Abstract
Background. The primary modes of transmission of severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) appear to be direct mucus membrane contact with infectious droplets and through exposure to formites. Knowledge of the survival characteristics of the virus is essential for formulating appropriate infection-control measures.
Methods. Survival of SARS-CoV strain GVU6109 was studied in stool and respiratory specimens. Survival of the virus on different environmental surfaces, including a laboratory request form, an impervious disposable gown, and a cotton nondisposable gown, was investigated. The virucidal effects of sodium hypochlorite, house detergent, and a peroxygen compound (Virkon S; Antec International) on the virus were also studied.
Results. SARS-CoV GVU6109 can survive for 4 days in diarrheal stool samples with an alkaline pH, and it can remain infectious in respiratory specimens for >7 days at room temperature. Even at a relatively high concentration (104 tissue culture infective doses/mL), the virus could not be recovered after drying of a paper request form, and its infectivity was shown to last longer on the disposable gown than on the cotton gown. All disinfectants tested were shown to be able to reduce the virus load by >3 log within 5 min.
Conclusions. Fecal and respiratory samples can remain infectious for a long period of time at room temperature. The risk of infection via contact with droplet-contaminated paper is small. Absorbent material, such as cotton, is preferred to nonabsorptive material for personal protective clothing for routine patient care where risk of large spillage is unlikely. The virus is easily inactivated by commonly used disinfectants.
In the early spring of 2003, a mysterious outbreak of severe pneumonia occurred in southern China and rapidly spread throughout the world. The causative agent was later found to be a novel coronavirus and was designated “severe acute respiratory syndrome (SARS) coronavirus” (SARS-CoV) [1–3]. As of 31 December 2003, a total of 8096 cases had been reported, of which 774 were fatal [4]. Altogether, 1706 health care workers were affected. More than 20% of the patients with SARS were themselves health care workers, which could be explained by the unique shedding pattern of SARS-CoV, with viral loads reaching a peak ∼2 weeks after onset of disease, when patients were in hospital care [5]. This shedding pattern of SARS-CoV also highlights the importance of control of nosocomial spread of the disease.
Soon after the isolation of SARS-CoV in our laboratory, we were able to perform a survival study of the virus, and partial results were reported on the World Health Organization Communicable Disease Surveillance and Response Web site on SARS [6]. Here, we provide a full report of our study of the survival characteristics of SARS-CoV in different clinical sample matrices, as well as on various environmental surfaces in the laboratory and hospital. The risk of acquisition of SARS-CoV attributed to the inanimate environment is also discussed. We also report the virucidal effect of 3 common liquid disinfectants on SARS-CoV.
Materials and Methods
Viruses and cell line. SARS-CoV strain GVU6109 was used in the present study. GVU6109 was isolated from a lung tissue specimen obtained from a patient during the SARS outbreak in 2003. The virus was inoculated into the Vero E6 cell line, which was grown in minimum essential medium (MEM) with 2% fetal calf serum at 37°C. All virus culture experiments were performed in a biosafety level 3 laboratory.
Survival of SARS-CoV in different stool specimens. Four different stool samples were used. The samples tested negative for SARS-CoV, norovirus, rotavirus, and other viral agents. Stool sample A was a normal stool specimen obtained from a 6-month-old baby, and samples B and C were 2 normal stool specimens obtained from adults. Sample D was a diarrheal stool specimen obtained from an adult. A 10% suspension of each stool specimen was prepared in PBS (pH, 7.4). After centrifugation at 1500 g for 20 min, the supernatant was collected, and the pH was checked with pH paper. Stool sample A had a pH of 6–7, sample B had a pH of 7–8, sample C had a pH of 8, and sample D had a pH of 9.
