Biochemistry of ACE2
The renin–angiotensin system (RAS) is a central regulator of cardiovascular and renal functions and plays a key role in the pathophysiology of various cardiovascular and renal diseases. The RAS consists of a series of enzymatic reactions culminating in the generation of angiotensin II (Ang II) in plasma as well as in various tissues including the heart and kidneys. The discovery of angiotensin-converting enzyme 2 (ACE2) had added a new dimension to the RAS [1–5]. Human and rodent ACE2 are similar proteins containing 805 amino acids that include an N-terminal signal sequence, a single active-site catalytic region and a C-terminal hydrophobic membrane-anchor region [2,3,5]. ACE2 functions predominantly as a carboxymonopeptidase with a substrate preference for hydrolysis between proline and a hydrophobic or basic C-terminal residue [6]. Both ACE and ACE2 are endothelium-bound carboxypeptidases with ACE2 protein being highly expressed in the heart and kidney, and in the kidney, ACE2 is expressed in renal tubular epithelium, vascular smooth muscle cells of the intrarenal arteries and in the glomeruli [1,2,5]. Both enzymes can be cleaved by distinct metalloproteases located on the cell surface and released as soluble forms [3]. Despite sharing many biochemical properties with ACE, ACE2 is insensitive to classical ACE inhibitors [2,5]. Intriguingly, the location of the ACE2 gene on the X-chromosome implies that gender differences in the RAS and cardiovascular physiology may be linked to the ACE2 gene. ACE2 can not only cleave Ang I to generate the inactive Ang 1-9 peptide ACE2 but also directly metabolize Ang II to generate Ang 1-7 [7,3,6]. Ang 1-7 functions essentially as a physiological antagonist of Ang II signalling in the cardiovascular system [8,3] and in the kidneys! [9,10]. ACE2 can potentially metabolize several other peptides including apelin and opioid peptides but their precise physiological role remains to be defined [3].
Role of ACE2 in lung diseases
During the 2002–2003 period, the SARS (Severe Acute Respiratory Syndrome) epidemic affected more than 8000 people with a mortality of ∼10% [11,12]. The identification of ACE2 as a functional receptor for the novel coronavirus (CoV) implicated as the causative agent of SARS [11]. The spike proteins of coronaviruses are one of the key elements of infectivity, mediating uptake by high-affinity binding to cellular receptors. ACE2 is a crucial SARS-CoV receptor in vivo and SARS-CoV infections and the spike protein of the SARS-CoV reduce ACE2 expression [13,12]. Notably, injection of SARS-CoV spike into mice worsens acute lung failure in vivo that can be attenuated by blocking the RAS. These results provide a molecular explanation why SARS-CoV infections cause severe and often lethal lung failure and suggest a rational therapy for SARS and possibly other respiratory disease viruses [13]. These findings establish a definitive role of the ACE2 in lung disease and confirm that ACE2 is a negative regulator of the RAS in the setting of acute lung injury and in response to pulmonary infection with the SARS-CoV.
Based on inhibitor experiments in rodents and ACE allelic correlation studies in humans, it has been suggested that the RAS could have a role in acute lung failure and pulmonary fibrosis [14,15]. Experimentally, a possible role of ANG II in lung disease has been implied from animal models of pulmonary fibrosis elicited by bleomycin or irradiation-mediated lung injury. In bleomycin-induced pulmonary fibrosis in rats or mice, ACE inhibitors or AT1 receptor blockers can attenuate epithelial apoptosis, interstitial fibrosis and collagen deposition [14]. In three different ARDS models, acid-aspiration-induced ARDS, endotoxin-induced ARDS, and peritoneal sepsis-induced ARDS, ACE2 knockout mice show very severe and exacerbated disease compared with wild-type mice [16]. Loss of ACE2 expression in mutant mice resulted in enhanced vascular permeability, increased lung oedema, neutrophil accumulation and worsened lung function. Importantly, treatment with catalytically active recombinant ACE2 protein improved the symptoms of acute lung injury in wild-type mice as well as in ACE2 knockout mice [16]. Thus, ACE2 plays a protective role in acute lung injury. Mechanistically, the negative regulation of ANG II levels by ACE2 accounts, in part, for the protective function of ACE2 in ARDS. Indeed, AT1 inhibitor treatment or additional ACE deficiency on an ACE2 knockout background rescues the severe phenotype of ACE2 single-mutant mice in acute lung injury [16]. Therefore, in acute lung injury, ACE, ANG II and AT1 receptor function as lung-injury-promoting factors, while ACE2 protects from lung injury consistent with its role as a negative regulator of the RAS [16]. Similarly, ACE2 also plays a key role in the pathophysiology of heart disease whereby loss of ACE2 can result in an age-dependent cardiomyopathy [1,4] and enhances the susceptibility to biomechanical stress [17].
