Abstract
Background. Community-acquired pneumonia (CAP) in young children is most commonly associated with viral infections; however, the role of viruses in CAP of school-age children is still inconclusive.
Methods. Seventy-five school-age children hospitalized with CAP were prospectively evaluated for the presence of viral and bacterial pathogens. Nasopharyngeal washes were examined by polymerase chain reaction for viruses and atypical bacteria. Antibody assays to detect bacterial pathogens in acute-phase and convalescent-phase serum samples were also performed.
Results. A viral infection was identified in 65% of cases. Rhinovirus RNA was detected in 45% of patients; infection with another virus occurred in 31%. The most common bacterial pathogen was Mycoplasma pneumoniae, which was diagnosed in 35% of cases. Chlamydia pneumoniae DNA was not detected in any patient; results of serological tests were positive in only 2 patients (3%). Mixed infections were documented in 35% of patients, and the majority were a viral-bacterial combination.
Conclusions. The high prevalence of viral and mixed viral-bacterial infections supports the notion that the presence of a virus, acting either as a direct or an indirect pathogen, may be the rule rather than the exception in the development of CAP in school-age children requiring hospitalization.
Community-acquired pneumonia (CAP) is a serious cause of morbidity among children in developed countries and has a considerable effect on the health care system. In the developing world, the incidence of pneumonia is higher, and this infection is one of the primary causes of death among young children [1].
Detailed information on the etiology of CAP is required for the formulation of treatment recommendations and the introduction of preventive measures. Evaluation of mixed infections and the relative importance of each potential pathogen may also contribute to improved understanding of the etiopathogenesis of this infection. However, identifying the cause of a lower respiratory tract infection remains a challenge for a number of reasons: adequate samples are difficult to obtain, and the differentiation between infection and colonization cannot always be made [1, 2]. Furthermore, the different responsible agents require multiple and complicated methodologies for their detection. As more diagnostic tests or combinations of them are performed, the number of potential causes directly or indirectly associated with pneumonia increases [1, 2].
Streptococcus pneumoniae has been identified as the most important cause of bacterial pneumonia in children [1, 2]. Mycoplasma pneumoniae and Chlamydia pneumoniae are most common causes in school-age children [1, 2], although recent studies have suggested their importance in younger age groups [3–5]. Viruses, with the predominance of respiratory syncytial virus (RSV), have been most commonly associated with pneumonia in infants and young children. However, the role of viruses in many respiratory tract diseases has been readdressed in recent studies that use new sensitive methodologies. This is mostly true for human rhinovirus (RV), which for many years has been considered as a uniquely upper-airway pathogen. Nevertheless, the development of sensitive diagnostic techniques has helped identify RV as significantly associated with lower respiratory tract diseases, such as asthma [6], bronchiolitis [7], and CAP [8].
We conducted a 12-month prospective study involving school-age children hospitalized with CAP to thoroughly investigate the role of viruses, and atypical and common bacteria by means of several detection techniques, including PCR and serological testing.
Patients and Methods
A total of 75 patients (37 of whom were boys) aged 5–14 years (median age, 86.5 months) who were consecutively admitted to our department with the diagnosis of CAP during 1 calendar year were enrolled in the study. Patients were included if they had fever (temperature, ⩾37.5°C) and an infiltrate visible on a chest radiograph. Children with any chronic underlying disorders were excluded from the study. A standard questionnaire, including demographic data, clinical symptoms and signs, laboratory and radiological findings, treatment, complications, and duration of hospitalization, was filled out for each patient. A serum sample and a nasopharyngeal wash were obtained from each patient within 48 h of admission. The samples were kept frozen at -70°C until further study. Forty-five patients returned for follow-up 1 month after discharge, at which time a convalescent-phase serum sample was obtained.
