Abstract
Acute otitis media and acute bacterial sinusitis are 2 of the most common indications for antimicrobial agents in children. Together, they are responsible for billions of dollars of health care expenditures. The pathogenesis of the 2 conditions is identical. In the majority of children with each condition, a preceding viral upper respiratory tract infection predisposes to the development of the acute bacterial complication. It has been shown that viral upper respiratory tract infection predisposes to the development of acute otitis media in 37% of cases. Currently, precise microbiologic diagnosis of acute otitis media and acute bacterial sinusitis requires performance of tympanocentesis in the former and sinus aspiration in the latter. The identification of a virus from the nasopharynx in either case does not obviate the need for antimicrobial therapy. Furthermore, nasal and nasopharyngeal swabs are not useful in predicting the results of culture of the middle ear or paranasal sinus. However, it is possible that a combination of information regarding nasopharyngeal colonization with bacteria and infection with specific viruses may inform treatment decisions in the future.
Acute otitis media (AOM) is the most common indication for the use of antimicrobial agents in children [1]. Acute bacterial sinusitis is the fifth most common indication for antibiotics [2]. Together, they are responsible for billions of dollars of health care expenditures.
AOM
Pathogenesis
The pathogenesis of AOM is directly related to a preceding viral infection that leads to impairment of the mucociliary apparatus and Eustachian tube dysfunction in very young children [3]. The peak age incidence for AOM is 3–24 months, which coincides with the peak incidence of community-acquired viral infections in children [4].
An elegant study performed by Chonmaitree et al revealed the usual sequence of events in children with viral upper respiratory tract infection (URI) [4]. Although similar investigations have been performed, the present study is representative and the most comprehensive. The aim was to study the incidence of AOM in children with viral URI. Infants 6–36 months of age were studied prospectively for 1 year. These otherwise healthy infants were examined as soon as possible after symptoms of a new URI developed. A combination of viral culture and molecular methods were used to identify the viruses causing the URI. A nasopharyngeal swab sample was obtained to determine the frequency of bacterial colonization with the usual pathogens associated with AOM.
A total of 1295 URI episodes were documented; a respiratory virus was identified in 63% of cases. Table 1 shows the respiratory viruses detected during 864 episodes of URI. Adenovirus and rhinoviruses were the most frequently detected viruses during the study period.
Table 1.
Respiratory Viruses Detected During 864 Episodes of Upper Respiratory Tract Infection (URI)
| Virus | Percentage of episodes |
| Adenovirus | 29 |
| Rhinovirus | 25 |
| Enterovirus | 18 |
| Coronavirus | 9 |
| Parainfluenza | 8 |
| RSV | 6 |
| Influenza A and B | 5 |
Overall, 37% of the episodes of URI were complicated by the development of AOM. Figure 1 depicts the rate of AOM and otitis media with effusion (OME), by virus, for all virus detection methods combined. For example, for adenovirus, 70% of cases were associated with the development of fluid in the middle ear; 45% were AOM and 25% were OME. Coronaviruses and respiratory syncytial virus (RSV) were most likely to be associated with the development of AOM. Of particular interest, AOM was more likely to develop when the virus was isolated by culture rather than by polymerase chain reaction (PCR), reflecting the higher inoculum of virus required for positive growth.
Figure 1.

Rate of acute otitis media and otitis media with effusion, by virus, for all detection methods.
Other groups of investigators have studied this same question in different locations and in different years. In a similar study conducted in Finland, rhinovirus was the most frequently detected by PCR in 63% of episodes, followed by RSV, influenza A, parainfluenza 3, and adenovirus [5]. Forty-five percent of cases of rhinovirus infection and almost 60% of cases of RSV infection were associated with AOM. In a longitudinal study of 102 families from Pittsburgh, Pennsylvania, and Charlottesville, Virginia, the most frequently recovered viruses were rhinovirus, RSV, coronavirus, adenovirus, and influenza A [6]. These authors did not discriminate between episodes of AOM and OME.
