Abstract
Viruses account for a substantial portion of respiratory illnesses, including pneumonia, in the elderly population. Presently, influenza virus A H3N2 and respiratory syncytial virus are the most commonly identified viral pathogens in older adults with viral pneumonia. As diagnostic tests such as reverse-transcription polymerase chain reaction become more widely used, the relative importance of additional viruses (such as parainfluenza, rhinoviruses, coronaviruses, and human metapneumovirus) will likely increase. Influenza virus should be considered as a cause of pneumonia during the winter months, especially during periods of peak activity. Patients with high-grade fever, myalgias, and cough should arouse the highest suspicion. Respiratory syncytial virus pneumonia should also be suspected during the winter in patients with coryza, wheezing, low-grade fever, and patchy infiltrates, especially if negative for influenza on rapid testing. Because clinical features and periods of activity for many viruses overlap, laboratory confirmation of influenza is recommended for cases involving seriously ill or institutionalized patients.
Viruses cause the largest proportion of cases of childhood pneumonia. In contrast, viral pneumonia is relatively uncommon during the reinfections during young adulthood. With advancing age and inevitable development of comorbidities, viruses once again may cause serious illness and pneumonia. Although acute respiratory infection rates steadily decrease with advancing age, rates of hospitalization and death increase substantially in persons aged >60 years. Multiple factors, such as declines in respiratory and immune function, likely contribute to increased morbidity (table 1) [1–3]. Immune dysregulation and waning cellular immune function may impair viral clearance, allowing spread of the virus to lower airways, with increased inflammation.
Table 1.
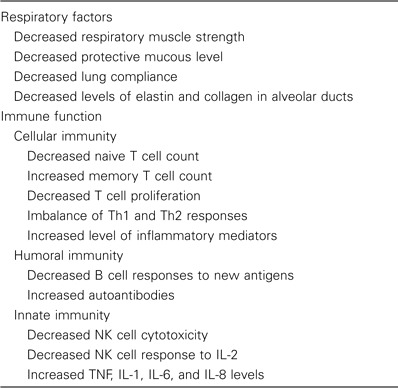
Factors that contribute to severe respiratory infections associated with aging.
Studies of community-acquired pneumonia (CAP) in adults indicate a viral etiology in 1%–23% of cases, with influenza virus being the most common virus [4]. Although distinguishing viral from bacterial pneumonia is difficult on clinical grounds, there are helpful epidemiologic and clinical clues to alert physicians to the possibility of viral pneumonia.
Epidemiology and Clinical Features
Influenza virus. Influenza viruses have segmented RNA genomes and are classified as type A, B, or C. The 2 envelope glycoproteins are important targets of neutralizing antibody [5]. Hemagglutinin (H) initiates infectivity by binding to cellular sialic acid residues and neuraminidase (N) protein cleaves newly synthesized virus from sialic acid on cell surfaces allowing spread of virus to adjacent cells. At least 16 antigenically distinct hemagglutinin (H1–H16) and 9 neuraminidase (N1–N9) molecules of influenza A have been identified. H1–H3 viruses cause most cases of human disease, whereas the others generally are pathogens of aquatic birds and other mammals. Antigenic variation in H and N contributes to the epidemic nature of influenza by 2 mechanisms. Antigenic “drift” is due to minor changes in neutralizing epitopes of H and results in epidemics and necessitates annual adjustment of vaccine formulations. Antigenic “shift” occurs when 2 influenza A viruses exchange H or N genes during infection of the same host. Shifts with resultant influenza pandemics occurred in 1918 (H1N1 virus), 1957 (H2N2 virus), and 1968 (H3N2 virus).
Although annual winter epidemics occur predictably, lasting 6–8 weeks in one locale, time of onset and severity are highly variable. Influenza is efficiently transmitted via small particle aerosols generated by coughing and sneezing and has a 2–3-day incubation period, which facilitates explosive outbreaks in closed settings, such as nursing homes (table 2). Since 1977, H3N2, H1N1, and B viruses have circulated, with epidemics of H3N2 infection causing the greatest morbidity in the elderly population. Older persons may be relatively resistant to severe H1N1 disease because of frequent exposure prior to 1957. The impact of influenza on the elderly population is substantial and is greatest among persons with chronic diseases. It is estimated that at least 63% of the 300,000 influenza-related hospitalizations and 85% of the 36,000 influenza-related deaths in the United States involve persons aged >65 years, a group that constitutes only 10% of the total population [6].
Table 2.
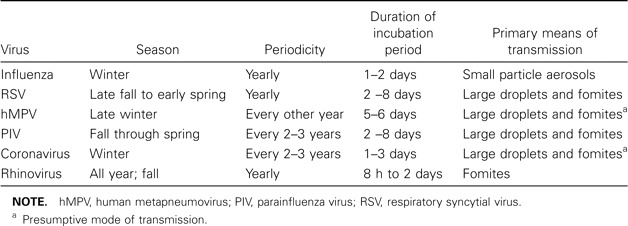
Characteristics of common respiratory viruses.
