized an increased BOLD response in the TPJ and mPFC in the placebo as compared to the control condition. Second, given that aversive learning circuits (Ozawa et al., 2017) play a role in observational learning of aversive stimuli (Haaker et al., 2017; Olsson et al., 2007; Olsson and Phelps, 2007), we hypothesized a higher BOLD response in the amygdalae and PAG during the control as compared to the placebo condition. Third, based on studies reporting the involvement of empathy related brain regions when someone else experiences pain (Lamm et al., 2011; Singer et al., 2004), we hypothesized an increased BOLD response in the anterior insula and posterior ACC during the control as compared to the placebo condition.
Fig. 3. Brain regions associated with observational learning and placebo hypoalgesia.
(A) While observing the pain experience of the demonstrator, participants showed a higher activation in the bilateral TPJ during the placebo as compared to the control condition. In the reverse contrast, a higher activation of the bilateral amygdalae and the PAG was observed. See also Fig. S1. (B) While observing the pain rating of the demonstrator, participants showed a higher activation in the right DLPFC in the placebo condition. (C) During the test phase, a higher activation of the left DLPFC was observed in the placebo condition. For visualization purposes, a voxels threshold of P < 0.005 uncorrected was used for the figure. Bar graphs indicate parameter estimates ± SEM within the ROIs for placebo (green) and control (blue).
Table 1. Overview of ROI results.
| ROI | T | P(corr) |
|---|---|---|
| Observation: pain stimulation | ||
| TPJ r | 2.83 | 0.034* |
| TPJ l | 3.23 | 0.012* |
| mPFC | 1.61 | 0.522 |
| anterior insula r | 0.85 | 1 |
| anterior insula l | 0.23 | 1 |
| posterior ACC | 0.58 | 1 |
| amygdala r | 4.17 | 0.001* |
| amygdala l | 3.21 | 0.012* |
| PAG | 4.86 | 0.000* |
| Observation: pain rating | ||
| DLPFC r | 2.31 | 0.040* |
| DLPFC l | 1.90 | 0.097 |
| rostral ACC | −0.39 | 1 |
| Test: pain stimulation | ||
| DLPFC r | 1.61 | 0.408 |
| DLPFC l | 2.94 | 0.020* |
| rostral ACC | −0.14 | 1 |
| PAG | 2.20 | 0.242 |
| MCC | 1.11 | 0.966 |
| posterior insula r | 1.35 | 0.654 |
| posterior insula l | 1.00 | 1 |
We investigated BOLD responses in preselected ROIs for the observation and test phases. ROI = region of interest, T = T-value, P(corr) = P-value, corrected for multiple comparisons, TPJ = temporoparietal junction, mPFC = middle prefrontal cortex, ACC = anterior cingulate cortex, PAG = periaqueductal grey, DLPFC = dorsolateral prefrontal cortex, MCC = middle cingulate cortex, r = right, l = left.
indicates significant P-values after multiple comparison correction.
We observed stronger BOLD responses in the left and right TPJ (placebo > control; T(37) = 2.8, P(corr) = 0.03 (peak: T(37) = 5.6, [−54 −50 34]); T(37) = 3.2, P(corr) = 0.01 (peak: T(37) = 4.7, [60 −46 40])) in the placebo condition compared to the control condition (Fig. S2). The mPFC was not significant after correcting for number of ROIs (T(37) = 1.6, P(corr) = 0.5 (peak: T(37) = 3.1, [−6 42 36])). In the reverse contrast, we observed stronger BOLD responses in the left and right amygdala (control > placebo; T(37) = 3.2, P(corr) = 0.01 (peak: T(37) = 4.3, [−20 −6 −10]); T(37) = 4.2, P(corr) < 0.001 (peak: T(37) = 4.8, [22 −6 −16])) and the PAG (control > placebo; T(37) = 4.9, P(corr) < 0.001 (peak: T(37) = 5.7, [2 −30 −8])). No significant BOLD signal differences were observed in brain regions previously associated with empathy for pain (anterior insula and posterior ACC).