The other important component of the susceptibility to the development of AOM, in the context of viral URI, is nasopharyngeal colonization with those bacterial pathogens associated with AOM, specifically Streptococcus pneumoniae, nontypeable Haemophilus influenzae, and Moraxella catarrhalis. Figure 2 shows the recovery of these pathogens from the nasopharynx at the onset of a new cold [7]. Only 14% of children did not have recovery of a middle ear pathogen from the nasopharynx. In some cases, >1 otopathogen was isolated. Figure 3 shows the risk of AOM after viral URI by nasopharygeal colonization status [8]. When a single pathogen is isolated, there is a 30% incidence of AOM; when 3 otopathogens are recovered, there is a 50% incidence of AOM. The greater number of otopathogens found colonizing the nasopharynx, the greater the risk of developing AOM [7].
Figure 2.
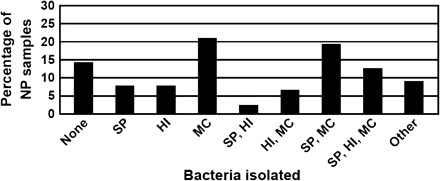
Recovery of middle ear pathogens from nasopharynx at onset of cold.
Figure 3.
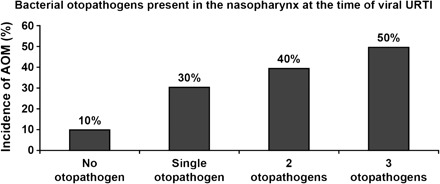
Risk of acute otitis media versus nasopharyngeal pathogens.
Clinical diagnosis
Current methods for the diagnosis of AOM rely entirely on the performance of accurate otoscopy [1]. The most effective strategy for limiting the use of antibiotics is to improve diagnostic accuracy and the ability to differentiate AOM from OME [9]. Of the 2 conditions, OME is more common, occurring both before and after AOM and also occurring without ever progressing to AOM [10]. The middle ear fluid in children with OME is sterile. OME is a nonbacterial inflammatory state that resolves spontaneously over time. The principal importance of OME is as a cause of hearing problems in young children and as a confounder in the diagnosis of AOM [10]. Antibiotics are neither appropriate nor beneficial in children with OME [11]. In contrast, in children with AOM, the probability of bacterial infection is very high, thereby enhancing the likelihood of a benefit from antibiotics.
How are OME and AOM distinguished? Middle ear effusion is common to both. To distinguish between OME and AOM, the tympanic membrane must be examined for signs of acute inflammation. The single most powerful sign of AOM is the presence of distinct fullness or bulging of the tympanic membrane [12]. Although adjunctive techniques, such as tympanometry and acoustic reflectometry, can confirm the presence of middle ear effusion, neither technique can distinguish between OME and AOM.
The most common and important error in diagnosis occurs when the clinician detects the presence of middle ear effusion and then uses a nonspecific marker of infection to classify the episode as AOM. Accordingly, middle ear effusion accompanied by fever, anorexia, nausea, irritability, and vomiting does not equal a diagnosis of AOM [9].
Figure 4 is an example of a normal tympanic membrane that is pearly gray in color, translucent, in normal position, and with clarity of the bony landmarks. Figures 5 and 6 are excellent examples of AOM demonstrating a bulging tympanic membrane. Figure 7 shows how a tympanocentesis is performed. The middle ear fluid is collected in the syringe or Senturia trap and sent to the laboratory for gram stain and culture [13].
Figure 4.

Normal tympanic membrane.
Figure 5.
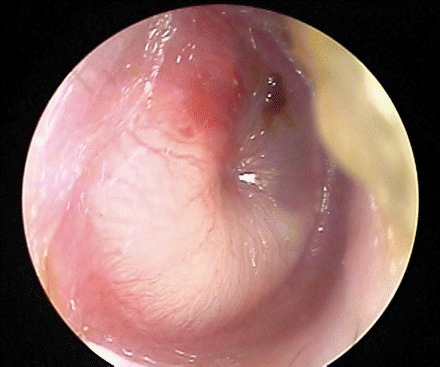
Bulging tympanic membrane in a case of acute otitis media.
Figure 6.

Bulging tympanic membrane in a case of acute otitis media.
Figure 7.

Tympanocentesis can be performed by using a needle attached to a tuberculin syringe (left) or by using an alden-senturia trap (Storz Instrument) with a needle attached (right).
