Abstract
Background. We examined the role of aerosol transmission of influenza in an acute ward setting.
Methods. We investigated a seasonal influenza A outbreak that occurred in our general medical ward (with open bay ward layout) in 2008. Clinical and epidemiological information was collected in real time during the outbreak. Spatiotemporal analysis was performed to estimate the infection risk among patients. Airflow measurements were conducted, and concentrations of hypothetical virus-laden aerosols at different ward locations were estimated using computational fluid dynamics modeling.
Results. Nine inpatients were infected with an identical strain of influenza A/H3N2 virus. With reference to the index patient's location, the attack rate was 20.0% and 22.2% in the “same” and “adjacent” bays, respectively, but 0% in the “distant” bay (P=.04). Temporally, the risk of being infected was highest on the day when noninvasive ventilation was used in the index patient; multivariate logistic regression revealed an odds ratio of 14.9 (95% confidence interval, 1.7–131.3; P=.015). A simultaneous, directional indoor airflow blown from the “same” bay toward the “adjacent” bay was found; it was inadvertently created by an unopposed air jet from a separate air purifier placed next to the index patient's bed. Computational fluid dynamics modeling revealed that the dispersal pattern of aerosols originated from the index patient coincided with the bed locations of affected patients.
Conclusions. Our findings suggest a possible role of aerosol transmission of influenza in an acute ward setting. Source and engineering controls, such as avoiding aerosol generation and improving ventilation design, may warrant consideration to prevent nosocomial outbreaks.
Nosocomial transmission of influenza is frequently reported [1β3]. It typically occurs during seasonal peaks, and may involve almost all types of healthcare facilities [1, 2]. Its consequences are considerable: it may result in significant disease and even fatality among hospitalized patients, because these patients often are older and/or have multiple comorbidities [1–3]. Health care professionals are frequently involved, and the affected hospital units may require temporary closure with service suspension [2–6]. Lack of preexisting immunity toward the recently emerged pandemic influenza H1N1 virus and its potential to cause serious disease in both young and older adults have further raised the importance of hospital infection control [7–9]. However, how influenza is transmitted in the health care setting and what control measures are effective have remained largely unclear [2, 8–12].
Under natural conditions, influenza virus is transmitted predominantly via droplets and direct contact [13]. Thus, adequate spacing, hand washing, and droplet precautions, including the use of face masks, are likely effective in preventing transmission [8, 9, 11]. However, in indoor health care settings, because of the special clinical and environmental conditions, aerosol transmission of diseases might become possible, as described for other viral and bacterial infections [1, 3, 13– 17]. Emerging evidence suggests that influenza infection can also be transmitted via the aerosol route, as shown in animal models and in experimental studies involving human subjects [3, 13, 18, 19]. In this study, we report an influenza outbreak that occurred in an acute medical ward. Epidemiological, airflow and computational fluid dynamics analyses were performed. The possible role of aerosol transmission of influenza was examined. The implications of the results on hospital infection control strategies will be discussed.
Methods
At Prince of Wales Hospital (PWH; Hong Kong), an outbreak of influenza A occurred in an adult general medical ward in April 2008. In Hong Kong, peak influenza activities occur in both spring (January–April) and summer (July–September) [20]. PWH is a 1350-bed acute care general hospital operated by the Hospital Authority of Hong Kong that serves an urban population of 1.5 million. Each year, >200 adult cases of confirmed influenza are being treated at PWH [5, 21, 22]. A major nosocomial outbreak of severe acute respiratory syndrome (SARS) occurred at PWH in 2003 [15, 23]; since then, all patients hospitalized with acute febrile respiratory illnesses are put on droplet precautions; if influenza is confirmed, the patients will be isolated or cohorted in designated wards [21, 22]. As a hospital policy, all health care personnel working in medical wards are required to wear surgical face masks, and an on-duty nursing officer is responsible for monitoring compliance [5].
