Abstract
Background
The microbial etiology of community-acquired pneumonia (CAP) is still not well characterized. During the past few years, polymerase chain reaction (PCR)-based methods have been developed for many pathogens causing respiratory tract infections. The aim of this study was to determine the etiology of CAP among adults—especially the occurrence of mixed infections among patients with CAP—by implementing a new diagnostic PCR platform combined with conventional methods.
Methods
Adults admitted to Karolinska University Hospital were studied prospectively during a 12-month period. Microbiological testing methods included culture from blood, sputum, and nasopharyngeal secretion samples; sputum samples analyzed by real-time quantitative PCR for Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis; nasopharyngeal specimens analyzed by use of PCR; serological testing for Mycoplasma pneumoniae, Chlamydophila pneumoniae, and viruses common in the respiratory tract; and urine antigen assays for detection of pneumococcal and Legionella pneumophila antigens.
Results
A microbial etiology could be identified for 67% of the patients (n = 124). For patients with complete sampling, a microbiological agent was identified for 89% of the cases. The most frequently detected pathogens were S. pneumoniae (70 patients [38]) and respiratory virus (53 patients [29]). Two or more pathogens were present in 43 (35%) of 124 cases with a determined etiology.
Conclusions
By supplementing traditional diagnostic methods with new PCR-based methods, a high microbial yield was achieved. This was especially evident for patients with complete sampling. Mixed infections were frequent (most commonly S. pneumoniae together with a respiratory virus).
Knowledge of pathogens causing community-acquired pneumonia (CAP) constitutes the basis for selection of empirical antimicrobial treatment, which has a substantial impact on the prognosis of the patient [1, 2]. Despite the development of improved microbiological methods during the past few years, the etiology of CAP has still not been well characterized [3]. Few recent studies with well-characterized patient groups, appropriate specimen collection prior to antibiotic treatment, and combined bacteriological and virological diagnostics have been published.
Viruses are recognized as important causes of CAP among infants and children [4], but the role these viruses play in causing CAP among adults is not well understood. Recent studies based on molecular diagnostics indicate that the viral etiology of CAP may have been underestimated [5–8] because of a previous limited range of diagnostic methods. It is still unclear whether a virus by itself can cause pneumonia or whether the virus can act in conjunction with other respiratory pathogens. Previous studies have shown that some respiratory viruses are capable of invading and replicating in the lower respiratory tract mucosa [9, 10]. Moreover, a few reports indicate that the incidence of mixed infections may be significant among patients admitted to hospital with CAP [11, 12].
Nucleic acid detection with the use of real-time polymerase chain reaction (PCR) has been developed for many bacterial and viral pathogens causing respiratory tract infections [13–20]. One of the advantages of molecular diagnostics is the possibility of identifying pathogens in patients already receiving antibiotic treatment [21]. To our knowledge, these new PCR methods have not been evaluated concurrently in a prospective study of patients with CAP. The aim of this study was to determine the etiology of CAP among patients admitted to hospital and, in particular, to assess the occurrence of mixed infections, by implementing a new diagnostic PCR platform combined with conventional methods.
Methods
Patients
The patient population and the inclusion criteria have been described in detail elsewhere [21]. During a 12-month period between the years 2004 and 2005, consecutive patients with CAP admitted to the Department of Infectious Diseases at Karolinska University Hospital in Solna, Stockholm, Sweden (n = 184), were included in a prospective study.
All patients provided written informed consent. The study was approved by the regional ethics committee in Stockholm, Sweden.
Specimen collection
Nasopharyngeal secretions, sputum, and blood were obtained for bacterial culture before start of antibiotic therapy in the emergency department. Because it is often difficult to obtain good quality sputum samples in the emergency department, induced sputum specimens were obtained with the assistance of a respiratory physiotherapist on the ward [21].
Nasopharyngeal secretion samples for bacterial and viral detection and urine samples for pneumococcal and Legionella pneumophila antigen detection were obtained 1 day after hospital admission. Blood samples were obtained 1 day after hospital admission and 4 weeks later for serological analysis of influenza virus, parainfluenza virus, adenovirus, respiratory syncytial virus (RSV), Mycoplasma pneumoniae, and Chlamy-dophila pneumoniae.