Microbiology.
Table 2 shows the current microbiologic characteristics of AOM [14−16]. S. pneumoniae now accounts for 35%–40% of isolates and nontypeable H. influenzae for 30%–35% of middle ear isolates. Licensure of the 7-valent pneumococcal conjugate vaccine in 2000 led to a temporary decrease in the prevalence of S. pneumoniae infection and a relative increase in H. influenzae infection. However, the emergence of the nonvaccine pneumococcal serotype 19A has reversed this trend [16]. M. catarrhalis is responsible for 15% of cases. In the postpneumococcal vaccine era, the prevalence of penicillin-resistant S. pneumoniae is quite variable; 35%–45% of H. influenzae strains are β-lactamase producing, and nearly 100% of the M. catarrhalis strains are β-lactamase producing [15].
Table 2.
Microbiologic Characteristics of Acute Otitis Media
| Bacterial species | Percentage of strains |
| Streptococcus pneumoniae | 35–40 |
| Haemophilus influenzae | 30–35 |
| Moraxella catarrhalis | 15–18 |
| Streptococcus pyogenes | 2–4 |
| Sterile | 20 |
Usefulness of Viral Diagnostics
Finding a virus in the nasopharynx or middle ear fluid of an individual child with AOM (using a commercially available multiplex reverse-transcriptase PCR assay) will not obviate the need for antibiotics, because it is understood that respiratory viruses are important in the pathogenesis of AOM. Antibiotics are appropriate if a diagnosis of AOM is made. However, epidemiologic studies that describe the frequency of recovery of various viruses can direct vaccine development. The frequency of AOM can be modulated by the prevention of viral URI. The best example of this is the impact of influenza vaccine in reducing the development of AOM [17].
Usefulness of Rapid Bacterial Diagnostics
There is a robust literature on the predictive value of nasopharyngeal cultures performed for patients with AOM. The overall positive predictive value of a nasopharyngeal swab culture (compared with bacterial cultures of middle ear fluid, usually obtained by tympanocentesis) for S. pneumoniae, H. influenzae, or M. catarrhalis is 43%, 52%, and 19%, respectively [18]. In contrast, the negative predictive value for a nasopharyngeal swab culture for which S. pneumoniae is not recovered is > 95% (ie, when the nasopharyngeal swab culture does not show S. pneumoniae, it is very unlikely to be found in the middle ear cavity) [18].
Unfortunately, surface cultures of specimens from the respiratory tract are not currently helpful in delineating the microbiologic characteristics of AOM. Precise microbiologic diagnosis requires a sample of middle ear fluid. However, new, rapid techniques that determine, on a molecular level. the presence, identity, and susceptibility of nasopharyngeal pathogens at diagnosis may be helpful in the future. For example, point-of-care testing that showed an absence of otopathogens in the nasopharynx would support observation rather than treatment of AOM. Likewise, if such a test showed either the absence of S. pneumoniae or the presence of penicillin-susceptible S. pneumoniae the selection and dose of antimicrobial agents could be influenced.
Acute Bacterial Sinusitis
Pathogenesis
Acute bacterial sinusitis (ABS) is less common than AOM as a complication of viral URI [19]. However, the pathogenesis of the 2 diseases is very similar. Figure 8 shows the relationship of the nose and the paranasal sinuses. The nose is divided in the midline by the nasal septum. From the lateral wall of the nose come 3 shelf-like structures designated according to their anatomic position as the inferior, middle and seen best on the saggital section, the superior nasal turbinates. The maxillary and ethmoid sinuses are the principal sinuses to be infected in children. They are present at birth but very small in caliber. The maxillary and ethmoid sinuses drain into the nose at the middle meatus, just beneath the middle turbinate.
Figure 8.
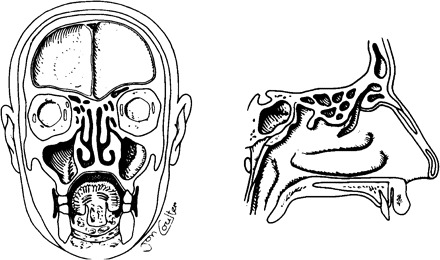
Coronal and sagittal sections of the head demonstrating the relationship between the nose and the paranasal sinuses.