Outbreak investigation. Nosocomial outbreaks of infectious diseases, once identified, would be investigated by the outbreak management team (which consisted of physicians, microbiologists, infection control practitioners, nurses, and hospital epidemiologists) as part of the management protocol [23]. Affected cases would be reviewed daily, and clinicoepidemiological information collected real time as the outbreak evolved. These data were studied and discussed in daily meetings throughout the whole outbreak period. Control measures, including ward closure, patient isolation, and use of antiviral prophylaxis, would then be recommended. Virological investigations for influenza have been described elsewhere [21, 22]. In brief, nasopharyngeal aspirate specimens were collected from symptomatic individuals and subjected to immunofluorescence assay and virus culture for diagnosis [21, 22]. In addition, for all confirmed cases, viral RNA was directly extracted for sequencing of the whole length of the hemaggultinin gene, as described elsewhere [24]; the nucleotide sequences in individual cases were then compared. Finally, a hemagglutination inhibition assay was performed (using the virus isolate obtained from the index patient as antigen source) to detect antibody rise in the paired serum samples collected from affected individuals [25].
Spatiotemporal analysis of epidemiological data. The epidemic curve for the outbreak was produced. The floor plan of the affected wards was studied; in PWH, the design of general medical wards followed an open bay ward layout [15]. For the analysis, all patients who had ever stayed with the index patient during the period of his presence in the ward (from admission to transfer or isolation) and all health care workers (HCWs) who had worked in the same ward during the period were included. Attack rates of influenza among inpatients were calculated each day on the basis of their locating “bay” [15]. Univariate relationships between risk of acquiring influenza infection and spatiotemporal variables were analyzed using the χ2 test or the Fisher exact test. Variables with a P value <.1 were entered into a multivariate (stepwise) logistic regression model to identify independent factors associated with infection. Odds ratio (ORs) and 95% confidence intervals (CIs) were reported for explanatory variables. In all analyses, a P value of <.05 was considered to indicate statistical significance. All probabilities were 2-tailed. Statistical analysis was performed using PASW Statistics software, version 17.0 (SPSS).
Airflow measurements and fluid dynamics analyses. Information on the ward's ventilation systems was collected during the outbreak investigation. These included the location and size of air supply diffusers, return grills, and the highefficiency particulate absorbing (HEPA) air purifier units that were present in each ward bay. Airflow rate through each supply diffuser, return grill, air purifier, and exhaust fan was measured in detail, as described elsewhere [15]. Dispersion of the hypothetical virus-laden aerosols, originated from the index patient's bed through the entire ward, was analyzed by computational fluid dynamics (CFD) method. The hypothetical virus-laden aerosols were modeled as gaseous and passive tracers, which have been shown to be able to model well the dispersion of droplet nuclei that were less than 5–10 microns in diameter. The commercial CFD software, Fluent, version 6.2, was used [15, 26]. The predicted concentration fields were compared with the locations of affected patients found in the outbreak.
Results
The ‘outbreak management team’ was being alerted of a possible outbreak in a general medical ward on 4 April 2008, when 7 inpatients were found to have developed fever and respiratory symptoms. The ward was immediately closed to new admissions, and transfer to other hospitals or institutes was suspended; sick patients were isolated. For all HCWs and the remaining patients, strict droplet precautions were implemented, and the individuals were required to wear surgical masks at all times. Hand hygiene was reinforced. Postexposure prophylaxis with oseltamivir was offered, and both groups were monitored for development of symptoms. Discharged patients were put under continuous home medical surveillance for 1 week. The ward returned to normal function on 12 April, when the outbreak was declared over.
An epidemiological investigation was started on 4 April. At the end of the outbreak, a total of 9 inpatients (patients A–I) were found to have symptoms that fulfilled the case definition of influenza-like illness (Figures 1 and 2). No visitor was known to be affected. The symptom onset dates of the first (index) case and the last case were 27 March and 10 April, respectively. All 9 inpatients tested positive for influenza A by immunofluorescence assay and culture of nasopharyngeal aspirate specimens. All isolates belonged to the H3N2 virus subtype (Influenza A/Brisbane/10/2007) and were 100% identical, as determined by nucleotide sequence analysis (Table 1). Nasopharyngeal aspiration was not performed for the 2 HCWs who had reported symptoms; however, serological test results did not suggest recent influenza infection (Table 1).