PCR-based microorganism detection. The presence of genomic material of the viruses and atypical bacteria in nasopharyngeal wash samples was examined by RNA or DNA extraction, followed by RT-PCR and direct PCR, respectively. PCR reactions with primer sets and conditions specific for RSV A and B, influenza viruses A (H1N1 and H3N2 subtypes) and B, parainfluenza viruses 1, 2, and 3, adenoviruses, human metapneumovirus, and C. pneumoniae and M. pneumoniae were performed as previously described [7, 9–11]. A single round and a seminested PCR were used for detection of RV [12, 13]. Primers were synthesized from MWG AG Biotech; Taq DNA polymerase and PCR buffers and supplements were purchased from Bioron. PCR products were electrophoresed on 2% agarose gels, and amplicons were visualized by ethidium bromide staining. When a PCR of nested or seminested type was used, it was conducted in a separate room to avoid intrasample contamination. cDNAs obtained from viral lysates served as positive controls, whereas several negative controls (virus transport medium or water) were included in each run to exclude the possibility of contamination.
Serological testing. IgG, IgA, and IgM antibodies against C. pneumoniae were measured by an in-house microimmunofluorescence method by means of purified, formalized elementary bodies, with strain K6 as antigen, as described elsewhere [14]. Diagnosis was based on a 4-fold increase in titer between paired serum samples or on the presence of IgM in any serum sample. A commercial immunoassay (Labsystems Oy) was also used for the detection of IgG and IgM antibodies to C. pneumoniae, as described elsewhere [15, 16].
For the detection of IgM antibodies against M. pneumoniae, the Platelia µ-capture immunoassay (Sanofi Diagnostics Pasteur) was used. For the detection of IgG antibodies against M. pneumoniae, the Platelia EIA was used. The results were expressed as positive or negative. In the case of a positive result, the antibody titer was determined with a conventional complement fixation test (Serion Immunodiagnostica). An increase of ⩾4-fold in antibody titer was considered an indicator of recent infection in paired samples. Antibodies to S. pneumoniae (pneumolysin and C-polysaccharide), Haemophilus influenzae, and Moraxella catarrhalis were measured in paired serum samples by EIA, as described elsewhere [17].
Statistical analysis. The association of clinical signs and symptoms and radiographic and laboratory findings with infection due to different pathogens was examined. The features considered included presence of chest pain and gastrointestinal symptoms, vital signs, maximum temperature, auscultation findings, presence of retractions, and oxygen saturation. Also included were chest radiography findings, such as type of infiltrate (alveolar vs. interstitial), size (segmental vs. lobar), and distribution (right vs. left), and presence of atelectasis or pleural fluid; simple laboratory findings, such as WBC count, total neutrophil count, C-reactive protein level, and erythrocyte sedimentation rate; duration of fever after admission; duration of hospitalization; and antibiotic therapy. Information regarding the patients' characteristics was also examined to adjust for potential confounding through regression models. Such characteristics included demographic characteristics, duration of fever and antibiotic use before admission, presence and duration of cough and rhinitis, and history of asthma and bronchodilator use. Linear and logistic regression models were used for symptoms that were examined through continuous and binary variables, respectively. First, univariate regressions were performed with RV alone, mycoplasma alone, S. pneumoniae alone, any virus, any single pathogen, and mixed infection for each symptom. Then, a multivariate analysis was performed with all the variables that exhibited statistically significant effect on that symptom. The level of significance was fixed at α = 5%. The statistical analysis was performed with the software product S-Plus 2000 (MathSoft).
Results
Infection with ⩾1 pathogen. Infection with ⩾1 pathogen was documented in 58 (77%) of 75 patients. One or more viruses was detected in 49 (65%) of 75 patients, whereas bacterial infection was documented in 30 (40%) of 75 patients (table 1). However, in the subgroup of cases in which paired serum samples were available for bacterial serological testing, infection with ⩾1 microorganism was identified in 41 (91%) of 45 cases.
Table 1.
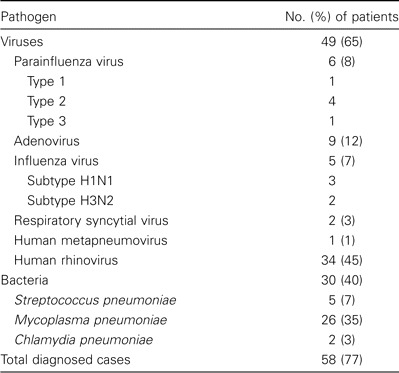
Infections caused by viruses and atypical bacteria diagnosed in 75 children (age, 5–14 years) hospitalized with community-acquired pneumonia.
