Abstract
We report the findings of high-resolution chest computed tomography of 6 hematopoietic stem cell transplant recipients with parainfluenza virus type 3 pneumonia who were not infected with any other pathogens. All patients had multiple small nodules (diameter, <5 mm) without cavitation in a peribronchial distribution. Changes preceded microbiological diagnosis in 4 of 6 cases.
Respiratory viruses are the most frequent causes of acute illness in the developed world. For 63% of adult recipients of a hematopoietic stem cell transplant (HSCT) with a respiratory illness, these viruses have been detected by the use of PCR [1]. Influenza, respiratory syncytial virus, rhinovirus, and parainfluenza virus (PIV) are the most common pathogens, and the mortality rate for viral pneumonitis is high [1]. Of the 4 serotypes of PIV, type 3 (PIV3) accounts for 90% of cases [2], with an overall incidence of 5%–7% [3]. Most cases of PIV3 infection among HSCT recipients have occurred during the spring and summer months, when PIV3 infection peaks in the community [4], and nosocomial outbreaks have been described elsewhere [5]. Pneumonia has been reported in 24%–50% of cases, with a 35% acute mortality rate [3] and a 75% mortality rate at 6 months [2]. Some of this mortality has been attributed to copathogens [2].
In the assessment of HSCT recipients with fever and suspected pulmonary disease, the net state of immunosuppression, the community respiratory viral activity, the onset and rate of evolution of symptoms, and the radiological appearance of the lesions help to predict a particular infectious etiology. Bacterial infections typically progress within 24 h, whereas viral, mycoplasma, nocardial, mycobacterial, and fungal infections evolve over 2–7 days. Pneumonia caused by respiratory viruses is often preceded by symptoms of upper respiratory tract infection; these symptoms have been reported in ∼85% of cases of PIV3 infection. However, during a nosocomial outbreak at Westmead Hospital (Westmead, Australia) in late 2003 that involved 7 allogeneic HSCT recipients and 1 autologous HSCT recipient, 3 patients presented with lower respiratory tract infection, 3 with upper respiratory tract infection that progressed within 19 days to lower respiratory tract infection, and 1 with an upper respiratory tract infection alone [6]. Radiological results indicate that areas of consolidation are typical of bacterial pneumonia, that peribronchovascular lesions are more likely due to viral or Pneumocystis pneumonitis, and that nodular (size, >1 cm) infiltrates are associated with fungal, nocardial, and tuberculous infections [7].
High-resolution computed tomography (CT) of the lungs is more sensitive than chest radiography for identifying pulmonary abnormalities and is particularly useful in the diagnosis of filamentous fungal infection in HSCT recipients. On the basis of a study of a heterogeneous group of 78 immunocompromised patients, 12 of whom had viral pneumonitis, it was suggested that the presence of multiple, small (size, <10 mm), noncavitating nodules with irregular margins was predictive of viral pneumonitis [8]. The appearance of these nodules (which were identifed by the use of high-resolution CT) in the lungs of patients with PIV3 pneumonia has not previously been described.
During a 3-year period (2003–2005), high-resolution CT scans of the lungs of 6 HSCT recipients who developed PIV3 pneumonia at Westmead Hospital, a quaternary referral center for HSCT, were made available. On the basis of these high-resolution CT scans, we now report that multiple small nodules are common in the lungs of HSCT recipients with PIV3 pneumonia.
Methods. HSCT recipients with PIV3 pneumonia who were not infected with any other pathogens and who had undergone high-resolution CT of their lungs at Westmead Hospital during the period 2003–2005 were identified, and their case reports were reviewed. Systematic screening for viral respiratory pathogens was performed in the hematology ward; in February 2005, a nurse-administered screening tool was used. Paired nose and throat swabs were collected if ⩾2 of the following symptoms were present: cough, fever, rhinorrhea or nasal stuffiness, dyspnoea, hypoxia (with room air oxygen saturations of <95%), or crackles observed during respiratory examination. Bronchoalveolar lavage (BAL) was performed at the discretion of the hematology and respiratory physicians. High-resolution CT was performed for patients with prolonged neutropenic fever and for patients with pneumonia who were not responding to antimicrobials.
A diagnosis of PIV3 pneumonia required a positive viral test result for PIV3 and the presence of ⩾2 of the following symptoms: cough, fever, shortness of breath, hypoxia, crackles on auscultation, or chest CT imaging consistent with pneumonia. For 4 of the 6 HSCT recipients with PIV3 pneumonia who were not infected with any other pathogens, the clinical data were collected retrospectively; for the other 2 recipients, the clinical data were collected prospectively.
