Abstract
Background. Human bocavirus is a newly discovered parvovirus. It has been detected primarily in children with acute lower respiratory tract infection, but its occurrence, clinical profile, and role as a causative agent of respiratory tract disease are not clear.
Methods. We investigated the presence of human bocavirus by quantitative polymerase chain reaction of nasopharyngeal aspirate specimens and selected serum samples obtained from 259 children (median age, 1.6 years) who had been hospitalized for acute expiratory wheezing. The samples were analyzed for 16 respiratory viruses by polymerase chain reaction, virus culture, antigen detection, and serological assays.
Results. At least 1 potential etiologic agent was detected in 95% of children, and >1 agent was detected in 34% of children. Human bocavirus was detected in 49 children (19%). A large proportion of the cases were mixed infections with other viruses, but human bocavirus was the only virus detected in 12 children (5%). High viral loads of human bocavirus were noted mainly in the absence of other viral agents, suggesting a causative role for acute wheezing. In addition, infections that had uncertain clinical relevance and low viral loads were prevalent. Human bocavirus DNA was frequently detected in serum specimens obtained from patients with acute wheezing, suggesting systemic infection.
Conclusions. Human bocavirus is prevalent among children with acute wheezing and can cause systemic infection. Results suggest a model for bocavirus infection in which high viral loads are potentially associated with respiratory symptoms and low viral loads indicate asymptomatic shedding. Therefore, quantitative polymerase chain reaction analysis may be important for additional studies of human bocavirus.
Acute viral respiratory tract infection is the leading cause of hospitalization for infants and young children in developed countries and is a major cause of death in developing countries [1, 2]. Human bocavirus (HBoV) is a newly discovered parvovirus that was first identified in Sweden from pooled nasopharyngeal aspirate specimens by large-scale molecular virus screening [3]. In the original study, 540 nasopharyngeal aspirate specimens obtained from hospitalized patients were analyzed for HBoV, and 3.1% yielded positive results. All HBoV-positive samples had been obtained from young children with respiratory distress (mainly acute expiratory wheezing). Many of the children had pneumonia, with interstitial infiltrates noted by chest radiography [3]. Recent studies have detected HBoV in 1.5%–11.3% of investigated respiratory tract samples in North America, Europe, Asia, and Australia, suggesting that the virus has a global distribution [4–10]. Respiratory distress and abnormal chest radiography findings have been frequent findings associated with HBoV in these studies. However, the causative role of HBoV remains unclear. In fact, HBoV was detected concurrently with other potential pathogens in 33%–56% of cases in which it was studied [5, 7, 9–11]. These findings have raised the question of whether HBoV is at all a causative agent of respiratory tract disease.
We investigated the prevalence of HBoV and the genome HBoV load in the respiratory tract and blood specimens obtained from children who had been hospitalized for acute expiratory wheezing—the most common manifestation of lower respiratory tract infection in children—to investigate the association between HBoV infection and acute respiratory tract illness. Importantly, the samples had earlier been investigated for evidence of different respiratory virus infections, and a potential causative agent had been detected in 88% of cases [12]. In the present, expanded study, we searched for a total of 16 respiratory viruses.
Materials and Methods
Study subjects and sample collection. As part of a randomized, placebo-controlled clinical trial evaluating the efficacy of systemic corticosteroids for the treatment of acute expiratory wheezing in children, we performed extensive diagnostic evaluations for respiratory virus infection. The study included 293 children who presented to the Department of Pediatrics, Turku University Hospital (Turku, Finland), during the period from September 2000 through May 2002 [12, 13]. Acute expiratory wheezing was considered to be bronchiolitis when it occurred for the first time in children aged <3 years. Asthma was diagnosed on the basis of the National Asthma Education and Prevention Program guidelines [14]. All other episodes of acute expiratory wheezing were considered to be recurrent wheezing.
All patients were examined by 1 of the 2 study physicians (T.J. and P.L.) twice daily during hospitalization. Signs and symptoms were recorded on a standardized form. Of the 293 children who were randomized, 259 children (median age, 1.6 years; range, 3 months to 15 years) who had sufficient sample material available for complete virus diagnostic evaluation (nasopharyngeal aspirate specimens were used for PCR [for 16 viruses], virus culture [for 9 viruses], and antigen detection [for 7 viruses]; acute- and convalescent-phase serum samples were used for serologic testing [for 7 viruses]) were included in the present study. Sixty-four asymptomatic children (median age, 4.1 years; range, 5 months to 14 years) who had been admitted to the Division of Pediatric Surgery, Turku University Hospital, during the period from September 2000 through October 2002 and who did not have respiratory symptoms during the preceding 4 weeks were also investigated for the presence of HBoV [15]. The study protocol was approved by the Ethics Committee of the Turku University Hospital.