Viral infections. Data on detection of different viruses in nasopharyngeal washes by PCR are shown in table 1. For RV, the results of single-round PCR were positive in 18 (24%) of 75 samples. The seminested PCR detected an additional 16 positive samples, bringing the total number of children infected with RV to 34 (45%).
Bacterial infections. A total of 26 (35%) of 75 patients were found to have M. pneumoniae infection by either PCR or serological testing (table 1). In patients with paired serum samples available, M. pneumoniae infection was diagnosed in 21 (46%) of 45 by PCR and/or serological tests. PCR was the only positive test result for 2 patients, both tests yielded positive results for 7, and PCR results were negative for 12, with the diagnosis based on the results of serological tests only. Among the 19 patients who received a diagnosis via serological testing, the presence of IgM antibodies was detected during the acute phase of illness in 11 patients (58%). Of the other 30 patients, M. pneumoniae infection was diagnosed in 5 with the use of PCR or a single serum examination. Overall, the results of PCR performed in all patients were positive for 11 (42%) of 26 patients with M. pneumoniae infection. Infection with C. pneumoniae was diagnosed serologically in 2 children; however, PCR results were negative in all cases.
S. pneumoniae infection was detected in 5 (7%) of 75 patients. These included 3 patients who received a diagnosis only serologically, 1 via positive blood culture and serological test results, and 1 with a positive blood culture result for whom paired serum samples were not available. Pneumococcal infection was diagnosed in 4 (9%) of 45 patients for whom paired serum samples were available. H. influenzae and M. catarrhalis antibodies were not detected in any of the patients.
Mixed infections. Mixed infections were documented in 26 (35%) of 75 patients. Infection with 2 pathogens was found in 20 children and with 3 pathogens in 6 children. The majority of these (21 [81%] of 26) were mixed viral-bacterial infections: 16 single-virus/single-bacterium combinations, 3 double-virus with a bacterium, and 2 double-bacteria with a virus. The remaining cases were 3 double-virus infections, 1 triple-virus infection, and 1 double-bacteria infection. The most common combination, found in 13 cases, was that of RV and M. pneumoniae, as expected from their high frequencies in the sample. Of the RV-positive patients, mixed infection was found in 20 (57%) of 35. Among bacterial infections, 21 (70%) of 30 were mixed with viruses.
Association of infection due to different pathogens with clinical and laboratory findings. Associations between clinical signs and laboratory and radiographic findings with infection due to different agents were examined in regression models. No significant associations were found with respect to the presence of RV, any virus, ⩾1 pathogen, or S. pneumoniae. Infections with multiple pathogens were associated with a lower WBC count (mean difference, -5047 cells/mm3; 95% CI, -9002 to -1092 cells/mm3; P < .001) and more frequent localization in the right hemithorax (OR, 1.7; 95% CI, 1.11–2.59; P = .01). However, the former lost its significance after controlling for the presence of M. pneumoniae. Infection with M. pneumoniae was associated with a reduced maximum temperature (mean difference, -0.4°C; 95% CI, -0.7°C to -0.1°C; P = .01), lower WBC count (mean WBC count for those with M. pneumoniae infection, 13,598 cells/mm3; mean WBC count for those without M. pneumoniae infection, 19,860 cells/mm3; mean difference, -7377 cells/mm3; 95% CI, -11,089 to -3664 cells/mm3; P < .001), and a lower C-reactive protein level (mean level for those with M. pneumoniae infection, 60.4 mg/L; mean level for those without M. pneumoniae infection, 128.6 mg/L; mean log-transformed difference: -0.75 mg/L; 95% CI, -1.27 to -0.23 mg/L; P = .005), all of which remained significant after controlling for the presence of viruses in multivariate models.