Next, we investigated the observation of the demonstrator’s pain ratings. During this phase, participants were presented rating information of the demonstrator, similar to previous social learning paradigms (Koban and Wager, 2016; Yoshida et al., 2013). Previous research suggests that learning from other’s rating information is mediated by prefrontal regions, especially the DLPFC (Koban et al., 2017). Additionally, the DLPFC and rostral ACC are important for learning and updating treatment outcome expectations during placebo (Krummenacher et al., 2010; Lui et al., 2010; Wager and Atlas, 2015). Therefore, we hypothesized that the DLPFC and rostral ACC would show a stronger BOLD response during the placebo as compared to the control condition. Consistently, we observed a stronger BOLD response in the right DLPFC (placebo > control; T(37) = 2.3, P(corr) = 0.04 (peak: T(37) = 4.0, [42 30 32]) Fig. 3B, Table 1, Fig. S1), in line with placebo hypoalgesia studies investigating the processing of treatment outcome values during conditioning (Lui et al., 2010; Watson et al., 2009). The left DLPFC failed significance after correcting for the number of ROIs (T(37) = 1.9, P(corr) = 0.1, (peak: T(37) = 3.2, [−46 26 36])).
3.3. Brain regions associated with placebo hypoalgesia after observational learning
Painful thermal stimulation (all pain conditions vs baseline) resulted in BOLD response increases in brain regions previously associated with pain processing (Fig. S3; Duerden and Albanese, 2013). We then investigated whether brain regions previously associated with placebo hypoalgesia (Atlas and Wager, 2014) were also modulated during the placebo test phase (Fig. 3C, Table 1, Fig. S1). We expected increased BOLD responses in brain regions involved in expectancy processing and top-down modulation (DLPFC, rostral ACC, PAG) as well as decreased BOLD responses in pain processing regions (posterior insula, anterior MCC). We observed an increase in BOLD response in the left DLPFC (placebo > control; T(36) = 2.9, P(corr) = 0.02 (peak: T(36) = 3.3, [−44 26 34])) in line with previous studies investigating placebo hypoalgesia after directly experiencing conditioning (Lui et al., 2010; Watson et al., 2009). The significant DLFPC ROI was located at the same location as the non-significant DLPFC ROI in the left hemisphere during the observation phase. No other ROIs reached statistical significance.
3.4. Functional DLPFC – TPJ connectivity during observational learning predicts placebo hypoalgesia
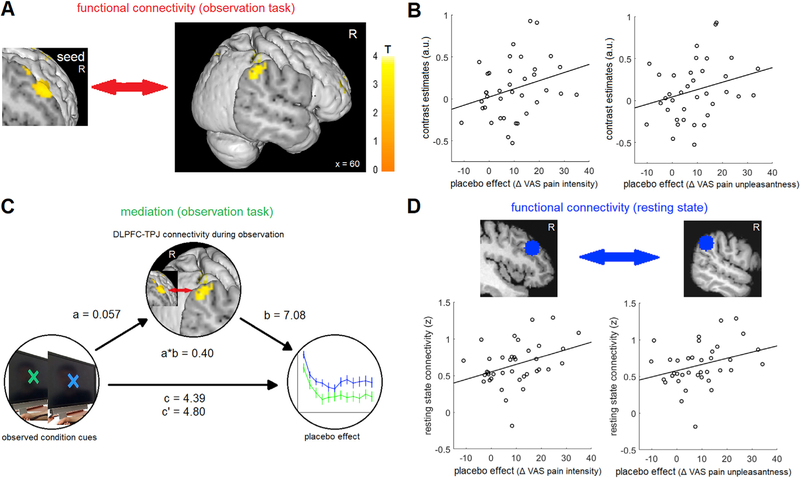
Studies investigating learning processes that combine conceptual information with direct-experience learning reported a critical role for integration processes between the prefrontal cortex (e.g. DLPFC) and brain regions involved in direct experience learning (e.g. ventral striatum, amygdala; Atlas et al., 2016; Li et al., 2011;Schenk et al., 2017b). In our case, the information gained from the demonstrator’s pain ratings had to be integrated with the processes relevant for the understanding of the observed state of pain relief in the demonstrator. The DLPFC is critical for instructional learning (Koban et al., 2017) and outcome expectations, especially in placebo hypoalgesia (Krummenacher et al., 2010; Lui et al., 2010; Watson et al., 2009), while the TPJ is critical for understanding mental states of others (Schurz et al., 2014), consistent with our observed modulation of the DLPFC and the TPJ. Additionally, DLPFC and dorsal/posterior TPJ are strongly interconnected (Makris et al., 2005; Seghier, 2013). We therefore conducted a psychophysiological interaction (PPI) analysis (Friston et al., 1997) to investigate a modulation of connectivity between the DLPFC and TPJ. We observed a stronger functional connectivity between the right DLPFC and the right TPJ during the placebo as compared to the control condition (T(37) = 2.1, P = 0.02 (peak: T(37) = 3.83, [62 −40 38]); Fig. 4A). The connectivity difference was correlated with the individual placebo effects (pain intensity, r = 0.30, P = 0.04; pain unpleasantness, r = 0.29, P = 0.04; Fig. 4B). This suggests that there was a stronger coupling between the two brain regions in the condition where participants observed a hypoalgesic treatment effect and that the coupling increase in the placebo condition predicted the subsequent pain relief in the placebo condition.