Figure 1.
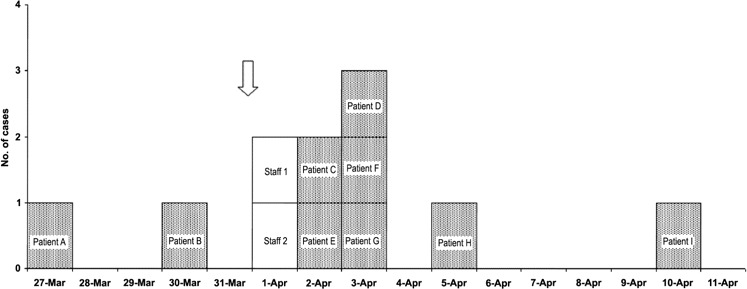
Epidemic curve of the influenza outbreak. Patients were shown according to their symptom onset date (fever or new respiratory symptoms); the order does not necessarily reflect the order in which they acquired infection. The arrow indicates the time when the index patient (patient A) commenced bi-level positive airway pressure (BiPAP) ventilation support. Prior to that, he was receiving supplemental oxygen therapy via nasal cannula. The BiPAP ventilation lasted for >16 h; he was subsequently transferred to the intensive care unit. Patient I started to receive oseltamivir prophylaxis on 4 April (the ward was closed and sick patients were isolated); however, he soon became unwell and developed fever on 10 April, despite receipt of prophylaxis. Staff 1 and 2 had symptoms; however, the results of serological tests for recent influenza infection were negative (Table 1).
Figure 2.
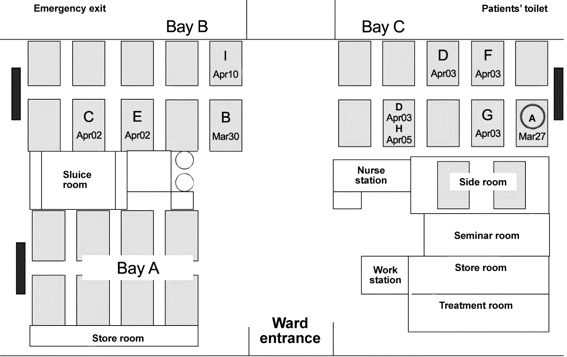
Figure showing layout of the outbreak ward and the locations of affected patients. Patient A (circled) was the index case. Dark-colored blocks represent high-efficiency particulate absorbing (HEPA) filters placed at the wall end of each ward bay. Dates of symptom onset were stated for all infected patients. Patient D had been staying at 2 bed locations (front row then back row).
Table 1.
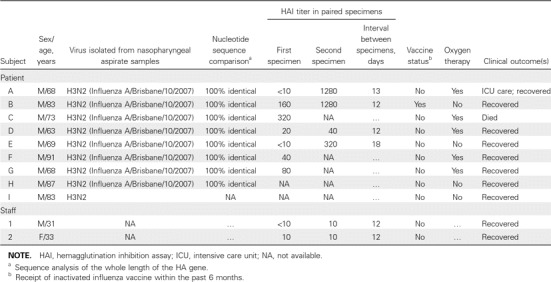
Clinical and Virological Data for Individuals Affected in the Outbreak
The index patient (patient A) (Figure 1) was a 68-year-old man who had underlying chronic obstructive pulmonary disease. He was admitted on 27 March with unresolved pulmonary shadows, despite having completed a course of antibiotic treatment, and increased dyspnea, which initially required supplemental oxygen at 1–2 L/min via nasal cannula. Although he was afebrile all along, his condition started to deteriorate, with increasing respiratory distress over the next few days. He became bed-bound. He developed type II respiratory failure on the evening of 31 March and underwent bi-level positive airway pressure (BiPAP) ventilation support (the inspiratory positive airway pressure [IPAP] level was set at 14 cm H2O; inspired oxygen concentration, 50% at a flow rate of 7.5 L/min). After 16 h of ventilation in the ward, the patient was transferred to the intensive care unit on 1 April for intubation and mechanical ventilation. Influenza H3N2 virus was subsequently isolated from a tracheal aspirate sample. He was given oseltamivir treatment and later recovered from his illness.