Discussion
Several serological and molecular diagnostic techniques were used in this study to thoroughly examine the etiology of CAP in hospitalized school-age children. By means of these methods, infection with 11 viruses and 5 bacteria was investigated, and the presence of ⩾1 of them was identified in 77% of the patients. When paired serum samples were available for bacterial antibody assays, the detection rate increased to 91%. These figures are among the highest reported etiological rates; in previous studies, the rate has been 43%–85% [8, 18–21,22,23].
Viruses have been most commonly associated with CAP in infants and younger children [2]. However, recent evidence suggests that, when sensitive detection methods are used, the prevalence of viral infections in older children with CAP is higher than was previously thought [8]. In our cohort, approximately one-third of patients were found to be infected with adenovirus, parainfluenza virus, influenza virus, RSV, or human metapneumovirus. The small number of RSV infections is of note and may be attributed to the older age (⩾5 years) of our study population. A low prevalence of RSV infection (5%–11.5%) has also been found in this age group in previous studies [21–23]. In addition, by use of sensitive detection methods, the presence of RV was documented in almost one-half of the patients, bringing the total proportion of cases with a confirmed viral infection to 65%.
RV has long been considered to be a benign virus causing mild upper respiratory tract infections. In recent years, both experimental and clinical evidence has accumulated that RV can infect the lower respiratory tract [24]. However, the role of this virus in CAP has not been adequately characterized. We have recently shown that RV can actually replicate in the higher temperatures of the lower airways [25, 26] and is able to infect cultured human bronchial epithelial cells and induce cytotoxicity [26] and a local inflammatory response [27].
RV infection has been associated with disease exacerbation in children with underlying pulmonary disease [28], as well as serious lower respiratory tract infections in hospitalized individuals, a considerable proportion of whom were healthy infants and children [29–32]. RV was also found to be only second to RSV in prevalence in infants with bronchiolitis, and its presence was associated with more severe disease [7]. The advent of sensitive PCR-based detection techniques may partly explain the increasing recognition of the involvement of RV in respiratory diseases [24]. Before the PCR era, RV was examined in a small number of studies of childhood CAP and was identified in 2%–12% of cases [18, 20]. More recently, RV was found in 25% of children with CAP with the use of 2 PCR assays [8]. Although the prevalence of RV in the asymptomatic population was not formally assessed in this study, it has been shown to be in the range of 6%–18% in previous studies that used PCR [33, 34] (N. G. Papadopoulos, unpublished data). Furthermore, we performed PCR for RV in 27 samples from children of the same age group, without an obvious respiratory infection, collected in our hospital during the same period of time and retrieved from our sample bank. Only 1 (3.7%) of those was found to yield a positive result, which is statistically significantly less than the proportion identified in children with CAP (P = .0001, by χ2 test).
The most frequently identified bacterium in this cohort was M. pneumoniae . The identification rate (34%) is among the highest reported, which have ranged from 9% to ⩾45% in hospitalized or ambulatory children ⩾5 years of age [3–5, 21–23, 34]. Similar to another recent study [36], PCR was less sensitive than serological testing for diagnosis of this infection. Detection of IgM antibodies during the acute phase of illness can be a useful tool for early diagnosis, because these antibodies were present in 58% of M. pneumoniae—positive patients. The use of a single positive IgM antibody titer for the diagnosis of M. pneumoniae infection has been questioned because of low specificity of the IgM response [37]. However, µ-capture EIA-Platelia has been shown to have an excellent specificity in the diagnosis of acute M. pneumoniae infection [37, 38].
In contrast to M. pneumoniae infection, C. pneumoniae infection was very rarely diagnosed in our patients, despite the use of both PCR and 2 different serological testing methods. Although high C. pneumoniae infection rates in persons with CAP have been reported elsewhere in hospitalized and ambulatory patients [3–5, 21], it is likely that C. pneumoniae is uncommon in childhood CAP in our settings, a finding in agreement with a previous report from our area [39].
A high prevalence of infections with “atypical” bacteria has also been found in adults hospitalized with CAP, often as part of a mixed infection with common bacteria. The importance of mixed infection is not clear, although several studies in adults have shown a benefit of adding a macrolide to the therapeutic regimen [40].