Fig. 4. DLPFC –TPJ connectivity.
(A) We observed a higher functional connectivity between the DLPFC and the TPJ in the placebo condition compared to the control condition. For visualization purposes, a voxels threshold of P < 0.005 uncorrected was used for the figure. (B) The connectivity difference predicted the subsequent placebo effect (pain intensity: r = 0.30, P = 0.04; pain unpleasantness r = 0.29, P = 0.04). (C) The mediation analysis indicated that the DLPFC-TPJ connectivity mediated the effect of the treatment condition cues on the behavioral placebo effect (a*b = 0.40, P = 0.002). (D) Resting state connectivity between the DLPFC and the TPJ also predicted the subsequent placebo effect (pain intensity: r = 0.37; P = 0.01; pain unpleasantness r = 0.34, P = 0.02).
3.5. Functional DLPFC –TPJ connectivity mediates observational acquisition of placebo hypoalgesia
We subsequently performed a formal mediation analysis to determine the link between the effect of the treatment condition and brain responses, as well as the relationship between brain and pain perception (Wager et al., 2009, 2008). We tested whether the connectivity between the right DLPFC and right TPJ during the observation mediates the effect of the observed treatment condition cues (placebo vs control) on subsequent placebo hypoalgesia. The indirect path a*b was significant, supporting a mediation of the effect (a = 0.057, P = 0.003’ ; b = 7.08, P = 0.002; a*b = 0.40, P = 0.002; c = 4.80, P = 0.003; c = 4.39, P = 0.002; path coefficient; Fig. 4C). This shows that DLPFC-TPJ connectivity mediates, at least partially, the effect of the treatment condition cues on the subsequent experience of placebo hypoalgesia.
3.6. Resting state DLPFC – TPJ connectivity predicts placebo hypoalgesia
Finally, based on the previous connectivity findings, we investigated the resting state data to see if we could find additional evidence that DLPFC-TPJ connectivity is important for learning placebo hypoalgesia through observation. If so, we expected that participants with a higher resting connectivity between the two brain regions would show better observational learning and therefore higher placebo effects. We extracted ROIs for the right DLPFC and the right TPJ from our resting state data to determine if the resting state connectivity between the two regions was correlated with the subsequent placebo hypoalgesic effect. We observed a positive correlation (pain intensity, r = 0.37, P = 0.01; pain unpleasantness, r = 0.34, P = 0.02; Fig. 4D) between individual DLPFC-TPJ connectivity and placebo hypoalgesia. This suggests that a higher baseline connectivity between DLPFC and TPJ supports the observational acquisition of placebo hypoalgesia, further supporting the importance of DLPFC-TPJ connectivity for observational learning.
4. Discussion
The data gathered in this study demonstrate the neural processes underlying the observational acquisition of placebo hypoalgesia. FMRI analysis shows significant modulation of the amygdalae, PAG, TPJ, and DLPFC during observational learning. The connectivity between the DLPFC and TPJ mediated the acquisition of placebo hypoalgesia through observation. Additionally, resting-state DLPFC-TPJ connectivity predicted subsequent placebo effects.
During the observation of the demonstrator, participants were able to accurately infer the pain of the demonstrator in both conditions. After the observation phase, participants expected less pain and were less anxious about the upcoming pain in the placebo treatment condition. During the following test phase, participants experienced lower pain intensity and unpleasantness during the placebo condition, confirming that observational learning can lead to placebo-induced reductions of pain, similar to pioneering research in this area (Colloca and Benedetti, 2009). Therefore, our behavioral results support that participants acquired and experienced placebo hypoalgesia, indicating that our experimental manipulation was successful.