Role of ACE2 in renal diseases
The ACE2 expression and activity are significantly increased in the spontaneously hypertensive rat (SHR) kidney at birth. With the onset of hypertension, the tubular expression of ACE2 falls in SHR compared to WKY and remains reduced in the adult SHR kidney [1,18]. The developmental pattern of ACE2 expression in the SHR kidney is altered before the onset of hypertension, consistent with the key role of the RAS in the pathogenesis of adult-onset hypertension. The colocalization of ACE2 and Ang 1-7 in renal tubules suggests functional interactions and the ability of Ang 1-7 to counteract the pressor, hypertrophic and antinatriuretic actions of Ang II coupled with low renal expression of Ang 1-7 in untreated essential hypertensive subjects collectively suggests that this vasodilator peptide may be a critical link in mediating the negative regulatory feedback between ACE and ACE2 [9,19]. However, loss of ACE2 in a mouse model leads to only mild and variable effect on blood pressure [1,20] suggesting that decreased renal expression of ACE2 may play a more important role in exacerbating hypertensive kidney injury rather than contributing directly to systemic hypertension. Increased intensity of Ang 1-7 immunocytochemical expression in association with increased ACE2 intensity of staining occurs in kidneys of pregnant animals [21]. The findings suggest that ACE2 may contribute to the local production and overexpression of Ang 1-7 in the kidney during pregnancy and in mediating the renal adaptations to the physiological changes of pregnancy.
Loss of ACE2 leads to an age- and gender-dependent glomerulosclerosis associated with expansion and increased deposition of glomerular mesangial matrix with an activation of ERK1/2 signalling [22]. These changes were completely prevented by long-term treatment with AT1 receptor blocker [22]. The absence of glomerular lesions in aged female ACE2 knockout mice is consistent with numerous studies that documented the protective effect of oestrogen in renal damage [23,24]. Activation of the RAS and Ang II plays an important role in the development of experimental and clinical diabetic nephropathy, and blockade of the RAS in both experimental and clinical diabetes mellitus attenuates the development of diabetic kidney injury [25,26,27]. Diabetic nephropathy in experimental models has been shown to alter both glomerular and tubular expressions of ACE2 (see Table 1). In a long-standing diabetic rat model, renal ACE2 expression is reduced [32], while there is an early increase in ACE2 expression and activity in the kidneys of the diabetic db/db [34,33] and Akita [27] mice (Table 1). The highest expression of the ACE2 mRNA found in renal proximal tubules was significantly reduced in the tubules from diabetic rats [32]. Deletion of the ACE2 gene and pharmacological inhibition of ACE2 is associated with accelerated glomerular injury in Akita diabetic mice [27] and in streptozocin-induced diabetes [36,35] providing definitive evidence that ACE2 is renoprotective and that reduced ACE2 expression might contribute to the progression of kidney disease [31,28,32]. Consistent with the increased activity of the RAS, treatment with an AT1 receptor blocker prevented the exacerbation of diabetic nephropathy in the absence of ACE2 [27]. The exacerbation of diabetic nephropathy in ACE2 knockout mice may also relate to the loss of the renoprotective action of cortical Ang 1-7 resulting from the absence of ACE2 [35].
Table 1.
ACE2 expression in glomeruli and renal tubules in diabetic nephropathy
| Glomeruli | Proximal tubules | ||||
|---|---|---|---|---|---|
| ACE2 mRNA | ACE2 protein | ACE2 mRNA | ACE2 protein | ACE2 activity | |
| Human | |||||
| Reich et al. [28] | ↓ | BD | ↓ | ↓ | NA |
| Lely et al. [29] | NA | ↑ | NA | ↔ | NA |
| Konoshita et al. [30] | NA | NA | ↔ | NA | NA |
| Mizuiri et al. [31] | ↓ | ↓ | ↓ | ↓ | NA |
| Animal models | |||||
| Tikellis et al. [32] Streptozocin rats (Type 1 DM) | ↑ | ↑ | ↓ | ↓ | NA |
| Ye et al. [33]; Wyscocki et al. [34] db/db mice (Type 2 DM) | NA | ↓ | ↔ | ↑ | ↑ |
| Wysocki et al. [34] Streptozocin mice (Type 1 DM) | NA | NA | ↔ | ↑ | ↑ |
| Tikelis et al. [35] Streptozocin mice (Type 1 DM) | NA | NA | ↓ | ↓ | ↑ |
| Wong et al. [27] Akita mice (Type 1 DM) | NA | NA | ↑ | ↑ | NA |
↓ = decreased; ↑ = increased; ↔ = no significant difference; NA = not analysed; BD = below detection.