A total of 1.8 mL of each 10% stool suspension was spiked with 0.2 mL of virus stock GVU6109 (107 TCID50/mL). As a control, 1.8 mL of viral transport medium was also spiked with 0.2 mL of the virus stock. The samples were incubated in closed containers at room temperature (20°C) for 0.5 h, 1 h, 3 h, 6 h, 1 day, 2 days, 4 days, 5 days, 6 days, and 7 days. Serial 10-fold dilutions of a different stool suspension and control were prepared in Earl's diluent. Fifty microliters of each dilution was inoculated into 4 wells of a 96-well plate. One hundred microliters of Vero E6 (105 cells/mL) was added to each well, and the plates were sealed and incubated at 37°C in 5% CO2 for 4 days. Virus concentration (in TCID50/50 µL) for each stool suspension at a different time was calculated on the basis of the Kärber method [7]. The whole experiment was repeated using a trivalent poliovirus vaccine to compare the effect of pH on a nonenveloped RNA virus.
Survival of SARS-CoV in different respiratory specimens. A total of 0.3 mL of virus stock GVU6109 (107 TCID50/mL) was added to 2.7 mL of a nasopharyngeal aspirate (NPA) specimen, throat and nasal swab (TNS) specimens, and viral transport medium as a control. The respiratory specimens had been determined to be negative for respiratory viruses. They were then incubated in closed containers at room temperature or 4°C for 3 h, 6 h, and 1, 3, 4, 5, 7, and 10 days. The virus concentration (TCID50/50µL) for each sample at various time points was determined as above.
Survival of SARS-CoV on paper, impervious disposable gowns, and cotton nondisposable gowns. To simulate the event of large droplets that contain SARS-CoV falling on paper and on cotton and disposable gowns, experiments were performed to determine whether SARS-CoV survived on these surfaces.
Paper. A paper laboratory request form was cut into small pieces (area, 1 × 1 cm), which were sterilized by autoclave at 121°C for 15 min. Stock virus GVU6109 (107 TCID50/mL) was serially diluted to 104 TCID50/mL with PBS. At each virus dilution, 5 µL was applied to the surface of each piece of sterilized paper. The sample was allowed to be absorbed at room temperature, and the paper pieces were left to stand for different durations (5 min, 1 h, 2 h, 3 h, 6 h, 1 day, and 2 days). Each piece of paper was then placed into a Vero E6 cell culture tube. For each virus dilution and at different intervals after absorption, 4 pieces of paper were inoculated into 4 cell culture tubes. All of the tubes were incubated at 37°C and were examined after 4 days. Sterilized paper without virus suspension was also included in the study to check for any toxicity to cell culture.
Disposable gown. For the disposable gown, the whole process used for paper was repeated, except that a disposable gown was used after treatment by irradiating it under UV light for 1 h. The gown is part of the personal protection equipment used in our laboratory when handling specimens that are potentially contaminated with SARS-CoV. It is made of polypropylene material (35 g/m2) coated with a polyethylene film (15 g/m2), and the waist and neck are tied when the gown is used to provide full-body protection.
Cotton gown. For testing of the cotton gown, a large piece of cloth cut from an ordinary cotton laboratory coat was soaked in distilled water overnight and was then boiled for 1 h. The whole process was repeated 3 times to remove chemical residue that was found to be toxic to the cell culture. After drying, the cloth was cut into small pieces (area, 1 × 1 cm). The pieces were then sterilized by autoclaving. The sterilized cotton cloth was then tested in the manner used for the paper and the disposable gown.
Effect of different disinfectants and detergents on the survival of SARS-CoV. Different dilutions of sodium hypochlorite solution (1 : 50 and 1 : 100 of the stock solution, which contains 50,000 ppm of active chlorine); a household detergent containing sodium lauryl ether sulphate, alkyl polyglycosides, and coco-fatty acid diethanolamide (1 : 50 and 1 : 100; AXE brand); and Virkon S (1%; Antec International) were made by dilution with distilled water. Fifty microliters of stock virus GVU6109 (107 TCID50/mL) was added to 450 µL of different dilutions of the hypochlorite solution, household detergent, and Virkon S and to viral transport medium as a control. After standing at room temperature for 5 min, 10 min, 20 min, and 30 min, serial 10-fold dilutions of different disinfectants or controls were made in Earl's diluent. Fifty microliters of each virus dilution was added to each of 4 wells of a 96-well plate. A total of 50 µL of MEM was added to each well. After adding 100 µL of Vero E6 cells to each well, the plates were sealed and incubated at 37°C in a 5% CO2 incubator. Cytopathic effect was recorded at day 4, and residual virus TCID50 was calculated from wells without showing cell toxicity.