At the neural level, we observed higher activation of the left and right TPJ during the observation of the demonstrator’s pain experience in the placebo as compared to the control condition, primarily located in the dorsal and posterior TPJ (Igelström et al., 2015). Furthermore, we observed a non-significant mean difference towards an activation of the mPFC. We have previously hypothesized the involvement of mentalizing processes during observational learning (Schenk et al., 2017a). TPJ and mPFC are core regions mediating mentalizing functions, and meta-analyses report the activation peak in the left and right posterior TPJ (Schurz et al., 2014). Previous investigations using resting state connectivity also support the theory that the posterior TPJ is part of the default mode network associated with mentalizing functions (Igelströmet al., 2015; Mars et al., 2012). In the placebo condition, participants had to understand the demonstrator’s treatment-induced pain reduction on top of the effects of the pain stimulation. The stronger BOLD response in the TPJ during placebo is consistent with this, indicating a higher mentalizing effort. A recent investigation on TPJ function using various tasks on mentalizing, memory, and attention concluded that only the mentalizing task involved both the dorsal and posterior areas of the TPJ (Igelström et al., 2016), which is consistent with our findings. However, although the posterior activation was specific for mentalizing, dorsal TPJ activation was reported for all other tasks. This suggests that the dorsal activation is related to more general processes of convergence and interaction that are required during attention, memory, and social cognition (Igelström et al., 2016), and potentially represents contextual updating and cognitive control (Duncan, 2010; Geng and Vossel, 2013).
We also observed reduced BOLD signals in the bilateral amygdalae and the PAG in the placebo condition. Both regions are a critical part of the well-established aversive learning circuits (Herry and Johansen, 2014), and recently these regions have been associated with the observational acquisition of fear (Haaker et al., 2017), similar to the acquisition of fear through conditioning (Olsson and Phelps, 2007). As expected, the activation was higher in the control condition in our experiment, indicating that the aversive value of pain was learned in a similar circuit, and that these circuits are downregulated when pain relief is observed.
By contrast, we did not observe a significant modulation of the anterior insula or the posterior ACC, which have been associated with affective empathy (Lamm et al., 2011) and therefore might have been involved in this observational learning study (Schenk et al., 2017a). Previous neuroimaging studies indicated that brain regions related to empathy may be involved when observing someone in pain (Singer et al., 2004), and a correlation between empathy scores and observationally learned placebo hypoalgesia was previously observed (Colloca and Benedetti, 2009). However, when observation occurred through a video empathy was not correlated with observationally learned placebo hypoalgesia (Hunter et al., 2014), suggesting that empathy may be context-dependent and not be the primary driving component for the findings related to this study. Future studies should investigate if and how interpersonal interaction between the demonstrator and the participant might be important to engage empathy processes during observational learning (Meyer et al., 2013) and should collect state empathy ratings (Betti et al., 2009).
While participants observed the demonstrator’s pain report, we detected a stronger activation of the right DLPFC and a trend in the left DLPFC in the placebo compared to the control condition. We also observed an increased BOLD signal in the left DLPFC during placebo hypoalgesia in the test phase. The DLPFC is important for maintaining and updating outcome expectancies and the processing of contextual information during placebo treatment (Lorenz et al., 2003; Wager and Atlas, 2015). Studies on the acquisition of placebo hypoalgesia through direct experience show a critical role for the DLPFC during conditioning and the subsequent test phase (Lui et al., 2010; Watson et al., 2009). Our results suggest that the DLPFC is also important for learning and maintaining outcome expectations during observational learning, similar to learning through direct experience. The fact that we observed right DLPFC during the observation phase and left DLPFC during the test phase is unlikely to reflect different lateralization, as in both instances a mean difference in the same direction on the contralateral side was also observed.
We did not observe a significantly decreased BOLD response in the posterior insula and anterior MCC during the test phase for placebo analgesia. While some studies would support the observation of a decrease in pain related brain regions, other studies observe no effect or even increased BOLD responses in pain processing regions (Amanzio et al., 2013; Koban and Wager, 2016). Additionally, the Neurologic Pain Signature (Wager et al., 2013), which represents a brain pattern based on perceived pain intensity, was not very sensitive to changes in pain perception due to placebo hypoalgesia (Zunhammer et al., 2018), suggesting that multiple mechanisms might be underlying placebo hypoalgesia.