At hospital admission, a nasopharyngeal aspirate sample was obtained using a standardized procedure, as described elsewhere [12]. Antigen detection and virus culture were performed immediately, and the samples obtained for PCR assays were stored in tubes at -70°C before processing. Blood samples were collected at the time of the patient's admission and 2–3 weeks after discharge from the hospital and were stored at -20°C. Nasal swab specimens were obtained from the asymptomatic children. Nasopharyngeal aspirate specimens could not be obtained from healthy children, because they did not have mucus in the nasopharynx.
Real-time PCR for HBoV. Nucleic acids were extracted from 400 µL of the nasopharyngeal aspirate samples using the MagAttract Virus Mini M48 kit and the Biorobot M48 instrument (Qiagen), and samples were eluted in 100 µL of RNase-free water. For serum samples, 100 µL of serum was processed using the QIAamp blood mini kit (Qiagen), and DNA was eluted in 50 µL. The PCR assay targeted the NP-1 gene of HBoV. The 20-µL amplification reaction contained 5 µL of sample DNA, 10 µL of TaqMan universal PCR master mix (PE Applied Biosystems), 0.1 µL of bovine serum albumin (20 mg/mL), 300 nmol/L each primer (Boca-forward, GGA AGA GAC ACT GGC AGA CAA; and Boca-reverse, GGG TGT TCC TGA TGA TAT GAG C), and 150 nmol/L Boca probe (FAM-CTG CGG CTC CTG CTC CTG TGA T-TAMRA). Amplification was performed using a LightCycler 1.2 instrument (Roche) with the following instrument settings: 95°C for 10 min, 50 cycles of 95°C for 0 s, and 60°C for 1 min. For standardizing the quantification, a plasmid (NPSC3.1) containing the HBoV NP-1 gene was used in serial dilutions covering a range of 6 logs. The criteria for a positive reaction were a cycle threshold <40 cycles and fluorescence count >0.5. The minimum genome viral load that would allow reproducible quantification was 10 copies per reaction, corresponding to 500 copies/mL for nasopharyngeal aspirate specimens or 1000 copies/mL for serum specimens. The analyses were performed at a diagnostic laboratory, with rigorous measures to prevent contamination. The real-time PCR assay was compared with the previously published single end-point PCR assay targeting the NP-1 gene [3] by parallel analysis of 74 samples. Fifteen samples yielded positive results with both assays, 5 yielded positive results with real-time PCR only, and the remaining 54 yielded negative results with both assays.
Means of diagnosis for other viruses. Virus culture was performed for adenovirus, influenza A and B viruses, parainfluenza virus types 1–3 (PIV 1–3), respiratory syncytial virus (RSV), enteroviruses, and rhinoviruses, in accordance with routine diagnostic protocols, in A549, HeLa, and LLC-MK2 and human foreskin fibroblast cells, as well as for human metapneumovirus in tertiary monkey kidney cells [16]. Viral antigens were detected for adenovirus, influenza A and B viruses, PIV 1–3, and RSV by time-resolved fluoroimmunoassay [17]. Levels of IgG antibodies specific for the same viruses (except for PIV 2 and human metapneumovirus) were analyzed in paired serum samples, in addition to IgM antibodies for enteroviruses. RT-PCR was used for the detection of enteroviruses and rhinoviruses, RSV, coronaviruses 229E and OC43, and human metapneumovirus. These results were reported elsewhere [12]. In addition to HBoV diagnostic evaluations, the present study included extended diagnostic evaluations by real-time PCR for influenza A and B viruses, adenovirus, PIV 1–4, and coronavirus NL63 (in Rotterdam) and coronavirus HKU-1 (in Stockholm). Total nucleic acids for these analyses were isolated using a MagnaPure LC isolation station (Roche). Amplification was performed with an ABI7700 instrument, using commercially available master mixes (Applied Biosystems) and standard protocols. The primer sequences for PIV 1–4 and coronavirus HKU-1 are available from the authors. Otherwise, the primer and probe sequences, PCR protocols, and other viral detection methods were described elsewhere [12, 16, 18–21]. The sequencing of the 12 previously reported, nontypable rhinoviruses and enteroviruses [12] identified them as rhinoviruses. A patient was considered to be positive for the virus if results of at least 1 of the tests used were positive.