Importantly, two recent studies have provided consistent data showing that in patients with type 2 diabetes associated with proteinuria and reduced GFR, renal, glomerular and tubular ACE2 mRNA and protein expression is reduced by at least 50% [31,28] with one study showing no overall change in renal cortical ACE2 expression (Table 1) [30]. Due to the concomitant increases in ACE expression, the ratio of ACE/ACE2 expression is dramatically increased in both the glomerular and tubulointerstitial compartments in patients with diabetic nephropathy [31,28]. The ACE/ACE2 mRNA and protein ratios correlate positively to the degree of proteinuria when analysed in diabetics in combination with healthy controls [31]. These results are consistent with findings from experimental models (see above) and indicate that the relative loss of ACE2 protective action can exacerbate glomerular pathology. However, when the analysis is confined to patients with diabetic nephropathy, this relationship is lost which may be related to technical issues such as limited sample sizes and the low level of ACE2 protein in the glomeruli [31,28]. The absence of changes in ACE2 mRNA and immunohistochemistry protein expression in other types of kidney diseases such as focal glomerulosclerosis and chronic allograft nephropathy suggests that ACE2 may have a limited role in these types of kidney diseases [28] while in transplanted kidneys and in a wide range of primary and secondary renal diseases, neo-expression of ACE2 occurs in glomerular and peritubular capillary endothelium [29]. Further studies will be needed to provide a more definitive role of ACE2 in other types of renal diseases.
In normal rats, ACE inhibition and angiotensin receptor blockade had no effect on ACE2 mRNA levels in the kidneys of normal rats [37]. In rodent models with type 1 diabetes, ACE2 mRNA levels were unaffected by treatment with the ACE inhibitor [35,32], in accordance with our observations in the kidneys of subjects with type 2 diabetes [28]. However, renal cortical ACE2 activity is augmented in normal rats treated with an ACE inhibitor or angiotensin receptor blocker [37], and ACE2 protein levels are markedly increased in response to ACE inhibitor treatment (in contrast to the effect on mRNA levels) in diabetic rats [32]. This uncoupling between ACE2 mRNA and protein/activity is supportive of a complex mechanism of ACE2 regulation in the kidney possibly via post-transcriptional mechanisms. Interestingly, blockade of the RAS (with ACE inhibitor/angiotensin receptor blocker) in subjects with either focal glomerulosclerosis or chronic allograft nephropathy is associated with an increase in the tubular expression of ACE2 [28], and it is tempting to speculate that ACE inhibitor/angiotensin receptor blocker use may increase ACE2 levels in subjects without diabetes.
Collectively, these data suggest that ACE2 plays a key role in pulmonary, cardiovascular and hypertensive and diabetic kidney diseases. ACE2 plays a pivotal role in maintaining a balanced status of the RAS synergistically with ACE by exerting counter-regulatory effects (Figure 1). ACE inhibitors and angiotensin receptor blockers provide only partial long-term benefits in patients with cardiovascular diseases [38], type 1 [39] and type 2 [40,25,26] diabetes, suggests that enhancing ACE2 action may serve to provide additional therapeutic benefits patients with cardiovascular and diabetic kidney disease. Increased ACE2 activity by the use of human recombinant ACE2 (unpublished data) and/or a small molecule activator (xanthenone) of ACE2 [41] may represent potential new therapies for lung, cardiovascular and kidney diseases by providing dual beneficial effects by antagonizing Ang II action while generating Ang 1-7.
Fig. 1.
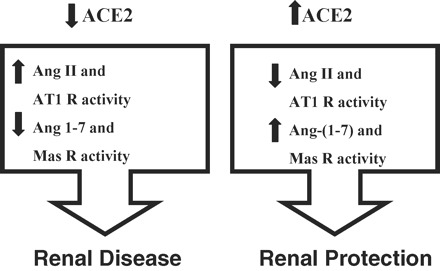
Angiotensin-converting enzyme 2 (ACE2) as a negative regulator of the renin–angiotensin system in the kidneys illustrating the opposing roles of Ang II/AT1 receptor and Ang 1-7/Mas receptor systems. R = receptor.
Acknowledgments
We acknowledge the financial support from the Canadian Institute for Health Research (G.Y.O., grant 86602; Z.K., grant 84279), Canadian Diabetes Association (J.W.S, G.Y.O and A.M.H., grant OG-3-08-2559-JS) and the EuGeneHeart (EU's 6th Framework Program), and a GEN-AU grant and IMBA (J.M.P.). G.Y.O. is a Clinician-Investigator Scholar of the Alberta Heritage Foundation for Medical Research.
Conflict of interest statement. None declared.