Microbiological methods
With regard to bacteriological methods, all sputum samples were examined by microscopy, and samples containing a preponderance of leukocytes and a few squamous epithelial cells were considered acceptable for culture, according to accepted criteria [22]. When reading the culture plates, we tried especially to recover and identify the bacteria indicated in the purulent parts of the slides. The number of colony-forming units per milliliter (cfu/mL) was assessed by use of a semiquantitative technique [20, 23]. Quantitative cultures of sputum samples and qualitative cultures of nasopharyngeal samples as well as blood samples were performed in accordance with accepted methods and criteria.
Real-time quantitative PCR (RQ-PCR) targeting the pneumolysin (ply) gene of Streptococcus pneumoniae, the fumarate reductase (frdB) gene of Haemophilus influenzae, and the outer membrane protein (copB) gene of Moraxella catarrhalis was performed as described by Kais et al [20]. Respiratory tract samples were also examined for L. pneumophila by use of culture and/or PCR and for M. pneumoniae and C. pneumoniae by use of PCR [13].
Immunochromatographic membrane tests (BinaxNOW S. pneumoniae and BinaxNOW Legionella; Inverness Medical Innovations) were performed on urine samples, for detection of pneumococcal and L. pneumophila antigens. Commercially available enzyme-linked immunosorbent assays (Ani Labsystems) were used according to the manufacturer's instructions for the detection of immunoglobulin G (IgG) and IgM antibodies to M. pneumoniae and C. pneumoniae.
With regard to virological methods, virus isolation was performed on all nasopharyngeal secretion samples in accordance with diagnostic practice [24]. The detection of adenovirus, enterovirus, human bocavirus, human coronaviruses 229E, HKU1, NL63, and OC43, human metapneumovirus, influenza viruses A and B, parainfluenza viruses 1–3, rhinovirus, and RSV (A and B) was performed retrospectively by a newly developed real-time PCR platform [19]. Three reactions were duplex assays, parainfluenza virus 1 and 3, parainfluenza virus 2, and human coronavirus 229E, and RSV A and RSV B (although only reported as RSV). All other reactions were performed as single-agent assays. For serology, an in-house enzyme-linked immunosorbent assay was used for the detection of IgG antibodies to adenovirus (using adenovirus type 2 antigen considered to react with all adenoviruses), influenza A and B viruses, parainfluenza 1, 2, and 3 viruses and RSV [25, 26].
Diagnostic criteria
A microorganism was considered to be of definite etiological significance if it was cultured from blood, pleural fluid, a protected specimen brush (cutoff, ⩾103 cfu/mL protected specimen brush broth), or bronchoalveolar lavage (cutoff, ⩾104 cfu/mL bronchoalveolar lavage fluid), or if the urine antigen assay for S. pneumoniae or L. pneumophila was positive. Detection of L. pneumophila by culture and/or PCR from sputum was also considered as definite support for the etiology.
In accordance with our previous findings of patients with bacteremic pneumococcal pneumonia, identification of ⩾105 cfu/mL of S. pneumoniae in sputum culture or nasopharyngeal culture was accepted as of probable significance [23, 27, 28]. Pneumococcal DNA corresponding to ⩾105 cfu/mL by use of RQ-PCR was also considered to be of probable significance [20]. For other bacteria, identification of ⩾106 cfu/mL in sputum culture [29] or DNA corresponding to ⩾106 cfu/mL by use of RQ-PCR for H. influenzae and M. catarrhalis were considered to be of probable significance. The identification of M. pneumoniae and C. pneumoniae by use of PCR, the presence of IgM antibodies, a 2-fold increase in IgG between acute and convalescent phase samples were all considered to be support for a probable microbial diagnosis. A viral etiology was deemed probable if at least one of the following criteria was met: detection of a virus in a respiratory secretion sample by use of isolation or PCR, or a 10-fold increase in IgG titer between acute and convalescent phase samples.
Results
Patients' characteristics
The baseline characteristics of the patients are shown in Table 1. The study population consisted of 94 males and 90 females. The mean age was 61.3 years (range, 18–93 years). A total of 74 patients had at least 1 underlying disease, with malignancy as the most frequent (19%). The average stay in the hospital was 7.1 days (range, 1–69 days). At hospital admission, 40 patients (22%) had already been started on antibiotic therapy.
Microbiological etiology of CAP
A definite or probable microbial etiology of CAP was established for 124 (67%) of the 184 patients when RQ-PCR assays for S. pneumoniae, H. influenzae, and M. catarrhalis from sputum samples and the PCR platform for respiratory viruses were added to the conventional methods. In contrast, the microbial yield was 60% (110 of 184 patients) when only conventional methods were used.