All secondary cases were detected to have fever and/or new onset of respiratory symptoms. Once influenza was confirmed, the patients started to receive oseltamivir treatment (75 mg twice per day for 5 days) within 24 h after symptom onset. The clinical courses of 7 patients remained uncomplicated, and the patients were subsequently discharged. The remaining patient (patient C) (Figure 1) was a 73-year-old man who had originally been admitted for Pseudomonas pneumonia that had complicated advanced bronchiectasis; although afebrile, he developed lower respiratory tract complication after influenza infection and died of progressive respiratory failure.
Spatiotemporal analysis. The outbreak ward was a male general medical ward consisting of 30 beds arranged in 3 major bays (2 adjacent rear bays B and C separated by a ∼2-m wide corridor and 1 front bay A) (Figure 2). The distance between adjacent beds was ∼1 m. The index patient's bed was located at the wall end of bay C, right next to a HEPA air purifier.
In total, 59 patients and 29 HCWs had stayed or worked on the ward during the period from 27 March to 1 April. The overall attack rate among patients was 13.6% (8 of 59 subjects). We found that patients who had stayed in the “adjacent” bay B (attack rate, 22.2%) were affected to a degree similar to those who stayed in the “same” bay C with the index patient (attack rate, 20.0%); however, no patients in the front, “distant” bay A or side room were affected (attack rate, 0%; P=.041, by the Fisher exact test). When analyzed according to date, presence in rear bays was associated with attack rates of 15.8%, 15.8%, 26.3%, and 26.3% on the dates 27–30 March, respectively. The risk of being infected was highest on 31 March (30.4%; P= .005) and 1 April (30.0%; P=.016), which coincided with the time of BiPAP ventilation use in the index patient. In a final multivariate logistic regression model, staying in the rear bays on 31 March was independently associated with a higher risk of acquiring influenza (OR, 14.9; 95% CI, 1.7–131.3; P=.015).
The attack rate among HCWs was not analyzed in detail, because only 2 were reported to have had symptoms, and neither had laboratory-confirmed infection. They reported close contact with patients staying at all 3 bays while performing their routine duties.
Airflow measurements and analysis. Air conditioning in the outbreak ward was provided by fan coil unit systems with a 4-way diffuser at the ceiling level in each of the 3 bays. The return air grills were located at the ceiling of the corridor. A HEPA air purifier was placed at the wall end of each bay (Figures 2 and 3); it functioned by drawing in air from a lower level, and after filtration, injecting the air back into the ward at an upper level. During the outbreak, the fan setting of the HEPA air purifiers was found to set to low in bays A and B and to medium in bay C inadvertently, with injection velocity measuring 1.47 m/s, 1.44 m/s, and 1.90 m/s respectively. Airflow measurements revealed that, under this situation, there was an imbalance in the air supply to and return from different bays, and the net flow toward the corridor was 70–100 L/s (Figure 3). The injecting air velocities from the HEPA air purifiers were substantially higher than that of the diffusers and thus dominated the overall airflow pattern. Because of the higher air injection velocity from the air purifier in bay C compared with that in the adjacent bay B, air from bay C was expected to be “pushed” into the corridor and toward bay B (Figure 4).
Figure 3.