Respiratory samples were tested by indirect immunofluorescence using monoclonal antibodies (Chemicon) for the presence of influenza A and B viruses; PIV type 1 (PIV1), type 2 (PIV2), and PIV3; respiratory syncytial virus; and adenovirus. Samples that tested negative by the use of immunofluorescence were cultured for viruses in monkey kidney cell lines (LLC-MK>2 and/or BGM) and human embryonic lung fibroblasts (MRC-5). Cultures were observed twice weekly (days 1–21) for the cytopathic effect. Immunofluorescence, viral culture, and early antigen testing for cytomegalovirus (CMV) were all performed on BAL samples. Other immunofluorescence samples that tested negative were cultured.
The use of PCR for the detection of respiratory viruses (PIV1–3; influenza A and B viruses; respiratory syncytial virus; human metapneumovirus [9, 10]; rhinoviruses; coronaviruses OC43, 229E, NL63, and HKU1 [11, 12]; polyomaviruses WU and KI [13, 14]; and human bocavirus [15]) was introduced in July 2005. CMV screening was performed weekly, using qualitative plasma PCR and pp65 antigen in 2003 and quantitative PCR in 2004 (Roche Cobas Assay).
BAL samples were examined for the presence of fungi by cytology and culture. Starting in 2005, Aspergillus PCR was performed on BAL samples, and a twice-weekly screening of serum was begun; galactomannan was not available. All allogeneic HSCT recipients received primary prophylaxis with fluconazole or secondary prophylaxis with voriconazole. High-resolution CT chest scans were reviewed independently, using a standardized format, by a radiologist who was blinded to the diagnosis [8].
Results. Fifteen episodes of PIV3 infection without copathogens were detected over the study period in 15 HSCT recipients. HRCT was performed for 6 of these episodes (table 1 and figure 1). All cases of infection occurred in allogeneic HSCT recipients, with illness beginning within 106 days of the HSCT. Three patients experienced respiratory failure, and 1 died. Diagnosis was based on positive results of immunofluorescence tests (4 cases), PCR (2 cases), and/or culture (2 cases). No copathogens were identified.
Table 1.
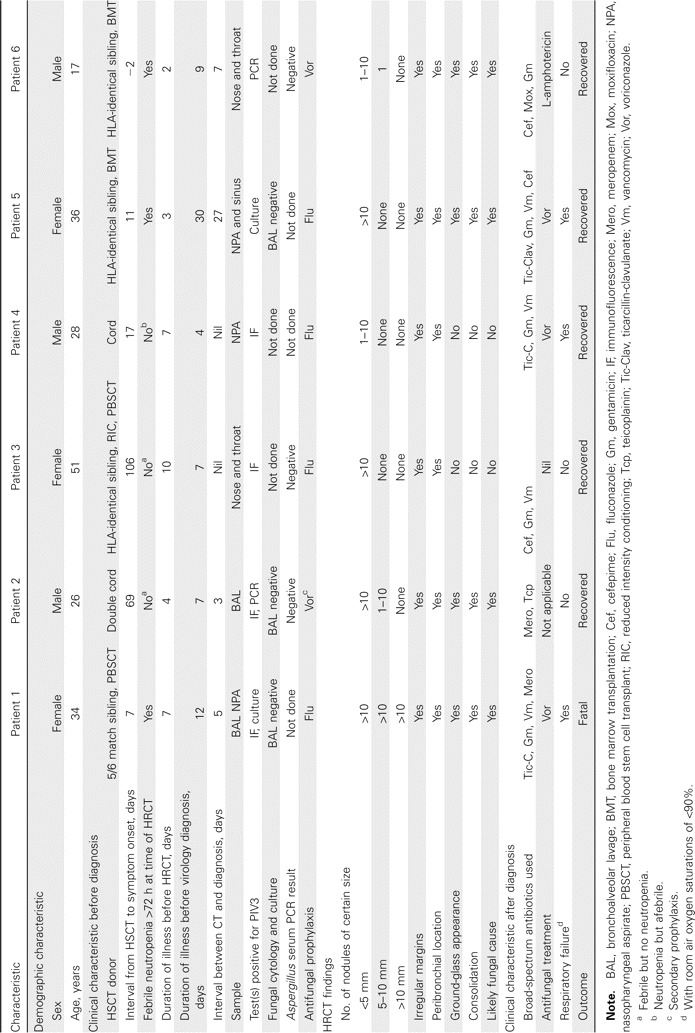
The demographic and clinical characteristics of the 6 hematopoietic stem cell transplant (HSCT) recipients with parainfluenza type 3 (PIV3) pneumonia and the radiological characteristics of their high-resolution computed tomography (HRCT) scans, at Westmead Hosptial, 2003–2005.
Figure 1.