A bacterial etiology was found for 106 patients (58%), 9 of whom had more than 1 bacterial species identified (Table 2). The most frequently detected bacteria were S. pneumoniae (70 patients [38]), M. pneumoniae (15 patients [8]), H. influenzae (9 patients [5]), and M. catarrhalis (7 patients [4]). A viral pathogen was identified for 53 (29%) of the 184 patients; 1 patient had 2 viral findings (Table 3). The most common viral findings were influenza virus (14 patients [8]), rhinovirus (12 patients [7]), RSV (7 patients [4]), and parainfluenza virus (7 patients [4]).
Table 2.
Bacterial Yield in the Study Population and the Contribution of Different Methods to the Determination of Etiology with Respect to Their Different Specificity
| Pathogen | No. (%) of patients with positive findings (n = 184) | Blood culture (n = 179) | Pleural fluid culture (n = 13) | Urine antigen assaya | BAL and/or protected specimen brush culture (n = 12) | Culture and/or PCR from sputum sample for L. pneumophila (n = 138) | Culture and PCR from respiratory sample for M. tuberculosis (n = 18) | Sputum culture (n = 128) | RQ-PCR from sputum sample (n = 126) | Nasopharyngeal secretion culture (n = 158) | PCR from nasopharyngeal secretion sampleb | Serology (n = 131) |
| Streptococcus pneumoniae | 70 (38) | 27 | … | 16 | … | … | … | 10 | 10 | 7 | … | … |
| Mycoplasma pneumoniae | 15 (8) | … | … | … | … | … | … | … | … | … | 8 | 7 |
| Haemophilus influenzae | 9 (5) | … | … | … | … | … | … | 4 | 5 | … | … | … |
| Moraxella catarrhalis | 7 (4) | … | … | … | … | … | … | 7 | … | … | … | … |
| Staphylococcus aureus | 4 (2) | 2 | 1 | … | … | … | … | 1 | … | … | … | … |
| Legionella pneumophila | 3 (1) | … | … | 2 | … | 1 | … | … | … | … | … | … |
| Streptococcus pyogenes | 2 (1) | 1 | … | … | 1 | … | … | … | … | … | … | … |
| Streptococcus milleri | 1 (0.5) | … | 1 | … | … | … | … | … | … | … | … | … |
| Nocardia cyriacigeorgica | 1 (0.5) | … | … | … | … | 1 | … | … | … | … | … | … |
| Fusobacterium necrophorum | 1 (0.5) | 1 | … | … | … | … | … | … | … | … | … | … |
| Mycobacterium tuberculosis | 2 (1) | … | … | … | … | … | 2 | … | … | … | … | … |
| Total | 115 | 31 | 2 | 18 | 1 | 2 | 2 | 22 | 15 | 7 | 8 | 7 |
NOTE. Date are no. of patients who received a diagnosis by use of a particular method, unless otherwise indicated. Additional patients received a diagnosis by use of different methods; for example, an additional 16 cases of S. pneumoniae infection were diagnosed by a urinary antigen test that were not diagnosed by blood culture, and another 10 cases were diagnosed by sputum culture that were not diagnosed by blood culture or a urinary antigen test. BAL, bronchoalveolar lavage; RQ-PCR, real-time quantitative polymerase chain reaction.
There were 168 patients who tested positive for L. pneumophila and 169 patients who tested positive for S. pneumoniae.
The were 99 patients who tested positive for Chlamydophila pneumoniae and 101 patients who tested positive for Mycoplasma pneumoniae.
Table 3.
Viral Yield in the Study Population and the Contribution of Different Methods to the Determination of Etiology with Respect to Their Different Specificity
| Pathogen | No. (%) patients with positive findings (n = 184) | Nasopharyngeal secretion culture (n = 157) | Serology (n = 131) | PCR from nasopharyngeal secretion sample (n = 156) |
| Influenza virus | 14 (8) | 3 | 7 | 4 |
| Rhinovirus | 12 (7) | … | … | 12 |
| Respiratory syncytial virus | 7 (4) | 1 | 5 | 1 |
| Parainfluenza virus | 7 (4) | 1 | 5 | 1 |
| Coronavirus | 4 (2) | … | … | 4 |
| Metapneumovirus | 4 (2) | 1 | … | 3 |
| Adenovirus | 3 (2) | … | 3 | |
| Herpes simplex 1 virus | 2 (1) | 2 | … | … |
| Enterovirus | 1 (0.5) | … | … | 1 |
| Total | 54 (29) | 8 | 20 | 26 |
NOTE. Date are no. of patients who received a diagnosis by use of a particular method, unless otherwise indicated. Additional patients received a diagnosis by use of different methods; for example, an additional 7 cases of influenza virus were diagnosed by serology that were not diagnosed by virus isolation, and another 4 cases were diagnosed by polymerase chain reaction (PCR) from nasopharyngeal secretion samples that were not diagnosed by virus isolation or serology.