Results
Survival of SARS-CoV in different stool specimens. Figure 1 shows the duration of SARS-CoV survival after incubation in stool specimens at different pHs. The virus was not recoverable within 1 day after incubation in normal adult stool specimens or within 3 h after incubation in the baby stool specimen with a slightly acidic pH. However, the virus survived for 4 days in a diarrheal stool specimen with a pH of 9. Poliovirus did not show these survival characteristics. Poliovirus spiked in the same baby stool specimen survived for >4 days, and it survived for even longer in the diarrheal stool specimen (data not shown). The duration of survival for SARS-CoV in the stool suspension was retested in another 2 diarrheal stool specimens, with the same results (data not shown).
Figure 1.
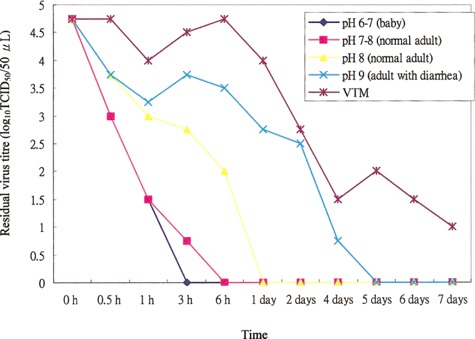
Survival time of severe acute respiratory syndrome coronavirus in different stool specimens at room temperature. VTM, viral transport medium.
Survival of SARS-CoV in different respiratory specimens. The virus can remain alive in respiratory specimens, such as NPA or TNS specimens, for >7 days at room temperature and for >20 days at 4°C (figure 2).
Figure 2.
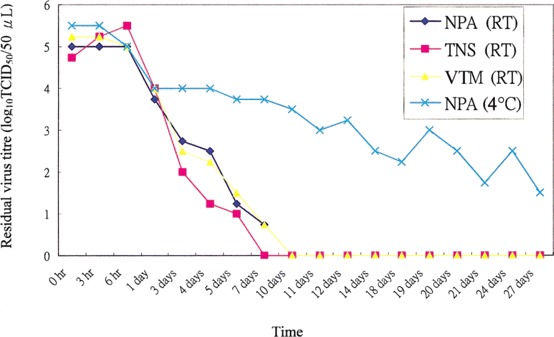
Survival time of severe acute respiratory syndrome coronavirus in nasopharyngeal aspirate (NPA) specimens, throat and nasal swab (TNS) specimens, or viral transport medium (VTM) at room temperature (RT) and at 4°C.
Survival of SARS-CoV on paper, the impervious disposable gown, and the cotton nondisposable gown. Table 1 shows the duration of survival for SARS-CoV on different materials. Even with a relatively high virus load in the droplet, rapid loss of infectivity was observed for paper and cotton material. Inactivation on impervious surface took much longer. No cell culture toxicity was observed for the paper, disposable gown, or cotton nondisposable gown.
Table 1.

Duration of survival of severe acute respiratory syndrome coronavirus (SARS-CoV) on paper, a disposable gown, and a cotton gown.
Effect of different disinfectants and detergents on SARS-CoV. After incubation with various disinfectants, a reduction in the virus load of >3 log was taken to indicate inactivation (table 2). All disinfectants reduced the virus load by >3 log within 5 min after incubation. Cell toxicity was observed in wells inoculated with a virus/disinfectant mixture at a 1 : 10 dilution. Thus, when trying to calculate the residual TCID50, results from wells with dilutions starting from 1 : 100 were used.