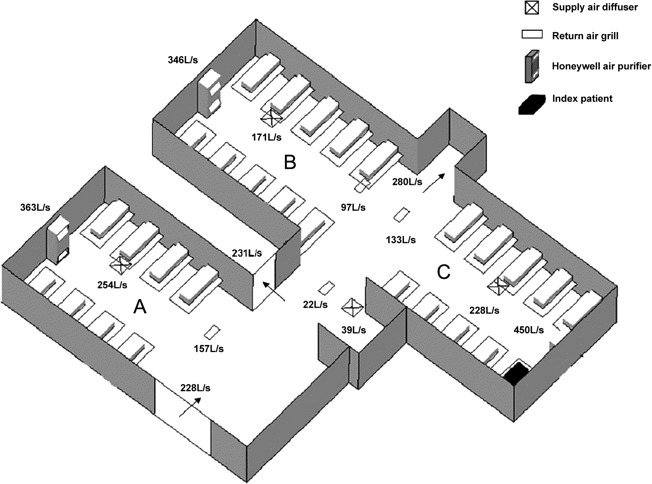
The measured airflow rates (in the unit of L/s) at different ward locations. High-efficiency particulate absorbing (HEPA) air purifiers were turned to the low setting in bay B and to the medium setting in bay C during time of measurement.
Figure 4.
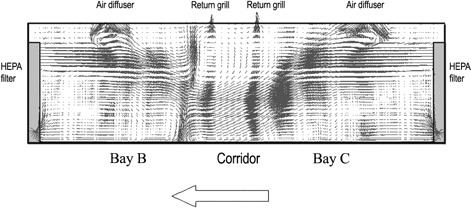
Airflow pattern at the mid-plane across the 2 high-efficiency particulate absorbing (HEPA) air purifiers in the rear bays B and C.
CFD simulations were performed, and the distributions of hypothetical virus-laden aerosols originated from the index patient are shown in Figure 5. The normalized concentration of the hypothetical virus-laden aerosols was found to be the highest in bay C (“same”), followed by bay B (“adjacent”), and the lowest in bay A (“distant”). The estimated spatial distribution was found to correspond to the locations of affected patients in the outbreak. Large droplets would not have accounted for such distribution because of their fast deposition onto surfaces [27, 28].
Figure 5.
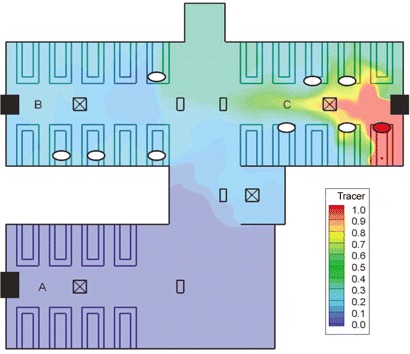
The spatial distribution of normalized concentration of hypothetical virus-laden aerosols (modeled as gaseous tracer) in the outbreak ward at a height of 1.1 m. The flow rates used in this model were those described in Figure 3. All high-efficiency particulate absorbing (HEPA) filters were assumed to function with 100% filtration of the modeled droplet nuclei. The 3 HEPA air purifiers are shown as black boxes, the 4 diffusers are shown by a square with an X, and the 4 returns are shown as a small rectangular filled box. Affected patients are represented by white ovals (the index patient is marked as a red oval).
Discussion
We report a nosocomial outbreak of seasonal influenza in an acute ward setting. It was temporally related to the use of an aerosol-generating device in the index patient. This had occurred together with an imbalanced indoor airflow; and the spatial distribution of cases was found to follow the directional airflow and coincided with a CFD-estimated aerosol dispersal pattern. Our findings suggest a possible role of aerosol transmission in this outbreak.