Previous research supports an important function of the TPJ in mentalizing processes (Frith and Frith, 2006), and the DLPFC is critical for acquiring and processing treatment expectations (Lui et al., 2010; Watson et al., 2009). Both regions were involved in understanding the treatment effect in our data. Additionally, DLPFC and dorsal/posterior TPJ are strongly interconnected (Makris et al., 2005; Seghier, 2013). We therefore conducted a PPI analysis between the DLPFC and the TPJ to investigate the coupling between the two regions. We observed a higher connectivity in the placebo than the control condition. The connectivity difference between the conditions in the observation phase was correlated with the pain rating difference in the test phase (placebo effect). The mediation analysis confirmed that the DLPFC-TPJ connectivity is formally mediating the effect of the treatment condition cues onto the placebo hypoalgesic effect. These results show that the treatment cue information influences the coupling between both regions and that the coupling between the DLPFC and TPJ is influencing the size of the observationally learned placebo hypoalgesia. This suggests that the coupling between the DLPFC and TPJ is critically involved during the observational acquisition of placebo hypoalgesia, potentially reflecting an integration of outcome expectations and the observed treatment effect in the demonstrator.
To corroborate these results, we used the resting state data collected prior to the experiment to explore whether resting state DLPFC-TPJ connectivity is important for observational learning. Participants with a higher connectivity between DLPFC and TPJ regions showed larger observationally learned placebo hypoalgesia. This provides additional evidence that DLPFC-TPJ connectivity is important for observational learning and suggests that in our experiment, participants with a higher integration between the DLPFC and TPJ would show better observational learning and therefore higher placebo responses.
The current findings expand existing research on the acquisition of placebo effects through direct experience (Lui et al., 2010; Watson et al., 2009), confirming the important role of the DLPFC. Our data also indicate an important role for social cognition areas and DLPFC-TPJ connectivity during observational learning, not previously observed in acquiring placebo effects through direct experience. It is important to note that in this study we used an ROI approach based on current literature (see results section for details). Although this allowed us to maximize power to detect significant effects in expected regions, it limits our explanatory power to the regions that were part of our hypotheses.
So far, neural observational learning processes have been investigated only in the field of fear (Haaker et al., 2017; Olsson et al., 2007). As the first study from a different field, our data indicates that the observational acquisition of fear and pain may be processed by similar aversive learning regions. However, we also provide evidence for a critical role of the coupling between DLPFC-TPJ not observed in previous observational learning research. This might be related to the more abstract concept of a treatment benefit as compared to a more reactive response to a fear stimulus (Jepma and Wager, 2015; Moerman and Jonas, 2002) and is consistent with the important role of higher order brain areas (i.e., DLPFC, TPJ) during more conceptual learning processes (Atlas et al., 2016; Koban et al., 2017; Li et al., 2011). Interestingly, physicians also show increased activation of the TPJ and DLPFC during patient treatment (Jensen et al., 2014), further supporting an important role of these regions in the social transmission of information about treatments.
In summary, we demonstrated the neural processes during the acquisition of placebo hypoalgesia via observational learning. Our results suggest that the circuits involved in learning the aversive value of pain (Haaker et al., 2017; Ozawa et al., 2017) are downregulated while observing someone receiving a pain treatment. Additionally, we provided evidence that the TPJ and the DLPFC, two brain regions critical for mentalizing and outcome expectations, are involved during the observational acquisition of placebo hypoalgesia. We also provide evidence that the coupling between the DLPFC and TPJ is critical during observational learning by mediating the effect of the treatment condition cues onto the placebo hypoalgesic effect, and this result is further supported by the resting state findings. Our results provide a neural understanding of observational learning and opens up new avenues for future research in placebo hypoalgesia and the emerging investigation of social learning processes in different fields.
Supplementary Material
Acknowledgements
This research was supported by the Dean Initiatives Funds University of Maryland School of Nursing, Baltimore (L.C.), the National Institute of Dental and Craniofacial Research (NIDCR, R01DE025946, L.C.), the National Center for Advancing Translational Sciences (1UL1TR00309801, L.C.), and the National Center for Complementary and Integrative Health (1R01AT010333-01A1, L.C.). The authors would like to thank Dr. David Seminowicz for advice on the resting state analysis; Samuel R. Krimmel for assistance with material creation and advice on the resting state analysis; Dr. Stephan Geuter for advice on the mediation analysis; and Nandini Raghuraman and Maxie Blasini for assistance during the data collection.