A preceding viral URI is very important in most cases of ABS. The preceding URI causes a mucositis of the membranes that line the nose and the paranasal sinuses [20]. Although in almost all instances, the mucositis resolves spontaneously, in some cases, it results in obstruction of the sinus ostia. When there is a functional or mechanical obstruction of the paranasal sinus ostia, there is a transient increase in pressure in the sinus cavity followed quickly by the development of negative pressure in the sinus cavity. This negative pressure evolves because the oxygen component of the air is rapidly absorbed, leaving the pressure in the sinus negative by the partial pressure of oxygen. This negative pressure relative to the positive or atmospheric pressure in the nose or nasopharynx favors aspiration of the mucus laden with bacteria, from the nasal cavity in to the maxillary sinuses during sniffing and nose-blowing, resulting in contamination of the paranasal sinus [21]. If the mucociliary apparatus was functioning normally, this material would be swept out again. However, in the face of obstruction of the sinus ostia, the bacteria begin to multiply and secondary bacterial sinusitis develops. Studies from the University of Texas Medical Branch, Galveston, have shown that ABS likely complicates viral URI in children 6–36 months of age in 8% of children [19]. These results are similar to those of Wald et al [22]. However, studies of the epidemiology of ABS have not been done in older children. There have not been comparable studies to those performed for children with AOM in which the precise viral isolates have been identified in children who subsequently develop ABS. Nor have there been elaborate studies of the nasopharyngeal colonization in children with ABS.
Microbiology
The gold standard in making a precise microbiologic diagnosis of ABS is the performance of a sinus aspirate. This is done in children by sterilizing the area beneath the inferior nasal turbinate and passing a trocar through the medial wall of the nose into the sinus cavity (Figure 9) [23]. Infection is defined as the recovery of bacterial species in high colony count to assure that the bacterial species implicated derive from the sinuses rather than from contaminating bacteria inadvertently picked up in the nose. Studies performed in the United States in the early 1980s defined the microbiology of ABS by performing this procedure in 50 children [23]. This is a potentially hazardous procedure in children because (1) there are no landmarks in the nasal cavity to guide the position of the needle and (2) the distance between the middle and lateral walls of the sinus may be <10 mm in small children. This procedure should only be done by a skilled pediatric otolaryngologist and requires sedation in most children [20].
Figure 9.
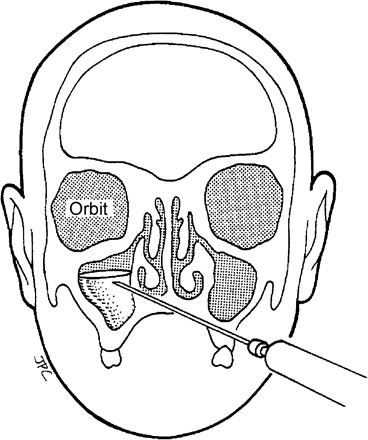
Technique for sinus aspiration after sterilization of the area beneath the inferior nasal turbinate.
The microbiology of acute sinusitis, as documented in the early studies, is as follows: S. pneumoniae in 30% of cases, H. influenzae and M. catarrhalis each in 20% of cases, S. pyogenes in ∼4%, and ∼25% were sterile. No studies have examined the microbiologic characteristics of uncomplicated ABS in children in the United States in >25 years.
Clinical Diagnosis
In clinical practice, the diagnosis of ABS is based almost entirely on patient or parent reported history. It is unfortunate that the physical examination is unhelpful in enabling differentiation of the patient with an uncomplicated viral URI from the patient with ABS. For the general practitioner or pediatrician, it is neither practical nor possible to examine the nose in an attempt to visualize the middle meatus and determine whether pus is present. In the best of circumstances, even if this procedure could be accomplished, there would still be tremendous barriers to obtaining useful information because (1) distinguishing mucus from pus visually is a very difficult if not impossible task and (2) even culture of the middle meatus does not appear to be helpful, because it has been shown that children without URI frequently demonstrate colonization of the middle meatus with the very same bacterial species that are known to be pathogens in ABS [24].