Diagnostic yield with different bacteriological diagnostic methods
The contribution of the different methods to the determination of etiology is illustrated in Tables 2 and 3. Blood cultures provided a microbial diagnosis for 31 (17%) of 179 patients. S. pneumoniae was detected by the urinary antigen assay for 33 of 169 patients tested. Seventeen of these patients were also diagnosed by use of blood culture, whereas 16 patients were diagnosed by the use of a urinary antigen test alone (Table 2). A probable diagnosis was established with sputum culture for 33 (26%) of 128 patients, which increased the diagnostic yield by an additional 22 cases. Positive findings with sputum RQ-PCR for S. pneumoniae, H. influenzae, and M. catarrhalis were obtained for 42 (33%) of 126 patients (1 patient tested positive for both S. pneumoniae and H. influenzae), which increased the diagnostic yield by an additional 15 cases. S. pneumoniae was found in the nasopharyngeal secretion samples of 42 (27%) of 158 patients, which increased the microbial diagnostic yield (as well as the diagnostic yield of other methods) by only an additional 7 cases.
Diagnostic yield with different viral diagnostic methods
Eight cases were detected by viral isolation. Serology accounted for 21 positive findings, in 1 case for both the influenza and the parainfluenza virus. The PCR results of the nasopharyngeal secretion samples of 35 patients were positive, and 5 of these patients also had serum samples that tested positive. Influenza virus was the most common finding (ie, 14 [8] of 184 patients) (Table 3). Nine different viral agents were identified.
Mixed infections
Conventional diagnostic methods identified 2 potential pathogens for 20 (11%) of the 184 patients and 3 pathogens for 2 patients (1%). PCR testing for S. pneumoniae, H. influenzae, M. catarrhalis, and respiratory viruses increased the total diagnostic yield to 42 (23%) and 4 (2%) patients with 2 and 3 pathogens, respectively. Of the 106 patients with a definite or probable bacterial etiology, 42 (40%) presented with mixed infections. When S. pneumoniae was identified, a copathogen was found in the samples of 34 of 70 patients: 6 bacterial pathogens (3 of which were isolates of H. influenzae) and 29 viral agents (7 of which were isolates of influenza virus and 7 of which were isolates of rhinovirus). Of the 53 patients with documented viral findings, two-thirds (ie, 35 patients) had at least 1 additional pathogen identified, of whom 30 (86%) had S. pneumoniae isolated.
Microbiological yield with different methods among patients with complete sample collection
A total of 38 patients had complete samples collected: blood, sputum, and nasopharyngeal secretion samples for culture; sputum samples analyzed by RQ-PCR for S. pneumoniae, H. influenzae, and M. catar-rhalis; nasopharyngeal specimens analyzed by use of PCR; serological testing for M. pneumoniae, C. pneumoniae, and viruses common in the respiratory tract; and urine antigen assays for detection of pneumococcal and L. pneumophila antigens. More over, none of these patients had been given antibiotics before admission to the hospital (Figure 1).
Figure 1.
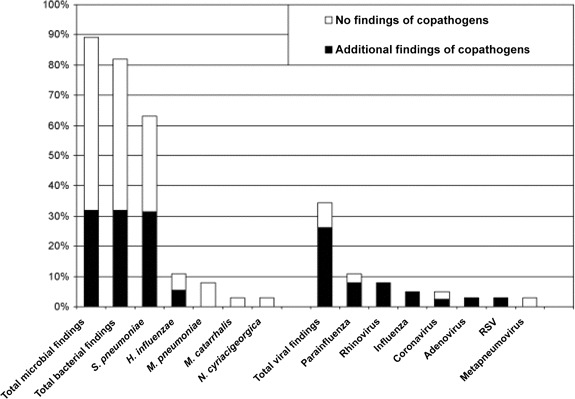
Percentage of patients with complete samples collected (n = 38) whose case of infection was etiologically determined and percentage of mixed infections. H. influenzae, Haemophilus influenzae; M. catarrhalis, Moraxella catarrhalis; M. pneumoniae, Mycoplasma pneumoniae; N. cyriacigeorgica, Nocardia cyriacigeorgica; RSV, respiratory syncytial virus; S. pneumoniae, Streptococcus pneumoniae.