The prevalence of S. pneumoniae infection in our study could be evaluated only in patients for which paired serum samples were available, which is a weak point of the study. The rate of pneumococcal infection in these patients was low (4 [9%] of 45), compared with previous studies that used serological testing; the rate in these studies was 5%–33% among children aged ⩾5 years with CAP [8, 20–23]. In addition, PCR was not used for detection of pneumococcus. A high rate of S. pneumoniae infections (44%) was diagnosed in a recent study among children with lower respiratory tract infection with the use of PCR [41].
Mixed infections were very common (35%), the majority of which were mixed viral-bacterial infections. These findings are in accordance with recent studies suggesting that >1 microorganism may often contribute to the pathogenesis of pneumonia [8, 19–21]. The exact role of each of these pathogens remains to be clarified. However, it has been speculated that viruses may induce pneumonia, either directly or by rendering the host more susceptible to bacterial infection. The high prevalence of viral infection as well as mixed viral-bacterial infection found in our study suggests that this possibility may be the rule rather than the exception in the development of pneumonia.
It has been previously shown that clinical, laboratory, and radiographic findings are poor indicators of an etiological diagnosis in childhood pneumonia [3, 15]. This was also the case in our study, in which correlation of patients' symptoms and findings with infection due to different agents revealed only a few associations, mostly in relation to M. pneumoniae, with limited clinical value.
The limitations of this study should be mentioned. As in previous reports [3–5, 8, 18, 20, 23, 35], documentation of infection in the upper respiratory tract does not necessarily prove an etiological association with pneumonia. Although thoracocentesis may provide more solid evidence of the local presence of pathogens, it is an invasive procedure and carries certain risks [42]. Second, year-to-year variations in the prevalence of different pathogens may affect their association with CAP. Finally, the results reported herein cannot be extrapolated to ambulatory children with CAP.
An extensive laboratory workup was undertaken to determine the etiology of pneumonia in this study. Presently, it is unlikely that results of viral diagnosis would influence treatment decisions if they were available to clinicians. Withholding antibiotics would only be feasible if bacterial (mainly pneumococcal) infection could be ruled out with certainty. Identification of a viral infection does not rule out the coexistence of a bacterium, because mixed infections are common. Determination of IgM antibodies to M. pneumoniae with µ-capture ELISA may be useful in the selection of antibiotic treatment, because these antibodies are detected in a high proportion of patients during the acute phase of illness.
In conclusion, the use of multiple assays, including PCR, may increase sensitivity in documenting infection with different pathogens in school-age children hospitalized with CAP. Viruses are commonly associated with CAP even in this age group, with RV present in almost one-half of these patients. Although it is possible that a viral infection may be an initial pathogenetic event, or even a prerequisite, for the development of pneumonia, additional studies are required to look into the epidemiological and mechanistic aspects of this hypothesis.
Acknowledgments
Financial support. Special Research Account of the University of Athens (grant 70/4/6595; to M.N.T.) and by the 2002 Klosterfrau Award (to N.G.P.).
Conflict of interest. All authors: No conflict.
Footnotes
Presented in part: 21st Annual Meeting of the European Society for Paediatric Infectious Diseases, Taormina, Sicily, Italy, 9–11 April 2003 (abstract 147).