A, High-resolution CT scan of the lungs of patient 1 that show peribronchial nodules <5 mm, 5–10 mm, and >10 mm in diameter. B, High-resolution CT scan of the lungs of patient 1 that show nodules with associated consolidation. C, High-resolution CT scan of the lungs of patient 3 that shows very small (<5 mm) peribronchial nodules in the left upper lobe. D, High-resolution CT scan of the lungs of patient 5 that show multiple small (⩽5 mm) peribronchial nodules and the appearance of ground-glass consolidation.
High-resolution CT revealed multiple small nodules (diameter, <5 mm) in the lungs of all 6 recipients, but there were also nodules 5–10 mm in diameter in the lungs of 3 recipients and nodules >1 cm in diameter in the lungs of 1 recipient. All nodules were peribronchial, with no evidence of cavitation. Surrounding ground-glass attenuation and airspace consolidation were present in the CT scans of one-half of the patients. Four of the 6 recipients had an initial radiological diagnosis that was considered most likely to be fungal pneumonitis.
Fungal infection was excluded because the test results from BAL culture or cytology (3 cases) or Aspergillus serum PCR (3 cases) were negative for the presence of fungi. One patient received secondary prophylaxis with voriconazole following an episode of probable invasive pulmonary aspergillosis 4 months earlier. Antifungal therapy was given to 4 patients following high-resolution CT.
No patients showed evidence of CMV pneumonitis. Four patients had plasma CMV PCR test results that were negative. Patients 4 and 5 had positive PCR test results and titer levels that remained low at the time of high-resolution CT (865 and 1400 copies/mL, respectively), with levels decreasing subsequently; patient 5 had a BAL sample that was negative for the presence of CMV (table 1).
Discussion. This small case series supports the contention that multiple small, noncavitary, pulmonary nodules are suggestive of viral pneumonitis. Notably, peribronchial nodules with irregular margins were consistently present and varied in size from <5 mm (all 6 patients) to >1 cm (1 patient). Most the nodules were surrounded by a ground-glass appearance. Consolidation, which is more characteristic of nonviral pathogens, was noted in the images of the lungs of 2 patients. For 4 patients, the presumptive radiological diagnosis was that of a fungal pneumonitis.
The presence of nodules in the lungs has historically been associated with fungal infection, particularly the presence of nodules with cavitation and the air-crescent or halo sign [16]. However, these manifestations require functioning neutrophils [16] and are late radiological signs in the HSCT recipient with prolonged neutropenia who may not develop cavitation until neutrophil recovery many days or weeks after nodules are first seen. In a recent report, it was determined that there were multiple nodules (diameter, <10 mm) in the lungs of 12 patients with viral pneumonitis (cytomegalovirus, influenza A virus, or adenovirus) associated with HSCT, hematological malignancies, or AIDS [8] and that there were multiple nodules (diameter, >10 mm) in the lungs of 19 patients with fungal infection (Aspergillus and Cryptococcus neoformans). The association between the presence of multiple small nodules and the diagnosis of viral pneumonitis was statistically significant [8]. Nodules <5 mm in diameter may be a more discriminatory finding for patients with viral pneumonitis, based on our case series. These changes were readily apparent for all CT scans, which were performed on days 2–10 of respiratory illness and preceded the microbiological diagnosis by 3–27 days in 4 of the 6 patients. Although nucleic acid–based tests are more sensitive than immunofluorescent antibody methods, culture, or immunofluorescence for the detection of respiratory viruses in respiratory specimens [10], they may not provide a diagnosis within the 2 days of clinical onset of pneumonia seen with CT scans. Multiplex real-time PCR has a minimum turnaround time of 6 h [17], but processing and staffing constraints can delay results by a mean (±SD) period of 30 (±13) h after sample collection [18], with further delays if the testing was done off-site.
The limitations of our study included nonsystematic screening, the introduction of new diagnostic testing during the study period, and the small sample size. It is possible that PIV3 infections were missed prior to 2005 and that other viral pathogens may have been present in cases not tested by PCR. However, this case series was not intended to suggest that all HSCT recipients with PIV3 infection will manifest nodular changes on high-resolution CT scans but to highlight that respiratory viruses, including PIV3, are potential causes of multiple small nodules. Currently, published diagnostic strategies recommend BAL for the investigation of nodular infiltrates on high-resolution CT scans of the lungs of allogeneic HSCT recipients, with testing for fungi, bacteria, and herpes viruses, but these strategies do not mention specific testing for respiratory viruses [19]. The presence of multiple small nodules on high-resolution CT scans should prompt clinicians to test for respiratory viruses, including PIV3.
In conclusion, high-resolution CT of the lungs may provide an important radiological clue to the presence of respiratory viruses, either as single pathogens or as copathogens, particularly in the absence of upper respiratory symptoms and particularly during spring and summer months, when viruses such as PIV3 are active but clinical suspicion may be low. Furthermore, because of the high potential for person-to-person transmission of respiratory virus infection, the consequent risk of nosocomial infection [5], and the increased risk of morbidity and/or mortality from HSCTs [2, 3, 6], the identification of multiple, small, peribronchial nodules on high-resolution CT scans should stimulate an assessment and, if necessary, a review of infection control practices in HSCT units.