The clinical manifestations of influenza in elderly persons may differ from those of “classic” influenza (i.e., abrupt fever, myalgias, and headache) seen in younger adults, with older persons having a lower frequency of upper respiratory tract symptoms. Among elderly outpatients, cough, fever, and acute onset of illness had only a 30% positive predictive value, in contrast to a positive predictive value of 78% for young adults [7]. In a study of older adults hospitalized with documented influenza, cough was nearly universal, and fever (temperature, >38°C) was seen in 70% [8]. Fever and altered mental status may be the only signs of influenza pneumonia in older persons with cognitive impairment. Gastrointestinal complaints, fever, and myalgias help to distinguish influenza from other winter respiratory viruses, such as respiratory syncytial virus (RSV) (table 3).
Table 3.
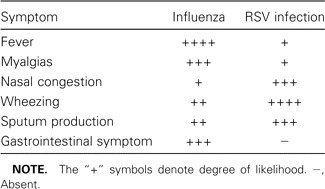
Clinical features of influenza and respiratory syncytial virus (RSV) infection.
In the classic description of influenza-associated lower respiratory tract complications during the H2N2 pandemic of 1957–1958, Louria et al. [9] described 4 syndromes: (1) no radiographic pneumonia, (2) viral infection followed by bacterial pneumonia, (3) rapidly progressive diffuse viral pneumonia, and (4) concomitant viral-bacterial pneumonia. Today, pure viral pneumonia is uncommon, perhaps because prior infection and vaccination has induced immunity among older persons, as well as the rarity of rheumatic heart disease. We found that 2%–6% of highly vaccinated elderly persons (i.e., >90%) in the community developed influenza each season, of whom 5% had pneumonia. Only 30% of hospitalized patients with influenza have radiographic infiltrates, whereas a equal percentage have findings consistent with congestive heart failure or other diseases [10]. The true incidence of secondary bacterial pneumonia during influenza is unknown, largely because there are difficulties involved in accurate diagnosis of bacterial infection. In our recent study [10], bacterial infection was identified in 10% of hospitalized patients with influenza, with only 1 episode of Streptococcus pneumoniae bacteremia. Dual viral-bacterial infections were more severe than were viral infections alone, as evidence by higher rates of intensive care use and morbidity.
RSV. RSV is the second—most commonly identified cause of viral pneumonia in older persons. First isolated in 1956 from a chimpanzee with a cold, it was subsequently shown to be the leading cause of lower respiratory infections in young children. RSV is an RNA virus belonging to the Paramyxoviridae family, and it causes characteristic syncytia formation in cell culture. Two major antigenic groups of RSV (A and B) cause annual winter epidemics of respiratory disease in temperate climates [11]. Unlike influenza virus, RSV does not undergo major periodic antigenic changes, and yet, immunity is incomplete. Reinfection in young adults usually produces mild illness; however, the likelihood of severe disease and pneumonia increases with advancing age.
RSV pneumonia was first described in older adults in the 1960s. However, it was not appreciated as a significant pathogen until outbreaks of infection in nursing homes were reported in the 1980s [12]. Rates of pneumonia and death ranged from 5% to 55% and 0% to 53%, respectively. In composite, these studies indicate that ∼10% of nursing home residents develop RSV infection annually, of whom 10% develop pneumonia.
RSV infection is also a problem among elderly persons who live in the community. Investigators at the Centers for Disease Control and Prevention linked viral surveillance data with national death databases and estimated that RSV infection accounts for ∼10,000 deaths among elderly persons each year [13]. In a study of 1200 adults with CAP, RSV (4.4% of cases) was the third—most commonly identified pathogen, compared with S. pneumoniae (6.2% of cases), influenza viruses A and B (5.4% of cases), and Mycoplasma pneumonia (4.1%) [14]. We found that, during each season, RSV infection occurred in 3%–7% of prospectively monitored healthy elderly persons and in 4%–10% of high-risk adults; pneumonia occurred in 2%–7% of infected persons [10]. In addition, RSV infection accounted for 11% of winter discharge diagnoses for pneumonia.
The clinical manifestations of RSV pneumonia are difficult to distinguish from those of other viral or bacterial pneumonias. Dyspnea and cough are common (60%–80% of cases) but are not unique to RSV infection. The typical RSV illness begins with nasal congestion, which gradually progresses to wheezing and difficulty breathing. Compared with influenza, RSV infection is more often associated with rhinorrhea, sputum production, and wheezing, whereas fever and gastrointestinal complaints are more common with influenza (table 3) [14, 15].
Chest radiographs typically show patchy bilateral alveolar infiltrates and interstitial changes. In culture-proven cases of RSV pneumonia, infiltrates tend to be small and ill-defined, although consolidation has been described [14]. Seventy percent of patients with RSV pneumonia have a normal WBC count, compared with 55% of patients with atypical pneumonia and 40% of those with bacterial pneumonia. Eleven percent to 30% of RSV-infected patients may have evidence of mixed viral-bacterial infection.