Table 2.
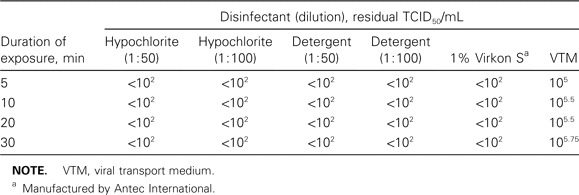
Effect of disinfectants on severe acute respiratory syndrome coronavirus.
Discussion
A recent study showed evidence that SARS-CoV has contaminated a variety of environmental surfaces in some hospital settings [8]. The presence of SARS-CoV on surfaces is always a concern, although few studies in which live virus has been successfully isolated from an environmental surface have been reported. Surfaces were usually contaminated with patient's droplets or by indirect transfer of virus from gloves that were contaminated with excreted virus. The increase in the isolation rate of methicillin-resistant Staphylococcus aureus at the intensive care unit of a Hong Kong hospital during the SARS outbreak suggested that increased cross-contamination could occur if gloves and gowns were worn all of the time [9]. It is important to gather evidence of the survival of SARS-CoV on surfaces so that appropriate infection-control measures can be taken.
The present study demonstrates that SARS-CoV can survive in respiratory samples for 5 days at room temperature and for up to 3 weeks at 4°C. Although normal fecal material seems to have a deleterious effect on its survival, the present study shows that the virus could have a prolonged survival when present in diarrheal stool. The virus can survive for 4 days at room temperature after being spiked in diarrheal stool with an alkaline pH. This observation lends evidence that fecal droplets containing SARS-CoV remain infectious for a period of time. This may explain the Amoy Gardens outbreaks, in which the drainage and sewage system was implicated in facilitating the spread of SARS, as was pointed out in the SARS Expert Committee study [10] and in a simulation study by Yu et al. [11].
On the basis of quantitative data obtained from our own study [5], stool samples contain a much higher viral load than do NPA samples. The mean virus concentration may reach 105 TCID50/mL at 2 weeks after onset of disease in stool samples, compared with 102.2 TCID50/mL for NPA samples. Our present data show that, at a high concentration of virus (106 TCID50/mL), SARS-CoV can survive for 4–5 days at room temperature in both respiratory and diarrheal stool samples. From the point of view of infection control of SARS, it is important to know that excreta from patients with SARS (especially those who have diarrhea) may remain highly infectious for a considerably long period, and appropriate precautions must be taken to prevent formation of aerosols, because of probable airborne transmission of SARS.
During the SARS outbreak in 2003, contamination of paper documents was a concern for health care workers, who frequently had to handle such documents in their daily work. The present study simulates a situation in which large respiratory droplets (volume, 5 µL; radius, ∼1 mm) that contain the virus fall onto paper. Even with a higher concentration of virus (104 TCID50/mL) than would normally occur in NPA samples (102.2 TCID50/mL), no virus infectivity remained after the paper was dried. Paper contaminated with a higher concentration of virus (equivalent to that of fecal excreta [i.e., 105 TCID50/mL]) was not infectious after 3 h, and no viral infectivity was shown after 24 h, even with a concentration of 106 TCID50/mL. Our study shows that the risk of infection through contact with a droplet-contaminated paper is small. Standard infection-control measures, such as hand washing after touching any potential infectious material, are effective against nosocomial transmission of SARS [12].
A previous study reported that coronavirus 229E and OC43 can survive for a few hours after drying on 3 different surfaces (aluminium, cotton gauze sponges, and latex gloves) [13]. In the present study, we compared the survival of SARS-CoV on 2 types of gowns: disposable gowns and cotton gowns. Our results showed that, even with a high concentration of virus (105 TCID50/mL), the droplets will lose all infectivity after 1 h on cloth, compared with 24 h needed for the disposable gown. Apparently, droplets will be absorbed more quickly on cotton material than on fluid-repellent material. The present data show that an ordinary cotton gown offers reasonable protection against small droplets containing SARS-CoV. Our study also raises the possibility that any droplets that hang on a nonabsorbent disposable gown may pose a risk of contaminating the environment when health care workers wear the gown all of the time or when they try to remove the gown. A similar conclusion may also be drawn for gloves, although gloves were not tested in the present study. A specially designed disposable garment with a fluid-repellent lamination that has an outer fluid-absorbing sheet may offer better protection for the personnel.