Acknowledgments
We thank Theo Sloots and Michael Nissen of the Queensland Paediatric Infectious Diseases laboratory, Royal Children's Hospital, Brisbane, Australia, for their PCR work and Ken McPhie and staff at the respiratory virology laboratory, Centre for Infectious Diseases and Microbiology, Westmead Hospital, Westmead, Australia.
Financial support. National Health and Medical Research Council of Australia (NHMRC; Centre of Clinical Research Excellence grant 264625 and NHMRC postgraduate medical award to P.F.).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Roghmann M, Ball K, Erdman D, Lovchik J, Anderson LJ, Edelman R. Active surveillance for respiratory virus infections in adults who have undergone bone marrow and peripheral blood stem cell transplantation. Bone Marrow Transplantation. 2003;32:1085–8. doi: 10.1038/sj.bmt.1704257. [DOI] [PubMed] [Google Scholar]
- 2.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–8. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 3.Lewis VA, Champlin R, Englund J, et al. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clin Infect Dis. 1996;23:1033–7. doi: 10.1093/clinids/23.5.1033. [DOI] [PubMed] [Google Scholar]
- 4.Marx A, Gary HE, Jr, Marston BJ, et al. Parainfluenza virus infection among adults hospitalized for lower respiratory tract infection. Clin Infect Dis. 1999;29:134–40. doi: 10.1086/520142. [DOI] [PubMed] [Google Scholar]
- 5.Zambon M, Bull T, Sadler CJ, Goldman JM, Ward KN. Molecular epidemiology of two consecutive outbreaks of parainfluenza 3 in a bone marrow transplant unit. J Clin Microbiol. 1998;36:2289–93. doi: 10.1128/jcm.36.8.2289-2293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson P, Gilroy N, Dwyer D, Bradstock K. High mortality outbreak from parainfluenza virus type 3 on a haematopoietic stem cell transplant unit. Intern Med J. 2005;35:86. [Google Scholar]
- 7.Rubin RH, Greene R. Clinical approach to the compromised host with fever and pulmonary infiltrates. In: Rubin RH, Lowell S, eds. Clinical approach to infection in the immunocompromised host. 4th ed. New York: Young Kluwer Academic/Plenum. 2002. p. 116. [Google Scholar]
- 8.Franquet T, Müller NL, Giménez A, Martçnez S, Madrid M, Domingo P. Infectious pulmonary nodules in immunocompromised patients: usefulness of computed tomography in predicting their etiology. J Comput Assist Tomogr. 2003;27:461–8. doi: 10.1097/00004728-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Lambert SB, Whiley DM, O'Neill NT, et al. Comparing nose-throat swabs and nasopharyngeal aspirates collected from children with symptoms for respiratory virus identification using real-time polymerase chain reaction. Pediatrics. 2008;122:e615–20. doi: 10.1542/peds.2008-0691. [DOI] [PubMed] [Google Scholar]
- 10.Maertzdorf J, Wang CK, Brown JB, et al. Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J Clin Microbiol. 2004;42:981–6. doi: 10.1128/JCM.42.3.981-986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–40. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloots TP, McErlean P, Speicher DJ, Arden KE, Nissen MD, Mackay IM. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol. 2006;35:99–102. doi: 10.1016/j.jcv.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaynor AM, Nissen MD, Whiley DM, et al. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64–0000. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bialasiewicz S, Whiley DM, Lambert SB, Gould A, Nissen MD, Sloots TP. Development and evaluation of real-time PCR assays for the detection of the newly identified KI and WU polyomaviruses. J Clin Virol. 2007;40:9–14. doi: 10.1016/j.jcv.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bialasiewicz S, Whiley DM, Lambert SB, et al. Presence of the newly discovered human polyomaviruses KI and WU in Australian patients with acute respiratory tract infection. J Clin Virol. 2008;41:63–8. doi: 10.1016/j.jcv.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh YW, Effmann EL, Godwin JD. Pulmonary infections in immunocompromised hosts: the importance of correlating the conventional radiologic appearance with the clinical setting. Radiology. 2000;217:647–56. doi: 10.1148/radiology.217.3.r00dc35647. [DOI] [PubMed] [Google Scholar]
- 17.Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004;42:1564–9. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oosterheert JJ, van Loon AM, Schuurman R, et al. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41:1438–44. doi: 10.1086/497134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maertens J, Theunissen K, Verhoef G, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005;41:1242–50. doi: 10.1086/496927. [DOI] [PubMed] [Google Scholar]