Footnotes
Declaration of competing interest
The authors declare no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2019.116510.
References
- Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F, 2013. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum. Brain Mapp 34, 738–752. 10.1002/hbm.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Doll BB, Li J, Daw ND, Phelps EA, 2016. Instructed knowledge shapes feedback-driven aversive learning in striatum and orbitofrontal cortex, but not the amygdala. eLife 5, e15192. 10.7554/eLife.15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Wager TD, 2014. A meta-analysis of brain mechanisms of placebo analgesia: consistent findings and unanswered questions In: Benedetti F, Enck P, Frisaldi E, Schedlowski M. (Eds.), Placebo, Handbook of Experimental Pharmacology. Springer Berlin; Heidelberg, pp. 37–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A, 1971. Social Learning Theory. General Learning Press, New York. [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT, 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti V, Zappasodi F, Rossini PM, Aglioti SM, Tecchio F, 2009. Synchronous with your feelings: sensorimotor {gamma} band and empathy for pain. J. Neurosci. Off. J. Soc. Neurosci 29, 12384–12392. 10.1523/JNEUROSCI.275909.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Geuter S, Sprenger C, Eippert F, 2014. Placebo analgesia: a predictive coding perspective. Neuron 81, 1223–1239. 10.1016/j.neuron.2014.02.042. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB, 2012. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct. Funct. 217, 783–796. 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Benedetti F, 2009. Placebo analgesia induced by social observational learning. Pain 144, 28–34. 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Albanese M-C, 2013. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum. Brain Mapp 34, 109–149. 10.1002/hbm.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, 2010. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci 14, 172–179. 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ, 1994. An Introduction to the Bootstrap. CRC Press. [Google Scholar]
- Fairhurst M, Fairhurst K, Berna C, Tracey I, 2012. An fMRI study exploring the overlap and differences between neural representations of physical and recalled pain. PLoS One 7, e48711. 10.1371/journal.pone.0048711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H, 2004. State-dependent opioid control of pain. Nat. Rev. Neurosci 5, 565–575. 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ, 1997. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6, 218–229. 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U, 2006. The neural basis of mentalizing. Neuron 50, 531–534. 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H, Lindblom U, Schmidt WC, 1976. Method for quantitative estimation of thermal thresholds in patients. J. Neurol. Neurosurg. Psychiatry 39, 1071–1075. 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ, Vossel S, 2013. Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci. Biobehav. Rev 37, 2608–2620. 10.1016/j.neubiorev.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Castro V, Olsson A, 2015. Social learning of fear and safety is determined by the demonstrator’s racial group. Biol. Lett 11, 20140817. 10.1098/rsbl.2014.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Olsson A, 2017. The interplay of social group biases in social threat learning. Sci. Rep 7, 7685 10.1038/s41598-017-07522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Yi J, Petrovic P, Olsson A, 2017. Endogenous opioids regulate social threat learning in humans. Nat. Commun 8, 15495. 10.1038/ncomms15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Johansen JP, 2014. Encoding of fear learning and memory in distributed neuronal circuits. Nat. Neurosci 17, 1644–1654. 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Hunter T, Siess F, Colloca L, 2014. Socially induced placebo analgesia: a comparison of a pre-recorded versus live face-to-face observation. Eur. J. Pain 18, 914–922. 10.1002/j.1532-2149.2013.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelström KM, Webb TW, Graziano MSA, 2015. Neural processes in the humantemporoparietal cortex separated by localized independent component analysis. J. Neurosci 35, 9432–9445. 10.1523/JNEUROSCI.0551-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelström KM, Webb TW, Kelly YT, Graziano MSA, 2016. Topographicalorganization of attentional, social, and memory processes in the human temporoparietal cortex. eNeuro 3 10.1523/ENEURO.0060-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Petrovic P, Kerr CE, Kirsch I, Raicek J, Cheetham A, Spaeth R, Cook A, Gollub RL, Kong J, Kaptchuk TJ, 2014. Sharing pain and relief: neural correlates of physicians during treatment of patients. Mol. Psychiatry 19, 392–398. 10.1038/mp.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepma M, Wager TD, 2015. Conceptual conditioning: mechanisms mediating conditioning effects on pain. Psychol. Sci 26, 1728–1739. 