Results
Viral findings in children with acute wheezing. A potential viral pathogen was identified in 245 children (95%) (table 1). PCR results were positive for ⩾1 virus in 95% of the children, virus culture results were positive in 40%, antigen detection results were positive in 28%, and serologic test results were positive in 38%. In 3% of the cases, diagnosis of viral infection was based only on serological findings. Notably, 34% of the patients had test results positive for >1 virus. Forty-nine patients (19%) tested positive for HBoV in the nasopharynx. HBoV was the only virus detected in 12 patients (4.6% of all patients and 24% of the HBoV-positive patients), whereas ⩾1 additional viral agent was detected in the remaining 37 HBoV-positive patients. The viruses most frequently found concordantly with HBoV were respiratory picornaviruses (in 21 children), of which 14 were classified as rhinovirus and 7 were classified as enterovirus, followed by adenovirus (in 8 children) and RSV (in 7 children).
Table 1.
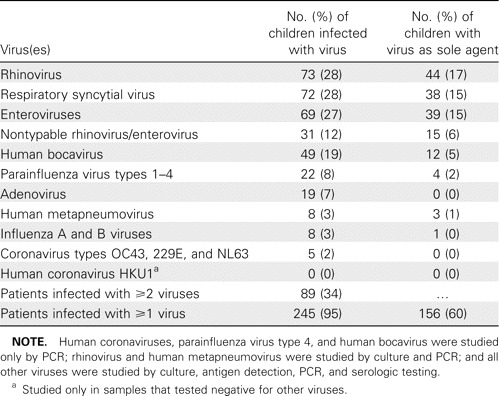
Viral etiology of respiratory infection in 259 children with acute wheezing during a 20-month study period.
Quantitative analysis of HBoV DNA in the nasopharynx. Genome viral loads in the nasopharyngeal aspirates ranged from <500 to 1.0 × 108 copies per mL of sample material. The genome counts appeared to define 2 nonoverlapping populations: one group of 28 samples with a high viral load (>104 copies/mL) and another group of 21 samples with a low viral load (<104 copies/mL) (figure 1). A high HBoV load was detected in 11% of wheezing patients. The nasal swab samples obtained from the 64 asymptomatic children were HBoV negative.
Figure 1.
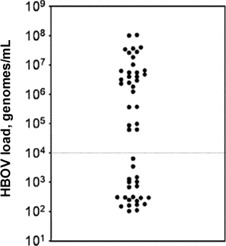
Distribution of human bocavirus (HBoV) genome loads among all 49 nasopharyngeal aspirate samples that tested positive for HBoV. Each sample is represented by a single dot. The dotted line indicates the cutoff between the high and low HBoV load groups discussed in the text.
Association of HBoV in the nasopharynx and acute wheezing. HBoV was found significantly more frequently among children with acute wheezing than among asymptomatic children (19% vs. 0%; P < .001, by Fisher's exact test). However, because age distributions and sampling techniques differed between the 2 groups, this comparison should be interpreted with caution. We searched for additional evidence of causality by studying whether detection of HBoV was unrelated or inversely correlated to the occurrence of other viruses in children with acute wheezing [3]. HBoV was indeed more prevalent among children with symptoms of an otherwise unexplained etiology than among those in which other viruses were detected (46% vs. 16%; P < .001) (table 2). Stratification of the patients on the basis of a nasopharynx HBoV load of <104 copies/mL versus >104 copies/mL revealed that only the patients with a high HBoV load had this association (table 2). This suggests that the presence of HBoV at high—but not at low—viral loads is associated with previously unexplained acute wheezing.
Table 2.
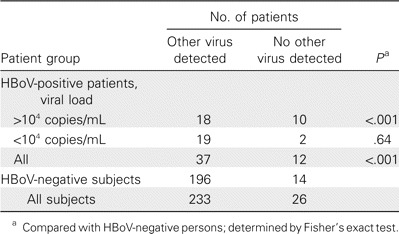
Distribution of patients by the presence of human bocavirus (HBoV), the HBoV load in the nasopharynx, and the presence or absence of other viruses.