References
- 1.McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429–37. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]
- 2.British Thoracic Society. Guidelines for the management of community-acquired pneumonia in childhood. Thorax. 2002;57(Suppl 1):i1–24. doi: 10.1136/thorax.57.90001.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block S, Hedrick J, Hammerschlag MR, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471–7. doi: 10.1097/00006454-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Harris JA, Kolokahtis A, Campbell M, Cassell G, Hammerschlag MR. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia. Pediatr Infect Dis J. 1998;17:865–71. doi: 10.1097/00006454-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Principi N, Esposito S, Blasi F, Allegra L. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Mowgli Study Group. Clin Infect Dis. 2001;32:1281–9. doi: 10.1086/319981. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos NG, Psarras S. Rhinoviruses in the pathogenesis of asthma. Curr Allergy Asthma Rep. 2003;3:137–45. doi: 10.1007/s11882-003-0026-5. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulos NG, Moustaki M, Tsolia M, et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–9. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 8.Juven T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–8. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Xepapadaki P, Psarras S, Bossios A, et al. Human metapneumovirus as a causative agent of acute bronchiolitis in infants. J Clin Virol. 2004;30:267–70. doi: 10.1016/j.jcv.2003.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham AF, Johnston SL, Julious SA, et al. Chronic Chlamydia pneumoniae infection and asthma exacerbations in children. Eur Respir J. 1998;11:345–9. doi: 10.1183/09031936.98.11020345. [DOI] [PubMed] [Google Scholar]
- 11.Abele-Horn M, Busch U, Nitschko H, et al. Molecular approaches to diagnosis of pulmonary diseases due to Mycoplasma pneumoniae. J Clin Microbiol. 1998;36:548–51. doi: 10.1128/jcm.36.2.548-551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland DC, Kent J, Nicholson KG. Improved detection of rhinoviruses in nasal and throat swabs by seminested RT-PCR. J Med Virol. 1993;40:96–101. doi: 10.1002/jmv.1890400204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadopoulos NG, Hunter J, Sanderson G, et al. Rhinovirus identification by BglI digestion of picornavirus RT-PCR amplicons. J Virol Methods. 1999;80:179–85. doi: 10.1016/S0166-0934(99)00045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S. The microimmunofluorescence test for Chlamydia pneumoniae infection: technique and interpretation. J Infect Dis. 2000;181:421–5. doi: 10.1086/315622. [DOI] [PubMed] [Google Scholar]
- 15.Tuuminen T, Varjo S, Ingman H, Weber T, Oksi J, Viljanen M. Prevalence of Chlamydia pneumoniae and Mycoplasma pneumoniae immunoglobulin G and A antibodies in a healthy Finnish population as analyzed by quantitative enzyme immunoassays. Clin Diagn Lab Immunol. 2000;7:734–8. doi: 10.1128/cdli.7.5.734-738.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ossewaarde JM, Tuuminen T, Boersma WG, Sandstrom M, Palomaki P, Boman J. A preliminary evaluation of a new enzyme immunoassay to detect Chlamydia pneumoniae-specific antibodies. J Microbiol Methods. 2000;43:117–25. doi: 10.1016/s0167-7012(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 17.Nohynek H, Eskola J, Kleemola M, Jalonen E, Saikku P, Leinonen M. Bacterial antibody assays in the diagnosis of acute lower respiratory tract infection in children. Pediatr Infect Dis J. 1995;14:478–84. doi: 10.1097/00006454-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Turner RB, Lande AE, Chase P, Hilton N, Weinberg D. Pneumonia in pediatric outpatients: cause and clinical manifestations. J Pediatr. 1987;111:194–200. doi: 10.1016/S0022-3476(87)80066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nohynek H, Eskola J, Laine E, et al. The causes of hospital treated lower respiratory tract infection in children. Am J Dis Child. 1991;145:618–22. doi: 10.1001/archpedi.1991.02160060036016. [DOI] [PubMed] [Google Scholar]
- 20.Ruuskanen O, Nohynek H, Ziegler T, et al. Pneumonia in childhood: etiology and response to antimicrobial therapy. Eur J Clin Microbiol Infect Dis. 1992;11:217–23. doi: 10.1007/BF02098083. [DOI] [PubMed] [Google Scholar]