10.1177/0956797615597658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Jepma M, Geuter S, Wager TD, 2017. What’s in a word? How instructions, suggestions, and social information change pain and emotion. Neurosci. Biobehav. Rev 81, 29–42. 10.1016/j.neubiorev.2017.02.014. The power of instructions: the influence of instructions on cognition, behaviour and physical states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Wager TD, 2016. Beyond conformity: social influences on pain reports and physiology. Emotion 16, 24–32. 10.1037/emo0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G, 2010. Prefrontal cortex modulates placebo analgesia. Pain 148, 368–374. 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T, 2011. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54, 2492–2502. 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Li J, Delgado MR, Phelps EA, 2011. How instructed knowledge modulates the neural systems of reward learning. Proc. Natl. Acad. Sci. U.S.A 108, 55–60. 10.1073/pnas.1014938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL, 2003. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126, 1079–1091. 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA, 2010. Neural bases of conditioned placebo analgesia. Pain 151, 816–824. 10.1016/j.pain.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Pandya DN, 2005. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebr. Cortex 15, 854–869. 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schüffelgen U, Jbabdi S, Toni I, Rushworth MFS, 2012. Connectivity-based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cerebr. Cortex 22, 1894–1903. 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- Meyer ML, Masten CL, Ma Y, Wang C, Shi Z, Eisenberger NI, Han S, 2013. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Soc. Cogn. Affect. Neurosci 8, 446–454. 10.1093/scan/nss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman DE, Jonas WB, 2002. Deconstructing the placebo effect and finding the meaning response. Ann. Intern. Med 136, 471–476. 10.7326/00034819-136-6-200203190-00011. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Johnson H, Henry JD, Mattingley JB, 2016. Understanding the minds of others: a neuroimaging meta-analysis. Neurosci. Biobehav. Rev 65, 276–291. 10.1016/j.neubiorev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA, 2007. Learning fears by observing others: the neural systems of social fear transmission. Soc. Cogn. Affect. Neurosci 2, 3–11. 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Phelps EA, 2007. Social learning of fear. Nat. Neurosci 10, 1095–1102. 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Ycu EA, Kumar A, Yeh L-F, Ahmed T, Koivumaa J, Johansen JP, 2017. A feedback neural circuit for calibrating aversive memory strength. Nat. Neurosci 20, 90–97. 10.1038/nn.4439. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FBM, 2002. Empathy: its ultimate and proximate bases. Behav. Brain Sci 25, 1–20 discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Schenk LA, Krimmel SR, Colloca L, 2017a. Observe to get pain relief: current evidence and potential mechanisms of socially-learned pain modulation. Pain 158, 2077–2081. 10.1097/j.pain.0000000000000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk LA, Sprenger C, Onat S, Colloca L, Büchel C, 2017b. Suppression of striatal prediction errors by the prefrontal cortex in placebo hypoalgesia. J. Neurosci 1101–17 10.1523/JNEUROSCI.1101-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J, 2014. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev 42, 9–34. 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Seghier ML, 2013. The angular gyrus multiple functions and multiple subdivisions. The Neuroscientist 19, 43–61. 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD, 2004. Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162. 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, 2015. The neuroscience of placebo effects: connecting context, learning and health. Nat. Rev. Neurosci 16, 403–418. 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E, 2013. An fMRIbased neurologic signature of physical pain. N. Engl. J. Med 368, 1388–1397. 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN, 2008. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050. 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF, 2009. Brain mediators of cardiovascular responses to social threat: Part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage, Brain Body Medicine 47, 821–835. 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AKP, 2009. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain 145, 24–30. 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A, 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Yoshida W, Seymour B, Koltzenburg M, Dolan RJ, 2013. Uncertainty increases pain: evidence for a novel mechanism of pain modulation involving the periaqueductal grey. J. Neurosci 33, 5638–5646. 10.1523/JNEUROSCI.498412.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunhammer M, Bingel U, Wager TD, Placebo Imaging Consortium, 2018. Placebo effects on the neurologic pain signature: a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurol. 75, 1321–1330. 10.1001/jamaneurol.2018.2017002E [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.