Detection of HBoV DNA in serum specimens. Acute- and convalescent-phase serum samples were available in sufficient quantity from 43 of the 49 patients who tested positive for HBoV in the nasopharynx. HBoV DNA was detected in 23 (53%) of the 43 acute-phase serum samples and in 8 (19%) of the 43 convalescent-phase serum specimens (P < .001, by Fisher's exact test). Detection of HBoV in serum was mainly, although not exclusively, associated with a high viral load in the nasopharynx (table 3). Viral loads in serum samples ranged from <1.0 × 103 to 5.9 × 105 copies /mL and did not correlate with genome viral loads in the corresponding nasopharyngeal aspirate specimens (r = -0.24; data not shown). We also analyzed 45 randomly selected serum samples obtained from the 196 wheezing patients who tested negative for HBoV in the nasopharynx but who had demonstrated infection with some other virus. Three tested positive for HBoV DNA (2 had low viral loads, and 1 had a viral load of 5.9 × 105 copies/mL).
Table 3.
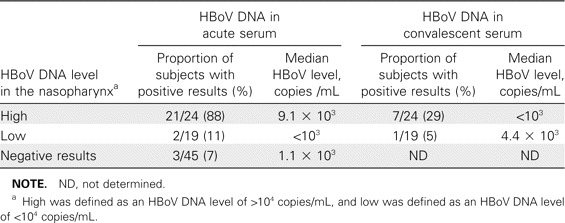
Prevalence of patients with human bocavirus (HBoV) in the serum and median serum HBoV DNA level, in relation to the presence of HBoV in the nasopharynx, among patients with acute wheezing.
Clinical characteristics of patients positive only for HBoV. HBoV was the only virus detected in 12 children (table 4). The median age of these children was 1.3 years. Eight children received a diagnosis of bronchiolitis, 3 had recurrent wheezing, and 1 had acute exacerbation of asthma. Acute otitis media was diagnosed in 5 (42%) of the children, and chest radiographs taken from 9 patients all showed interstitial infiltrates. One child had leukocytosis (16.3 × 109 cells/L), and 2 children had increased levels of serum C-reactive protein (73 and 78 mg/L). In all other cases, total WBC counts and serum C-reactive protein levels were within normal limits. The reported duration of symptoms varied from 2 days to >20 days. When characteristics of the children positive only for HBoV were compared with those of children with other demonstrated virus infections, no clinically significant differences were recorded (table 4).
Table 4.
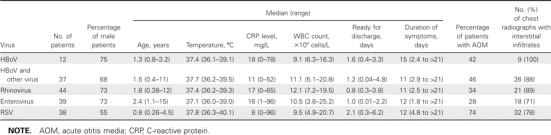
Characteristics of children with acute wheezing caused solely by human bocavirus (HBoV), enterovirus, rhinovirus, or respiratory syncytial virus (RSV) or by bocavirus associated with other viruses.
Discussion
We found HBoV in the nasopharynx of 19% of children with acute wheezing illness, and it was the fourth most frequent virus detected after rhinoviruses, enteroviruses, and RSV. These findings show that HBoV is a common virus in the community. The prevalence of HBoV was higher in our study than in previous reports [3–11]; this may have been the result of technical factors and study design rather than true differences in virus prevalence. A large number of the samples had low HBoV loads, making evaluation of prevalence highly dependent on assay sensitivity. Differences in PCR assay sensitivity may, of course, similarly have affected the relative prevalence estimates for all 16 of the viruses studied. It should also be recognized that nasopharyngeal sampling is not a standardized procedure. Finally, the present study included only hospitalized patients with acute wheezing illness, which may be the major manifestation of HBoV infection, whereas previous studies have included a broader selection of samples.
Despite the large number of mixed HBoV infections, our findings are still consistent with a potential etiologic role for HBoV in respiratory tract disease in young children. In the absence of in vitro virus propagation systems and animal models, final proof of causality will remain difficult to obtain. Fredricks and Relman [22] proposed molecular diagnostic criteria for causality for use in this situation, and the present study provides data relevant for many of these criteria:
1. HBoV was present at the site of the symptoms (i.e., in the respiratory tract). Genome viral loads were generally higher in the respiratory tract specimens than in the serum specimens.