- 21.Heiskanen-Kosma T, Korpi M, Jokinen C, et al. Etiology of childhood pneumonia: serologic results of a prospective, population-based study. Pediatr Infect Dis J. 1998;17:986–91. doi: 10.1097/00006454-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Wubbel L, Munitz L, Ahmed A, et al. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr Infect Dis J. 1999;18:98–104. doi: 10.1097/00006454-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Claesson BA, Trollfors B, Brolin I, et al. Etiology of community-acquired pneumonia in children based on antibody responses to bacterial and viral antigens. Pediatr Infect Dis J. 1989;8:856–62. doi: 10.1097/00006454-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos NG, Johnston SL. Rhinoviruses as pathogens of the lower respiratory tract. Can Respir J. 2000;7:409–14. doi: 10.1155/2000/169814. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos NG, Sanderson G, Hunter J, Johnston SL. Rhinoviruses replicate effectively at lower airway temperatures. J Med Virol. 1999;58:100–4. doi: 10.1002/(sici)1096-9071(199905)58:1<100::aid-jmv16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 26.Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–84. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulos NG, Papi A, Meyer J, et al. Rhinovirus infection upregulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin Exp Allergy. 2001;31:1060–6. doi: 10.1046/j.1365-2222.2001.01112.x. [DOI] [PubMed] [Google Scholar]
- 28.Chidekel AS, Rosen CL, Bazzy AR. Rhinovirus infection associated with serious lower respiratory tract illness in patients with bronchopulmonary dysplasia. Pediatr Infect Dis J. 1997;16:43–7. doi: 10.1097/00006454-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Krilov L, Pierik L, Keller E, et al. The association of rhinoviruses with lower respiratory tract disease in hodpitalized patients. J Med Virol. 1986;19:345–52. doi: 10.1002/jmv.1890190407. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt HJ, Fink RJ. Rhinovirus as a lower respiratory tract pathogen in infants. Pediatr Infect Dis J. 1991;10:700–2. doi: 10.1097/00006454-199109000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Kim JO, Hodinka RL. Serious respiratory illness associated with rhinovirus infection in a pediatric population. Clin Diagn Virol. 1998;10:57–65. doi: 10.1016/s0928-0197(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 32.El-Sahly HM, Atmar RL, Glezen WP, Greenberg SB. Spectrum of clinical illness in hospitalized children with “common cold” virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston SL, Sanderson G, Pattemore PK, et al. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993;31:111–7. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nokso-Koivisto J, Kinnari TJ, Lindahl P, et al. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol. 2002;66:417–20. doi: 10.1002/jmv.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gendrel D, Raymond J, Moulin JL, et al. Etiology and response to antibiotic therapy of community-acquired pneumonia in French children. Eur J Clin Microbiol Infect Dis. 1997;16:388–91. doi: 10.1007/BF01726370. [DOI] [PubMed] [Google Scholar]
- 36.Hardy RD, Michelow IC, Rios AM, et al. Program and abstracts of the 43rd Meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy (Chicago) Washington, DC: American Society for Microbiology; 2003. Comparison of nasopharyngeal and oropharyngeal PCR for the diagnosis of Mycoplasma pneumoniae pneumonia in children [abstract D-1859] p. 187. [Google Scholar]
- 37.Petitjean J, Vabret A, Gouarin S, Freymuth F. Evaluation of four commercial immunoglobulin G (IgG)- and IgM-specific enzyme immunoassays for diagnosis of Mycoplasma pneumoniae infections. J Clin Microbiol. 2002;40:165–71. doi: 10.1128/JCM.40.1.165-171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waris ME, Toikka P, Saarinen T, et al. Diagnosis of Mycoplasma pneumoniae pneumonia in children. J Clin Microbiol. 1998;36:3155–9. doi: 10.1128/jcm.36.11.3155-3159.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Triga MG, Anthracopoulos MB, Saikku P, Syrogiannopoulos GA. Chlamydia pneumoniae infection among healthy children and children hospitalized with pneumonia in Greece. Eur J Clin Microbiol Infect Dis. 2002;21:300–3. doi: 10.1007/s10096-002-0710-8. [DOI] [PubMed] [Google Scholar]
- 40.Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. The American Thoracic Society. Am J Respir Crit Care Med. 2001;163:1730–54. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 41.Michelow IC, Lozano J, Olsen K, et al. Diagnosis of Streptococcus pneumoniae lower respiratory infection by culture, polymerase reaction, serological testing and urinary antigen detection. Clin Infect Dis. 2002;34:1–11. doi: 10.1086/324358. [DOI] [PubMed] [Google Scholar]
- 42.Vuori-Holopainen E, Salo E, Saxen H, et al. Etiological diagnosis of childhood pneumonia by use of transthoracic needle aspiration and modern microbiological methods. Clin Infect Dis. 2002;34:583–90. doi: 10.1086/338642. [DOI] [PubMed] [Google Scholar]