It is also unfortunate that imaging is not useful diagnostically, except to rule out the presence of sinusitis. Many studies have shown that children with viral URI show changes on imaging that are identical to those typical of cases of ABS [25−27].
Therefore, the history of the illness becomes critical and we can diagnose ABS by comparing the characteristics of the illness to those of the common cold [20]. When the illness differs substantially from the common cold in duration and severity, there are strong data to support the premise that an acute bacterial superinfection is present [28]. However, this does not provide microbiologic information.
Value of Viral Diagnostics
In the individual patient, viral diagnostics are not of value. Again, it is recognized that viral URI is the most frequent predisposing condition to the occurrence of ABS. Finding a virus or its nucleic acid fingerprint in an individual child who fits criteria for ABS would not preclude the necessity for antibiotic therapy. Epidemiologic studies detailing the viruses that are most likely to lead to cases of ABS may be of value to guide vaccine development.
Value of Rapid Bacterial Diagnostics
Although culture of the maxillary sinus aspirate is regarded as the gold standard for a diagnosis of ABS, it is not performed for uncomplicated infection because it is technically difficult, requires anesthesia, and is substantially more hazardous than tympanocentesis. Techniques that are easier to perform with lower risks need to be developed.
Unfortunately, nasal and nasopharyngeal swabs are not useful in predicting the results of the cultures of sinus aspirate in children with ABS [23]. However, the negative predictive value of culture of nasopharyngeal swab has not been tested and should be evaluated in the future.
In summary, precise microbiologic diagnosis of ABS requires a sample of sinus contents. Rapid bacterial diagnosis of cases of ABS in children does not seem to be feasible at this time. However, it is possible that a combination of information regarding colonization with bacteria and infection with specific viruses may inform treatment decisions in the future.
Acknowledgments
Supplement sponsorship. This article was published as part of a supplement entitled “Workshop on Molecular Diagnostics for Respiratory Tract Infections.” The Food and Drug Administration and the Infectious Diseases Society of America sponsored the workshop. AstraZeneca Pharmaceuticals, Bio Merieux, Inc., Cepheid, Gilead Sciences, Intelligent MDX, Inc., Inverness Medical Innovations, and Roche Molecular Systems provided financial support solely for the purpose of publishing the supplement.
Potential conflicts of interest. Author certifies no potential conflicts of interest.
References
- 1.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 2.McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA. 1995;273:214–9. [PubMed] [Google Scholar]
- 3.Bluestone CD. Pathogenesis of otitis media: role of Eustachian tube. Pediatr Infect Dis J. 1996;15:281–91. doi: 10.1097/00006454-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Chonmaitree T, Revai K, Grady JJ, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–23. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vesa S, Kleemola M, Blomqvist S, Takala A, Kilpi T, Hovi T. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J. 2001;20:574–81. doi: 10.1097/00006454-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Alper CM, Winter B, Mandel EM, Hendley JO, Doyle WJ. Rate of concurrent otitis media in upper respiratory tract infections with specific viruses. Arch Otolaryngol Head Neck Surg. 2009;135:17–21. doi: 10.1001/archotol.135.1.17. [DOI] [PubMed] [Google Scholar]
- 7.Revai K, Mamidi D, Chonmaitree T. Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin Infect Dis. 2008;46:e34–7. doi: 10.1086/525856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelton ST, Leibovitz E. Recent advances in otitis media. Pediatr Infect Dis J. 2009;28:S133–7. doi: 10.1097/INF.0b013e3181b6d81a. [DOI] [PubMed] [Google Scholar]
- 9.Wald ER. Acute otitis media: more trouble with the evidence. Pediat Infect Dis J. 2003;22:103–4. doi: 10.1097/01.inf.0000050363.97163.d8. [DOI] [PubMed] [Google Scholar]