According to our diagnostic criteria, a microbial etiology was established for 34 (89%) of the 38 patients. Multiple pathogens were detected in the samples of 12 patients (32%). There were 31 patients (82%) who received a diagnosis of bacterial infection. For 24 patients (63%), S. pneumoniae was the most common bacterial finding, and a second microbial agent was found for 12 (50%) of the 24 patients; of these 12 patients, 10 (83%) had a respiratory virus infection. A total of 13 (34%) of the 38 patients received a diagnosis of viral infection. For 10 (77%) of these 13 patients, a bacterial pathogen (predominantly S. pneumoniae) was also found.
Treatment in intensive care unit and mortality
Of the 184 patients, 11 (6%) were treated in the intensive care unit. An etiological agent was found in the samples of 10 of these 11 patients, and multiple pathogens were detected in the samples of 5 of these 10 patients. Eight patients had S. pneumoniae infection. For 4 (50%) of these 8 patients, at least 1 viral agent was also detected. From 1 patient's sample, Streptococcus pyogenes was found together with metapneumovirus; from another patient's sample, M. pneumoniae was identified. The overall case fatality rate was 3.8% (ie, 7 of 184 patients died). Microbial infection was diagnosed for 3 of the patients who died; S. pneumoniae and RSV was found in the samples of the first patient who died, S. pyogenes and metapneumovirus in the samples of the second patient who died, and M. catarrhalis in the samples of the third patient who died.
Discussion
Three major findings are reported in this study. First, the total microbial yield was high. The yield improved with the implementation of PCR for respiratory virus and RQ-PCR for S. pneumoniae, H. influenzae, and M. catarrhalis. Second, S. pneumoniae was the leading causative agent. Third, respiratory viruses were found most often and at a high frequency as part of a mixed infection, usually in combination with S. pneumoniae.
Establishing a microbial diagnosis for patients with CAP is challenging. Although many etiological studies have been performed, the etiology most often remains unknown in approximately one-half of the cases [2, 3, 30]. In the present study, at least 1 etiological agent was found in two-thirds of all cases, and the yield increased to ∼90% for patients with complete samples collected and no antibiotics given prior to hospital admission. The yield improved by adding to the conventional procedures RQ-PCR testing of sputum samples for S. pneumoniae, H. influenzae, and M. catarrhalis and real-time PCR testing of nasopharyngeal secretion samples for 15 respiratory viruses.
Blood samples for culture are easy to collect and provide a definite microbial diagnosis when the results are positive, but in the present study, they revealed the etiology of infection for only 17% of all patients, a number similar to previous studies [30, 31]. Urine specimens are also easy to obtain, and the pneumococcal and L. pneumophila antigen assays are generally considered specific. However, these tests provided a diagnosis for only an additional 11% of patients (ie, nonbacteremic cases). A pneumococcal antigen assay was performed for 65 patients with a pneumococcal infection; 51% of the specimens were positive, 65% from bacteremic patients and 40% from nonbacteremic patients, and no more than one-third (13 of 37 patients) tested positive when the sputum samples positive for S. pneumoniae were studied alone. Our results indicate that the sensitivity of the pneumococcal urine antigen assay may be lower than recently reported [32]. Sputum samples for both culture and RQ-PCR were obtained from ∼70% of all patients. Sputum samples for culture provided a probable etiology for one-fourth of the patients, and sputum samples for RQ-PCR provided a probable etiology for one-third of the patients. Furthermore, for patients with negative test results by culture from blood, sputum, or urine antigen assays, RQ-PCR provided a probable microbial diagnosis in 12% of the cases, confirming that this method is a valuable complement to conventional methods. The ply gene used for the RQ-PCR has recently been identified in Streptococcus mitis as well [33], and thus the specificity of our analysis may be questioned. However, our analysis was quantitative, and any amount of DNA from S. mitis would probably be low. Moreover, our previous data support the good specificity of the negative results from throat samples [20] and the positive findings determined by use of other diagnostic methods [21]. However, in future studies, it will be important to take into account the choice of primers.