2. HBoV was more prevalent in wheezing patients than in asymptomatic children. The significance of this observation alone should not be exaggerated. First, the age distribution differed between symptomatic and asymptomatic children. Second, sampling techniques were not matched. However, these results are consistent with the recent findings of Kesebir et al. [23], who reported that nasal wash samples obtained from 96 asymptomatic children were negative for HBoV. Nevertheless, the comparison of symptomatic and asymptomatic individuals remains problematic. The large number of mixed infections observed with HBoV may indicate that HBoV is reactivated—or its detection enhanced—by other infections. This would result in a higher prevalence among symptomatic patients than among control subjects, even in the absence of an etiologic role. Respiratory secretions recovered during acute infection are also inherently very different from those recovered from asymptomatic subjects (e.g., with regard to cell counts). Therefore, evidence of a virus-disease association must rely also on criteria other than differences in the prevalence between case patients and control subjects.
3. HBoV was more prevalent among patients with previously unexplained wheezing than among patients who tested positive for other viruses.
4. This association was seen only for patients with a high viral load (i.e., there was a dose-response relationship).
5. Occurrence of HBoV in serum specimens was linked in time with an episode of acute wheezing, because HBoV became less prevalent in the same patients after clinical recovery.
Taken together, these findings suggest an association between high HBoV load and acute wheezing of otherwise unknown etiology, but they do not prove a causal relationship. At the same time, shedding of HBoV (continuous or secondary to other infections) also appears to be common. This model for HBoV biology may prove to be valuable for additional studies of the possible causative role of HBoV in respiratory tract disease. Applying this model to the present study would suggest that HBoV is a potential etiologic agent in 28 patients (11%) with a high viral load, of whom 10 (3.9%) had HBoV infection alone. However, some studies have reported that dual viral infections are associated with more-severe disease than are infections with a single agent, so the clinical relevance of HBoV infection with a low viral load will also require additional study [24–26].
Detection of HBoV DNA in serum suggests that HBoV is a systemic infection, like most parvovirus infections, including animal bocavirus infection [27, 28]. It is possible that children with high-titer HBoV in both the respiratory tract and the blood had a primary HBoV infection, and this hypothesis is supported by the finding that serum viral loads generally became low or undetectable after clinical recovery. However, confirmation of a primary infection will have to await the development of an HBoV antibody assay. An HBoV infection with a low viral load may reflect long-term virus persistence after clinical recovery, as has been reported for parvovirus B19 [29, 30]. The findings of HBoV in a few convalescent-phase serum samples and in serum samples obtained from some patients whose respiratory tract specimens tested negative for HBoV are also consistent with virus persistence. Cases of possibly symptomatic primary HBoV infection (in the patients with HBoV infection alone) seemed to be particularly common among children aged ∼1 year (i.e., soon after the loss of maternal antibodies). Such cases emerged throughout the year (table 4). These findings, together with the high prevalence rate, suggest that HBoV is an endemic virus in the study area.
We only studied children with bronchiolitis, recurrent wheezing, and acute asthma. Thus, possible other manifestations of HBoV infection were not addressed. Isolated HBoV positivity was associated with acute otitis media in nearly one-half of the subjects, and interstitial infiltrates were seen in all chest radiographs, suggesting that HBoV could be a frequent causative agent of acute otitis media and childhood community-acquired pneumonia. HBoV-associated acute wheezing did not differ clinically from that induced by rhinoviruses, enteroviruses, or RSV.
Our results indicate that acute wheezing is almost invariably associated with virus infection—and often with multiple-virus infection. Many new respiratory viruses, such as human metapneumovirus and 3 new types of coronaviruses, have been identified over the past few years [3, 16, 18, 31, 32]. Our results suggest that 5% of suspected respiratory virus infections are still unaccounted for, but that estimate is, of course, highly uncertain. On one hand, 5% of patients may not experience virus infections or may be infected by a known virus not detected because of assay limitations. On the other hand, the large number of mixed infections that we observed indicates that additional respiratory agents may also be found in other patients in addition to the 5% who still had negative results.
In conclusion, we found evidence of virus infection in nearly all children with acute wheezing. HBoV was detected in one-fifth of patients, and it was the fourth most prevalent virus detected. Results suggest that HBoV at a high viral load could be an etiologic agent of respiratory tract disease, whereas little support was found for the etiologic role of HBoV at a low viral load. Quantitative analysis may, therefore, be important for future studies of HBoV infection. Like other parvoviruses, HBoV can cause a systemic infection and may persist after resolution of symptoms.
Acknowledgments
We thank Kaisu Kaistinen for traveling with the samples between Turku and Stockholm.