- 10.Paradise JL, Rockette HE, Colborn DK, et al. Otitis media in 2253 Pittsburgh area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–33. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld RM, Culpepper L, Doyle KJ, et al. Clinical practice guideline: otitis media with effusion. Otolaryngol Head Neck Surg. 2004;130:S95–S118. doi: 10.1016/j.otohns.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Hoberman A, Paradise JL. Acute otitis media: diagnosis and management in the year 2000. Pediatr Ann. 2000;29:609–20. doi: 10.3928/0090-4481-20001001-06. [DOI] [PubMed] [Google Scholar]
- 13.Bluestone CD, Klein JO. Otitis Media and Eustachian Tube Dysfunction. Pediatric Otolaryngology. 4th ed. Philadelphia, PA: Saunders; 2003. pp. 474–685. [Google Scholar]
- 14.Casey JR, Pichichero ME. Changes in the frequency and pathogens causing acute otitis media 1995–2003. Pediatr Infect Dis J. 2004;23:824–8. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 15.Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J. 2004;23:1142–52. [PubMed] [Google Scholar]
- 16.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis medic six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2009 doi: 10.1097/INF.0b013e3181c1bc48. [Epub PMID:19935445] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchisio P, Esposito S, Bianchini S, et al. Efficacy of injectable trivalent virosomal-adjuvanted inactivated influenza vaccine in preventing acute otitis media in children with recurrent complicated or noncomplicated acute otits media. Pediatr Infect Dis J. 2009;28:855–9. doi: 10.1097/INF.0b013e3181a487b4. [DOI] [PubMed] [Google Scholar]
- 18.Gehanno P, Lenoir G, Barry B, Bons J, Boucot I, Berche P. Evaluation of nasopharyngeal cultures for bacteriologic assessment of acute otitis media in children. Pediatr Infect Dis J. 1996;15:329–32. doi: 10.1097/00006454-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Revai K, Dobbs L, Nair S, Patel JA, Grady JJ, Chonmaitree T. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics. 2007;119:e1408–12. doi: 10.1542/peds.2006-2881. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics, Subcommittee on Management of Sinusitis and Committee on Quality Improvement. Clinical practice guidelines: management of sinusitis. [published corrections appear in Pediatrics 2001;108:A24; and Pediatrics 2002;109:40]. Pediatrics 2001; 108:798–808. [DOI] [PubMed] [Google Scholar]
- 21.Gwaltney JM, Jr, Hendley JO, Phillips CD, Bass CR, Mygind N, Winther B. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis. 2000;30:387–91. doi: 10.1086/313661. [DOI] [PubMed] [Google Scholar]
- 22.Wald ER, Guerra N, Byers C. Upper respiratory tract infections in young children: duration of and frequency of complications. Pediatrics. 1991;87:129–33. [PubMed] [Google Scholar]
- 23.Wald ER, Milmoe GJ, Bowen A, Ledesma-Medina J, Salamon N, Bluestone CD. Acute maxillary sinusitis in children. N Engl J Med. 1981;304:749–54. doi: 10.1056/NEJM198103263041302. [DOI] [PubMed] [Google Scholar]
- 24.Gordts F, Nasser IA, Pierard D, Meyvisch P, Clement PAR. Microbiology of the middle meatus in children requiring adenotonsillectomy. J Laryngol Otol. 1999;113:24–7. doi: 10.1017/s0022215100143075. [DOI] [PubMed] [Google Scholar]
- 25.Kovatch AL, Wald ER, Ledesma-Medina J, et al. Maxillary sinus radiographs in children with nonrespiratory complaints. Pedatrics. 1984;73:811–5. [PubMed] [Google Scholar]
- 26.Diament MJ, Senac MO Jr, Gilsanz V, et al. Prevalence of incidental paranasal sinses opacification in pediatric patients: a CT study. J Comput Assist Tomogr. 1987;11:426–31. doi: 10.1097/00004728-198705000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Kristo A, Uhari M, Luotonen J, et al. Paranasal sinus findings in children during respiratory infection evaluated with magnetic resonance imaging. Pediatrics. 2003;111:e586–e589. doi: 10.1542/peds.111.5.e586. [DOI] [PubMed] [Google Scholar]
- 28.Wald ER, Nash D, Eickhoff J. Effectiveness of amoxicillin/clavulanate potassium in the treatment of acute bacterial sinusitis in children. Pediatrics. 2009;124:9–15. doi: 10.1542/peds.2008-2902. [DOI] [PubMed] [Google Scholar]