We have previously reported aerosol transmission of SARSassociated coronavirus in a similar ward setting [15]. In that study, CFD modeling demonstrated a close relationship between concentration of virus-laden aerosols and the risk of acquiring infection in various ward locations. We studied this outbreak using similar approach, and our findings provide further evidence to support the hypothesis that, under suitable clinical and environmental conditions, aerosol transmission of influenza virus can occur [2, 3]. In this outbreak, we postulate that infectious aerosols were generated continuously through the use of noninvasive ventilation for the index patient (which projected for at least 1 m sagittally to bed end) [29–31] for 16 h and these aerosols were blown toward the adjacent bay by an imbalanced indoor airflow created by an air purifier's outflow jet, which was located at patient's bed end. We believe that droplets and contact routes of transmission cannot entirely explain the outbreak because (1) the epidemic curve suggested a point-source mode of infection, instead of successive propagation (especially for the aggregation of symptom onset on 2 and 3 April); (2) the index patient and most other inpatients were immobile during their illnesses and, therefore, direct contacts should have been minimal; (3) patients >2 m away were affected; and (4) locations of affected patients coincided with dispersal pattern of aerosols and the directional airflow. In this outbreak, patients in the “adjacent” and “same” bay were similarly affected, but the “distant” bay was spared. A more random distribution of infected cases would be expected if HCWs had acted as vectors, because they were responsible for the care of patients in all bays (duties were assigned on the basis of function and not location) [15]. Similarly, close patient-patient contact, visits to a contaminated common area, and transmission through a wandering, sick visitor are unlikely explanations in our setting [15]. Finally, high air velocities (>10 m/s), which can carry even large droplets beyond 2 m, were not found in our ward setting, and they would have been deposited too fast onto surfaces to account for the observed dispersal pattern [27, 28].
We hold the view that the predominant modes of influenza transmission are via droplets and direct contact [2, 3, 8, 13]. However, accumulating evidences suggest that the aerosol route may have a contributing role [3, 13, 18, 19]. In animal models, influenza virus (eg, H3N2 or H1N1) has been shown to transmit efficiently through air, whereas fomite or contact spread is relatively inefficient [13, 18, 19, 32–34]. In clinical studies, virus- laden particles less than 5–6 µm (ie, within the respirable aerosol fraction) have been detected in exhaled breaths of patients with influenza and in the air sampled from an acute healthcare setting during seasonal peak [19, 35, 36]. In contrast to natural coughing or sneezing, artificially generated respiratory particles are often much smaller in size ( less than 5–6 mm), can penetrate more readily into the lower respiratory tract, and can cause infection with a smaller dose [3, 13, 18, 19]. It has been shown that certain clinical procedures (eg, endotracheal intubation, cardiopulmonary resuscitation, noninvasive ventilation, and receipt of high-flow oxygen) can generate a large amount of respiratory aerosols [8, 9, 13, 16, 18, 29–31], and transmission of respiratory infection related to some of these procedures—despite implementation of droplet precautions— has been reported [37–39].
Since the outbreak, we have used a hierarchy of control measures to prevent influenza transmission in our hospital, such as administrative and source controls, engineering controls, and use of personal protective equipment [8, 9]. The policy of isolating or cohorting patients with suspected or confirmed influenza is reinforced; application of an aerosol-generating procedure is allowed only in adequately ventilated single rooms before influenza can be excluded; all patients with respiratory infections are required to wear face masks, which are freely provided, until symptoms subside; air-conditioning units and their settings are regularly checked to avoid airflow imbalance; and HCWs are advised to use N-95 respirators, face shields, gloves, and gowns while performing aerosol-generating procedures and to receive annual influenza vaccines [2, 5, 8, 9].We have adopted these measures on all medical wards, because there might be similar unsuspected or “invisible” patients with influenza acting as infection sources. We did not encounter another influenza outbreak in open wards during the subsequent 24 month period, which included the first wave of the influenza H1N1 pandemic [7, 20]. Although each institute's infrastructure may be different, our findings suggest that the strategies of source and engineering controls might be important considerations to prevent nosocomial influenza transmission.
Our study is limited by its descriptive nature. We could not analyze the impact of influenza vaccination on the size of outbreak, because such information was unavailable from many patients; however, the general vaccine uptake rate in our community was reported to be very low [40, 41]. Also, we could not entirely eliminate the role of sick HCWs in transmitting infection, albeit no case of influenza was eventually confirmed among them. However, because all HCWs were required to wear surgical face mask during work and to report any influenza- like illnesses through a daily reporting system (the presence of which would immediately exempt them from duty), the chance of HCWs cross-infecting patients should be rather small [5]. Furthermore, the spatial distribution of cases could not be easily explained by HCW-to-patient or patient-to-patient transmission, as discussed above [15].