Financial support. Academy of Finland (to T.J.), the Pediatric Research Foundation (to O.R. and T.J.), the Torsten and Ragnar Söderberg Foundation, the Swedish Cancer Foundation, Nana Svartz' Fund, and the Swedish Society for Clinical Microbiology (to T.A.). T.A. is a fellow of the Swedish Research Council.
Potential conflicts of interest. T.A. is a coinventor on a patent application related to HBoV and assigned to Karolinska Institutet Innovations. All other authors: no conflicts.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 3.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102:12891–6. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastien N, Brandt K, Dust K, Ward D, Li Y. Human bocavirus infection, Canada. Emerg Infect Dis. 2006;12:848–50. doi: 10.3201/eid1205.051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foulongne V, Rodiere M, Segondy M. Human bocavirus in children. Emerg Infect Dis. 2006;12:862–3. doi: 10.3201/eid1205.051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma X, Endo R, Ishiguro N, et al. Detection of human bocavirus in Japanese children with lower respiratory tract infections. J Clin Microbiol. 2006;44:1132–4. doi: 10.1128/JCM.44.3.1132-1134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloots TP, McErlean P, Speicher DJ, Arden KE, Nissen MD, Mackay IM. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol. 2006;35:99–102. doi: 10.1016/j.jcv.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold JC, Singh KK, Spector SA, Sawyer MH. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis. 2006;43:283–8. doi: 10.1086/505399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi EH, Lee HJ, Kim SJ, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006;43:585–92. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissbrich BB, Neske FF, Schubert JJ, et al. Frequent detection of bocavirus DNA in German children with respiratory tract infections. BMC Infect Dis. 2006;6:109. doi: 10.1186/1471-2334-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–40. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jartti T, Lehtinen P, Vuorinen T, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jartti T, Lehtinen P, Vanto T, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25:482–8. doi: 10.1097/01.inf.0000215226.69696.0c. [DOI] [PubMed] [Google Scholar]
- 14.National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma update on selected topics—2002. J Allergy Clin Immunol. 2002;110:141–219. [PubMed] [Google Scholar]
- 15.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–9. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 16.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mäkelä MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–42. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fouchier RA, Hartwig NG, Bestebroer TM, et al. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci U S A. 2004;101:6212–6. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heim A, Ebnet C, Harste G, Pring-Akerblom P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol. 2003;70:228–39. doi: 10.1002/jmv.10382. [DOI] [PubMed] [Google Scholar]
- 20.van Doornum GJ, Guldemeester J, Osterhaus AD, Niesters HG. Diagnosing herpesvirus infections by real-time amplification and rapid culture. J Clin Microbiol. 2003;41:576–80. doi: 10.1128/JCM.41.2.576-580.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward CL, Dempsey MH, Ring CJ, et al. Design and performance testing of quantitative real-time PCR assays for influenza A and B viral load measurement. J Clin Virol. 2004;29:179–88. doi: 10.1016/S1386-6532(03)00122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredricks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev. 1996;9:18–33. doi: 10.1128/cmr.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kesebir D, Vazquez M, Weibel C, et al. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194:1276–82. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greensill J, McNamara PS, Dove W, Flanagan B, Smyth RL, Hart CA. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003;9:372–5. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semple MG, Cowell A, Dove W, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–6. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadopoulos NG, Moustaki M, Tsolia M, et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–9. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 27.Carmichael LE, Schlafer DH, Hashimoto A. Minute virus of canines (MVC, canine parvovirus type-1): pathogenicity for pups and seroprevalence estimate. J Vet Diagn Invest. 1994;6:165–74. doi: 10.1177/104063879400600206. [DOI] [PubMed] [Google Scholar]
- 28.Durham PJ, Lax A, Johnson RH. Pathological and virological studies of experimental parvoviral enteritis in calves. Res Vet Sci. 1985;38:209–19. [PubMed] [Google Scholar]
- 29.Lefrere JJ, Servant-Delmas A, Candotti D, et al. Persistent B19 infection in immunocompetent individuals: implications for transfusion safety. Blood. 2005;106:2890–5. doi: 10.1182/blood-2005-03-1053. [DOI] [PubMed] [Google Scholar]
- 30.Lindblom A, Isa A, Norbeck O, et al. Slow clearance of human parvovirus B19 viremia following acute infection. Clin Infect Dis. 2005;41:1201–3. doi: 10.1086/444503. [DOI] [PubMed] [Google Scholar]
- 31.van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–73. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–95. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