S. pneumoniae was the most common microorganism found (38% of patients). For approximately two-thirds of the patients with complete diagnostic samples collected, there was support for a pneumococcal etiology. These findings support earlier suggestions that pneumococci cause a majority of CAP cases in which negative test results were found using conventional methods [11, 30, 34]. Viruses are the most common pathogens found in the samples obtained from young children with CAP, accounting for 14%–35% of the cases [35]. In contrast, a viral etiology has previously been reported for ∼10% of adult patients with CAP admitted to hospital [30]. However, in some recent publications, viral agents have been recognized as a more common cause of CAP among adults [1, 5, 8, 11, 36]. The results in the present study corroborate these findings, with support for a viral etiology for one-third of the patients.
The new real-time PCR platform provided a viral diagnosis for 35 (22%) of the 156 patients tested, and the addition of this method to the conventional microbial techniques improved the yield by nearly 50%. These results are in agreement with previous findings by Oosterheert et al [37] and indicate that PCR is not only more rapid than virus isolation and serology, but also more sensitive. The role that viruses play in causing CAP has not been fully elucidated. In particular, the possibility that the rhinovirus may cause CAP is still considered controversial. However, recent studies have established that rhinovirus replication can occur in the lower airways and that it can infect human respiratory epithelial cell lines in vitro, inducing the release of proinflammatory cytokines and chemokines [10, 38]. In some clinical reports, rhinovirus has also been associated with lower respiratory tract disease (including pneumonia) among adults [8, 38].
In a recent review on the prevalence of respiratory viral findings for asymptomatic subjects, definitive conclusions could not been drawn because of the lack of prospective studies [39]. However, available reports suggest that the prevalence is infrequent (⩽5%) overall and that the persistence of viral nucleic acids is rather short lasting, indicating that most of the viral findings in the present study have a clinical relevance.
Mixed infections among patients admitted to the hospital with CAP have previously been reported to account for 5%–11% of cases of CAP [1, 6, 30], which is comparable to the 11% of cases found in the present study if only conventional methods were considered. However, when all PCR results were added, mixed infections were found in the samples of one-third of the 124 patients with a determined etiology as well as in the samples of those patients with complete sampling.
Our data are in agreement with those from a recent study by Lim et al [11]. They found a second pathogen in the samples of 47% of the patients with a pneumococcal infection, and for 60% of these patients, it was a viral agent. In our study, S. pneumoniae was the most common bacterial agent in mixed infections, and a copathogen was found in one-half of the cases, with the majority of pathogens (ie, 83%–85%) being viral agents. Thus, viral agents in adults with CAP most often seem to be part of a mixed infection, usually with S. pneumoniae as the copathogen. Also, for patients with severe pneumonia, S. pneumoniae was the predominant pathogen, and the copathogen was frequently found to be a viral agent.
There were several limitations to our study. First, not all patients who met the criteria for inclusion were enrolled in the study. Second, the sample collection was not complete. These issues are inherent in all trials enrolling patients with CAP. We have no reason to believe that the group of patients who were not included would have been substantially different from the group of patients that we studied. Also, samples to be obtained at the emergency department were decided by the physician on call, and the patients on the ward were later included in the study by the investigators. Selection bias in the collection of samples is possible but unlikely.
In summary, by supplementing traditional diagnostic methods with new PCR-based techniques for both the most common bacteria and a number of respiratory viral agents, a higher microbial yield was achieved. S. pneumoniae was the leading causative agent, accounting for more than one-half of the cases with a determined etiology. Mixed infections were frequent, with the combination of S. pneumoniae and a respiratory virus being the most common finding.
Table 1.
Baseline Characteristics of 184 Patients with Community-Acquired Pneumonia Admitted to Hospital
| Characteristic | Patients |
| Mean age, years | 61.3 |
| Male | 94 (51) |
| Female | 90 (49) |
| Any comorbidity | 74 (40) |
| COPD and/or chronic bronchitis | 21 (11) |
| Heart failure | 12 (7) |
| Cerebrovascular disease | 16 (9) |
| Malignancy | 35 (19) |
| Liver disease | 7 (4) |
| Renal failure | 0 (0) |
| Antibiotic use prior to hospital admission | 40 (22) |
NOTE. Data are no. (%) of patients, unless otherwise indicated. COPD, chronic obstructive pulmonary disease.
Acknowledgments
We thank Dr Carl Spindler for the contribution of data regarding the sputum RQ-PCR results and Dr Rima Dandachi for her help with the data compilation. We also thank the respiratory physiotherapists Susanna Wennman and Annette Idengren for their help with the collection of sputum samples.