References
- 1.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 2.Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 3.Oudit GY, Crackower MA, Backx PH, et al. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 4.Oudit GY, Kassiri Z, Patel MP, et al. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75:29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 6.Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 7.Garabelli PJ, Modrall JG, Penninger JM, et al. Distinct roles for angiotensin-converting enzyme 2 and carboxypeptidase A in the processing of angiotensins within the murine heart. Exp Physiol. 2008;93:613–621. doi: 10.1113/expphysiol.2007.040246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dias-Peixoto MF, Santos RA, Gomes ER, et al. Molecular mechanisms involved in the angiotensin-(1-7)/Mas signaling pathway in cardiomyocytes. Hypertension. 2008;52:542–548. doi: 10.1161/HYPERTENSIONAHA.108.114280. [DOI] [PubMed] [Google Scholar]
- 9.Chappel MC, Ferrario CM. ACE and ACE2: their role to balance the expression of angiotensin II and angiotensin-(1-7) Kidney Int. 2006;70:8–10. doi: 10.1038/sj.ki.5000321. [DOI] [PubMed] [Google Scholar]
- 10.Su Z, Zimpelmann J, Burns KD. Angiotensin-(1-7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 2006;69:2212–2218. doi: 10.1038/sj.ki.5001509. [DOI] [PubMed] [Google Scholar]
- 11.Nicholls J, Peiris M. Good ACE, bad ACE do battle in lung injury, SARS. Nat Med. 2005;11:821–822. doi: 10.1038/nm0805-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peiris JS, Yuen KY, Osterhaus AD, et al. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 13.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuba K, Imai Y, Rao S, et al. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med. 2006;84:814–820. doi: 10.1007/s00109-006-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall RP, Webb S, Bellingan GJ, et al. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- 16.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto K, Ohishi M, Katsuya T, et al. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 18.Tikellis C, Cooper ME, Bialkowski K, et al. Developmental expression of ACE2 in the SHR kidney: a role in hypertension? Kidney Int. 2006;70:34–41. doi: 10.1038/sj.ki.5000428. [DOI] [PubMed] [Google Scholar]
- 19.Joyner J, Neves LA, Granger JP, et al. Temporal-spatial expression of ANG-(1-7) and angiotensin-converting enzyme 2 in the kidney of normal and hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R169–R177. doi: 10.1152/ajpregu.00387.2006. [DOI] [PubMed] [Google Scholar]
- 20.Gurley SB, Allred A, Le TH, et al. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest. 2006;116:2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brosnihan KB, Neves LA, Joyner J, et al. Enhanced renal immunocytochemical expression of ANG-(1-7) and ACE2 during pregnancy. Hypertension. 2003;42:749–753. doi: 10.1161/01.HYP.0000085220.53285.11. [DOI] [PubMed] [Google Scholar]
- 22.Oudit GY, Herzenberg AM, Kassiri Z, et al. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol. 2006;168:1808–1820. doi: 10.2353/ajpath.2006.051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 24.Reyes D, Lew SQ, Kimmel PL. Gender differences in hypertension and kidney disease. Med Clin North Am. 2005;89:613–630. doi: 10.1016/j.mcna.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 26.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 27.Wong DW, Oudit GY, Reich H, et al. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171:438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reich HN, Oudit GY, Penninger JM, et al. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- 29.Lely AT, Hamming I, van Goor H, et al. Renal ACE2 expression in human kidney disease. J Pathol. 2004;204:587–593. doi: 10.1002/path.1670. [DOI] [PubMed] [Google Scholar]
- 30.Konoshita T, Wakahara S, Mizuno S, et al. Tissue gene expression of renin-angiotensin system in human type 2 diabetic nephropathy. Diabetes Care. 2006;29:848–852. doi: 10.2337/diacare.29.04.06.dc05-1873. [DOI] [PubMed] [Google Scholar]
- 31.Mizuiri S, Hemmi H, Arita M, et al. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51:613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Tikellis C, Johnston CI, Forbes JM, et al. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41:392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- 33.Ye M, Wysocki J, Naaz P, et al. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension. 2004;43:1120–1125. doi: 10.1161/01.HYP.0000126192.27644.76. [DOI] [PubMed] [Google Scholar]
- 34.Wysocki J, Ye M, Soler MJ, et al. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 35.Tikellis C, Bialkowski K, Pete J, et al. ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes. Diabetes. 2008;57:1018–1025. doi: 10.2337/db07-1212. [DOI] [PubMed] [Google Scholar]
- 36.Soler MJ, Wysocki J, Ye M, et al. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72:614–623. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- 37.Ferrario CM, Jessup J, Gallagher PE, et al. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int. 2005;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 38.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 39.Suissa S, Hutchinson T, Brophy JM, et al. ACE-inhibitor use and the long-term risk of renal failure in diabetes. Kidney Int. 2006;69:913–919. doi: 10.1038/sj.ki.5000159. [DOI] [PubMed] [Google Scholar]
- 40.Yusuf S, Sleight P, Pogue J, et al. (the Heart Outcomes Prevention Evaluation Study Investigators). Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez Prada JA, Ferreira AJ, Katovich MJ, et al. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51:1312–1317. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]