Our findings indicate the need to evaluate the infection risks of aerosol-generating procedures or devices, especially when applied to the disease state [8, 9, 29, 30, 37]; the effectiveness of various source and administrative control strategies [2, 3, 8, 9, 39]; the ventilation systems in different healthcare settings; and the impact of airflow and humidity on nosocomial influenza transmission with an architectural aerodynamics approach [13, 17, 42, 43].
In conclusion, our findings suggest a possible role of aerosol transmission of influenza in an acute ward setting. Source and engineering controls, such as avoiding aerosol generation and improving ward ventilation design, may warrant consideration to prevent nosocomial outbreaks.
Acknowledgments
We thank Mr. Li Liu and Ms Caroline Xiaolei Gao for their participation in the field measurement in the outbreak ward.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Voirin N, Barret B, Metzger MH, Vanhems P. Hospital-acquired influenza: a synthesis using the Outbreak Reports and Intervention Studies of Nosocomial Infection (ORION) statement. J Hosp Infect. 2009;71(1):1–14. doi: 10.1016/j.jhin.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Salgado CD, Farr BM, Hall KK, Hayden FG. Influenza in the acute hospital setting. Lancet Infect Dis. 2002;2:145–155. doi: 10.1016/s1473-3099(02)00221-9. [DOI] [PubMed] [Google Scholar]
- 3.Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza:implications for control in health care settings. Clin Infect Dis. 2003;37:1094–1101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- 4.Sartor C, Zandotti C, Romain F, et al. Disruption of services in an internal medicine unit due to a nosocomial influenza outbreak. Infect Control Hosp Epidemiol. 2002;23(10):615–619. doi: 10.1086/501981. [DOI] [PubMed] [Google Scholar]
- 5.Ng TC, Lee N, Hui SC, Lai R, Ip M. Preventing healthcare workers from acquiring influenza. Infect Control Hosp Epidemiol. 2009;30(3):292–295. doi: 10.1086/595690. [DOI] [PubMed] [Google Scholar]
- 6.Horcajada JP, Pumarola T, Martínez JA, et al. A nosocomial outbreak of influenza during a period without influenza epidemic activity. Eur Respir J. 2003;21(2):303–307. doi: 10.1183/09031936.03.00040503. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Pandemic (H1N1) 2009. http://www.who.int/csr/disease/swineflu/en/index.html. Accessed 1 April 2010.
- 8.World Health Organization Infection prevention and control in health care for confirmed or suspected cases of pandemic (H1N1) 2009 and influenza-like illnesses. http://www.who.int/csr/resources/publications/swineflu/swineinfinfcont/en/index.html. Accessed 1 April 2010.
- 9.Centers for Disease Control and Prevention Interim guidance on infection control measures for 2009 H1N1 influenza in healthcare settings including protection of healthcare personnel. http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm. Accessed 1 April 2010. [PubMed]
- 10.Jefferson T, Del Mar C, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses:systematic review. BMJ. 2009;339:3675. doi: 10.1136/bmj.b3675. doi:10.1136/bmj.b3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers:a randomized trial. JAMA. 2009;302(17):1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 12.Shine KI, Rogers B, Goldfrank LR. Novel H1N1 influenza and respiratory protection for health care workers. N Engl J Med. 2009;361(19):1823–1825. doi: 10.1056/NEJMp0908437. [DOI] [PubMed] [Google Scholar]
- 13.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 14.Yu ITS, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 15.Yu IT, Wong TW, Chiu YL, Lee N, Li Y. Temporal-spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clin Infect Dis. 2005;40(9):1237–1243. doi: 10.1086/428735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth TF, Kournikakis B, Bastien N, et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis. 2005;191(9):1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Leung GM, Tang JW, et al. Role of ventilation in airborne transmission of infectious agents in the built environment—a multidisciplinary systematic review. Indoor Air. 2007;17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 18.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tellier R. Aerosol transmission of influenza A virus:a review of new studies. J R Soc Interface. 2009;6(suppl 6):783–790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centre for Health Protection, Hong Kong SAR Influenza page. http://www.chp.gov.hk/sentinel. Accessed 1 April 2010.