Finally, our study shows that 3 common liquid detergents/disinfectants are equally effective against the SARS-CoV. All demonstrated a minimum 103-fold reduction in the initial virus titer within 5 min after incubation in solution [14]. The household detergents tested in this study were shown to be effective against the SARS-CoV with a lipid envelope and could be used for cleaning common items and surfaces that are not grossly contaminated with secretions or excreta.
Although we did not perform specific neutralizing steps for the 3 detergents/disinfectants, the fact that the wells that we examined to calculate the residual virus TCID50 were free of cell toxicity highly suggests that nonneutralized disinfectants also have no effect on the virus during the 4 days of incubation.
The SARS-CoV is a newly discovered virus. Thus far, there have only been a few reports of its survival characteristics [15]. Here, we demonstrate that this deadly virus can remain infectious for a long period in stool specimens. The samples that we spiked with SARS-CoV were incubated in closed containers during the entire period of incubation, simulating the conditions in a sewage drainage pipe. Thus, our results showed that, in this situation, droplets may be a concern with regard to disease transmission, as occurred in the Amoy Gardens outbreaks. This has significant implications for sewage treatment in both domestic and hospital environments. Fortunately, this virus is also susceptible to drying. We showed that, when virus-containing droplets were dried, the virus was inactivated rapidly on paper and cotton cloth. Transmission through droplet-contaminated paper and cotton gowns is unlikely, and common household detergents can be effective decontaminating agents for use in the laboratory and hospital.
Acknowledgments
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 2.Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. SARS study group. Lancet. 2003;361:1319–25. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. SARS Working Group. N Engl J Med. 2003;348:1953–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. 2005. Available at: http://www.who.int/csr/sars/country/table2004_04_21/en/. Accessed 12 August 2005. [Google Scholar]
- 5.Cheng PK, Wong DA, Tong LK, et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699–700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network. Available at: http://www.who.int/csr/sars/survival_2003_05-04/en/. Accessed 22 December 2004. [Google Scholar]
- 7.Schmidt NJ, Emmons RW, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. 6th ed. Washington, DC: American Public Health Association; 1989. [Google Scholar]
- 8.Dowell SF, Simmerman JM, Erdman DD, et al. Severe acute respiratory syndrome coronavirus on hospital surfaces. Clin Infect Dis. 2004;39:652–7. doi: 10.1086/422652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yap FHY, Gomersall CD, Fung KSC, et al. Increase in methicillin-resistant Staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin Infect Dis. 2004;39:511–6. doi: 10.1086/422641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SARS Expert Committee. SARS in Hong Kong: from experience to action. Report of the SARS Expert Committee. Hong Kong: Health, Welfare, and Food Bureau, Hong Kong Government; 2003. [Google Scholar]
- 11.Yu ITS, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–9. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 12.Seto WH, Tsang D, Yung RWH, et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Advisors of Expert SARS group of Hospital Authority. Lancet. 2003;361:1519–20. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sizun J, Yu MWN, Talbot PJ. Survival of human coronavirus 229E and OC43 in suspension and after drying on surfaces: a possible source of hospital-acquired infections. J Hosp Infect. 2000;46:55–60. doi: 10.1053/jhin.2000.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell AD, Hugo WB, Ayliffe GAJ. Principles and practice of disinfection, preservation and sterilization. 2nd ed. Oxford, UK: Blackwell Scientific Publications; 1992. [Google Scholar]
- 15.Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol (Berl) 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