Financial support. The study was supported by grants from Karolinska Institutet.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.File TM. Community-acquired pneumonia. Lancet. 2003;362(9400):1991–2001. doi: 10.1016/S0140-6736(03)15021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiz M, Ewig S, Marcos MA, et al. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160(2):397–405. doi: 10.1164/ajrccm.160.2.9808045. [DOI] [PubMed] [Google Scholar]
- 3.Jokinen C, Heiskanen L, Juvonen H, et al. Microbial etiology of community-acquired pneumonia in the adult population of 4 municipalities in eastern Finland. Clin Infect Dis. 2001;32(8):1141–1154. doi: 10.1086/319746. [DOI] [PubMed] [Google Scholar]
- 4.Samransamruajkit R, Hiranrat T, Chieochansin T, et al. Prevalence, clinical presentations and complications among hospitalized children with influenza pneumonia. Jpn J Infect Dis. 2008;61(6):446–449. [PubMed] [Google Scholar]
- 5.de Roux A, Marcos MA, Garcia E, et al. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125(4):1343–1351. doi: 10.1378/chest.125.4.1343. [DOI] [PubMed] [Google Scholar]
- 6.Angeles Marcos M, Camps M, Pumarola T, et al. The role of viruses in the aetiology of community-acquired pneumonia in adults. Antivir Ther. 2006;11(3):351–359. [PubMed] [Google Scholar]
- 7.Diaz A, Barria P, Niederman M, et al. Etiology of community-acquired pneumonia in hospitalized patients in chile: the increasing prevalence of respiratory viruses among classic pathogens. Chest. 2007;131(3):779–787. doi: 10.1378/chest.06-1800. [DOI] [PubMed] [Google Scholar]
- 8.Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63(1):42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 9.Panuska JR, Cirino NM, Midulla F, Despot JE, McFadden ER, Jr, Huang YT. Productive infection of isolated human alveolar macrophages by respiratory syncytial virus. J Clin Invest. 1990;86(1):113–119. doi: 10.1172/JCI114672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181(6):1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 11.Lim WS, Macfarlane JT, Boswell TC, et al. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax. 2001;56(4):296–301. doi: 10.1136/thorax.56.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhi SA, Ludewick H, Kuwanda L, et al. Pneumococcal coinfection with human metapneumovirus. J Infect Dis. 2006;193(9):1236–1243. doi: 10.1086/503053. [DOI] [PubMed] [Google Scholar]
- 13.Welti M, Jaton K, Altwegg M, Sahli R, Wenger A, Bille J. Development of a multiplex real-time quantitative PCR assay to detect Chlamydia pneumoniae, Legionella pneumophila and Mycoplasma pneumoniae in respiratory tract secretions. Diagn Microbiol Infect Dis. 2003;45(2):85–95. doi: 10.1016/s0732-8893(02)00484-4. [DOI] [PubMed] [Google Scholar]
- 14.Gunson RN, Collins TC, Carman WF. Real-time RT-PCR detection of 12 respiratory viral infections in four triplex reactions. J Clin Virol. 2005;33(4):341–344. doi: 10.1016/j.jcv.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunson RN, Collins TC, Carman WF. Practical experience of high throughput real time PCR in the routine diagnostic virology setting. J Clin Virol. 2006;35(4):355–367. doi: 10.1016/j.jcv.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza a and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004;42(4):1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watzinger F, Suda M, Preuner S, et al. Real-time quantitative PCR assays for detection and monitoring of pathogenic human viruses in immunosuppressed pediatric patients. J Clin Microbiol. 2004;42(11):5189–5198. doi: 10.1128/JCM.42.11.5189-5198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Pol AC, van Loon AM, Wolfs TF, et al. Increased detection of respiratory syncytial virus, influenza viruses, parainfluenza viruses, and adenoviruses with real-time PCR in samples from patients with respiratory symptoms. J Clin Microbiol. 2007;45(7):2260–2262. doi: 10.1128/JCM.00848-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiveljung-Lindell A, Rotzen-Ostlund M, Gupta S, et al. Development and implementation of a molecular diagnostic platform for daily rapid detection of 15 respiratory viruses. J Med Virol. 2009;81(1):167–175. doi: 10.1002/jmv.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kais M, Spindler C, Kalin M, Ortqvist A, Giske CG. Quantitative detection of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in lower respiratory tract samples by real-time PCR. Diagn Microbiol Infect Dis. 2006;55(3):169–178. doi: 10.1016/j.diagmicrobio.