- 21.Lee N, Choi KW, Chan PKS, et al. Outcomes of adults hospitalized with severe influenza. Thorax. 2010;65(6):510–515. doi: 10.1136/thx.2009.130799. [DOI] [PubMed] [Google Scholar]
- 22.Lee N, Chan PKS, Choi KW, et al. Factors associated with early hospital discharge of adult influenza patients. Antivir Ther. 2007;12:501–508. [PubMed] [Google Scholar]
- 23.Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 24.Tang JW, Ngai KL, Lam WY, Chan PK. Seasonality of influenza A(H3N2) virus:a Hong Kong perspective (1997–2006) PLoS ONE. 2009;3(7):2768. doi: 10.1371/journal.pone.0002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang JW, Ngai KL, Chan PK. Lack of cross-immune reactivity against influenza H5N1 from seasonal influenza vaccine in humans. J Med Virol. 2008;80(11):1992–1996. doi: 10.1002/jmv.21321. [DOI] [PubMed] [Google Scholar]
- 26.Fluent . Lebanon NH: Fluent Inc; 2005. Fluent 6.2 user's guide. [Google Scholar]
- 27.Qian H, Li Y. Removal of exhaled particles by ventilation and deposition in a multibed airborne infection isolation room. Indoor Air. 2010;20:284–297. doi: 10.1111/j.1600-0668.2010.00653.x. [DOI] [PubMed] [Google Scholar]
- 28.Xie X, Li Y, Chwang ATY, Ho PL, Seto WH. How far droplets can move in indoor environments—revisiting Wells evaporation-falling curve of droplets. Indoor Air. 2007;17(3):211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 29.Hui DS, Chow BK, Ng SS, et al. Exhaled air dispersion distances during noninvasive ventilation via different respironics face masks. Chest. 2009;136(4):998–1005. doi: 10.1378/chest.09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui DS, Hall SD, Chan MT, et al. Noninvasive positive-pressure ventilation:an experimental model to assess air and particle dispersion. Chest. 2006;130(3):730–740. doi: 10.1378/chest.130.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui DS, Hall SD, Chan MT, et al. Exhaled air dispersion during oxygen delivery via a simple oxygen mask. Chest. 2007;132(2):540–6. doi: 10.1378/chest.07-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowen AC, Mubareka S, Tumpey TM, Garcia-Sastre A, Palese P. The guinea pig as a transmission model for human influenza viruses. Proc Natl Acad Sci USA. 2006;103:9988–9992. doi: 10.1073/pnas.0604157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mubareka S, Lowen AC, Steel J, Coates AL, Garcia-Sastre A, Palese P. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J Infect Dis. 2009;199:858–65. doi: 10.1086/597073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maines TR, Jayaraman A, Belser JA, et al. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science. 2009;325(5939):484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blachere FM, Lindsley WG, Pearce TA, et al. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis. 2009;48(4):438–40. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 36.Fabian P, McDevitt JJ, DeHaan WH, et al. Influenza virus in human exhaled breath:an observational study. PLoSOne. 2008;3(7):2691. doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varia M, Wilson S, Sarwal S, et al. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ. 2003;169(4):285–292. [PMC free article] [PubMed] [Google Scholar]
- 38.Christian MD, Loutfy M, McDonald LC, et al. SARS Investigation Team Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg Infect Dis. 2004;10(2):287–293. doi: 10.3201/eid1002.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu IT, Xie ZH, Tsoi KK, et al. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44(8):1017–1025. doi: 10.1086/512819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lau JT, Kim JH, Yang X, Tsui HY. Cross-sectional and longitudinal factors predicting influenza vaccination in Hong Kong Chinese elderly aged 65 and above. J Infect. 2008;56(6):460–468. doi: 10.1016/j.jinf.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Chor JS, Ngai KL, Goggins WB, et al. Willingness of Hong Kong healthcare workers to accept pre-pandemic influenza vaccination at different WHO alert levels:two questionnaire surveys. BMJ. 2009;339:3391. doi: 10.1136/bmj.b3391. doi:10.1136/bmj.b3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3(10):1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