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Johansson N, Kalin M, Giske CG, Hedlund J. Quantitative detection of Streptococcus pneumoniae from sputum samples with real-time quantitative polymerase chain reaction for etiologic diagnosis of community-acquired pneumonia. Diagn Microbiol Infect Dis. 2008;60(3):255–261. doi: 10.1016/j.diagmicrobio.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis. 2000;31(2):347–382. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalin M, Lindberg AA. Diagnosis of pneumococcal pneumonia: a comparison between microscopic examination of expectorate, antigen detection and cultural procedures. Scand J Infect Dis. 1983;15(3):247–255. doi: 10.3109/inf.1983.15.issue-3.04. [DOI] [PubMed] [Google Scholar]
- 24.Ostlund MR, Wirgart BZ, Linde A, Grillner L. Respiratory virus infections in Stockholm during seven seasons: a retrospective study of laboratory diagnosis. Scand J Infect Dis. 2004;36(6–7):460–465. doi: 10.1080/00365540410015295. [DOI] [PubMed] [Google Scholar]
- 25.Grillner L. Screening of blood donors for cytomegalovirus(CMV) antibodies: an evaluation of different tests. J Virol Methods. 1987;17(12):133–139. doi: 10.1016/0166-0934(87)90076-0. [DOI] [PubMed] [Google Scholar]
- 26.Bidwell DE, Bartlett A, Voller A. Enzyme immunoassays for viral diseases. J Infect Dis. 1977;136:S274–S278. doi: 10.1093/infdis/136.supplement_2.s274. [DOI] [PubMed] [Google Scholar]
- 27.Hedlund J, Ortqvist A, Kalin M. Nasopharyngeal culture in the pneumonia diagnosis. Infection. 1990;18(5):283–285. doi: 10.1007/BF01647005. [DOI] [PubMed] [Google Scholar]
- 28.Kalin M. Bacteremic pneumococcal pneumonia: value of culture of nasopharyngeal specimens and examination of washed sputum specimens. Eur J Clin Microbiol. 1982;1(6):394–396. doi: 10.1007/BF02019941. [DOI] [PubMed] [Google Scholar]
- 29.Bartlett JG, Finegold SM. Bacteriology of expectorated sputum with quantitative culture and wash technique compared to transtracheal aspirates. Am Rev Respir Dis. 1978;117(6):1019–1027. doi: 10.1164/arrd.1978.117.6.1019. [DOI] [PubMed] [Google Scholar]
- 30.BTS guidelines for the management of community acquired pneumonia in adults. Thorax. 2001;56(Suppl 4):IV1–64. doi: 10.1136/thorax.56.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodhead MA, Macfarlane JT, McCracken JS, Rose DH, Finch RG. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet. 1987;1(8534):671–674. doi: 10.1016/s0140-6736(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 32.Marcos MA, Jimenez de Anta MT, de la Bellacasa JP, et al. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur Respir J. 2003;21(2):209–214. doi: 10.1183/09031936.03.00058802. [DOI] [PubMed] [Google Scholar]
- 33.Abdeldaim G, Herrmann B, Korsgaard J, Olcen P, Blomberg J, Stralin K. Is quantitative PCR for the pneumolysin (ply) gene useful for detection of pneumococcal lower respiratory tract infection? Clin Microbiol Infect. 2009;15(6):565–570. doi: 10.1111/j.1469-0691.2009.02714.x. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz-Gonzalez A, Falguera M, Nogues A, Rubio-Caballero M. Is Streptococcus pneumoniae the leading cause of pneumonia of unknown etiology? A microbiologic study of lung aspirates in consecutive patients with community-acquired pneumonia. Am J Med. 1999;106(4):385–390. doi: 10.1016/s0002-9343(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 35.British Thoracic Society guidelines for the management of community acquired pneumonia in childhood. Thorax. 2002;57(Suppl 1):i1–i24. doi: 10.1136/thorax.57.90001.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howard LS, Sillis M, Pasteur MC, Kamath AV, Harrison BD. Microbiological profile of community-acquired pneumonia in adults over the last 20 years. J Infect. 2005;50(2):107–113. doi: 10.1016/j.jinf.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Oosterheert JJ, van Loon AM, Schuurman R, et al. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41(10):1438–1444. doi: 10.1086/497134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol. 2004;14(1):17–31. doi: 10.1002/rmv.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jartti T, Jartti L, Peltola V, Waris M, Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J. 2008;27(12):1103–1107. doi: 10.1097/INF.0b013e31817e695d. [DOI] [PubMed] [Google Scholar]