Human metapneumovirus (hMPV). hMPV is a newly described respiratory pathogen that was identified by Dutch researchers in 2001 in young children with respiratory illnesses resembling RSV bronchiolitis [16]. hMPV is an RNA virus belonging to the Paramyxoviridae family and metapneumovirus genus, and it has worldwide distribution, with universal infection by the age of 5 years. In temperate climates, the virus circulates predominantly in the winter months, overlapping with other seasonal viruses. Several studies suggest there may be significant year-to-year variation in incidence rates [17].
Because hMPV is a newly identified virus, clinical data are still relatively limited, and most studies describe children with bronchiolitis and pneumonia. Asymptomatic infection, colds, asthma exacerbations, and flulike illnesses have been documented in older children and healthy young adults [18]. Comprehensive studies of hMPV as a cause of CAP among adults have not yet been published. In a 2-year study of elderly subjects and high-risk adults, hMPV infection was identified in 4.1%, with RT-PCR and serologic testing used for diagnoses [17]. Impact was greatest among those with cardiopulmonary diseases, who were ill twice as long as healthy elderly subjects. of the hospitalized persons, 25% had infiltrates noted on chest radiographs. Illness may be more severe in frail elderly subjects, as evidenced by a Canadian study in which pneumonia was documented in 40% of hMPV-infected nursing home residents [19].
The clinical characteristics of hMPV pneumonia in older adults do not appear to be distinctive from those of illness due to other winter respiratory viruses. hMPV infection may be somewhat less severe than RSV infection and influenza, with lower rates of mechanical ventilation and death noted in one study [17].
Parainfluenza viruses (PIVs). PIVs are paramyxoviruses that cause croup, bronchitis, and pneumonia in young children. PIV type 3 infection is endemic year round, and infections due to PIV types 1 and 2 tend to peak during the fall months [11]. Infection recurs throughout adulthood, accounting for 1%–15% of acute respiratory illnesses, with occasional reports of pneumonia in young adults. In the elderly population, PIV has been less well studied than RSV, but pneumonia has been reported [20]. Investigators in Sweden found that 11% of community-dwelling elderly persons with pneumonia and 7% of those with acute respiratory tract illness had serologic evidence of recent infection due to PIV type 1 or 3 [21]. Prospective studies in nursing homes have documented PIV infection in 4%–14% of respiratory illnesses, and fatal cases of bronchopneumonia have been reported [22]. PIV has also been implicated as a precursor to an outbreak of invasive pneumococcal disease in a long-term care facility. Clinical syndromes, characterized by fever, rhinorrhea, cough, and sore throat, are not distinctive.
Coronaviruses. Coronaviruses are RNA viruses that derive their name from the crown-like appearance of club-shaped surface proteins. They were discovered in 1965 and are second to rhinoviruses as a cause of the common cold. Studies of human illness have been limited by the inability to grow the virus under routine conditions. Over the past 4 decades, OC43 and 229E strain infections have been diagnosed in all age groups by serologic testing [23]. Recently, 2 new human strains (NL63 and HKU1) were identified by RT-PCR [24, 25]. Interest in coronaviruses intensified in 2002 with the severe acute respiratory syndrome epidemic, which spread quickly from China and was proven to be due to a novel coronavirus, SARS-CoV.
OC43 and 229E most often occur in late winter and early spring, demonstrating 2–3-year periodicity [26]. In healthy adults, infection is characterized by low-grade fever, malaise, and nasal symptoms. Pneumonia has been reported in young children, immunocompromised patients, and elderly persons [27, 28]. Coronaviruses were identified by RT-PCR in 17% of elderly persons in the community with acute respiratory illnesses in a recent Dutch study, and 50% of patients had lower respiratory tract symptoms [28]. Most studies to date have not reported radiographic findings; thus, the frequency of coronavirus pneumonia remains unknown.
Rhinoviruses. Rhinoviruses, the most frequent cause of the common cold, circulate throughout the year, but activity peaks in the fall and spring. Infections are common among persons of all ages, including elderly persons, and account for ∼25%–50% respiratory illnesses among community-dwelling elderly individuals [29]. Outbreaks have also been documented in long-term care facilities and senior daycare centers [30, 31]. Prominent nasal congestion, cough, and constitutional symptoms characterize illnesses. The role of rhinoviruses in pneumonia remains somewhat controversial. Because replication is restricted at core body temperature, rhinoviruses were once dismissed as a cause of pneumonia. Recent evidence indicates that rhinoviruses can be recovered from lower airways after experimental challenge, and cases of pneumonia have been described in very young children and severely immunocompromised patients [32]. Comprehensive studies of older adults with CAP using new, sensitive molecular techniques have yet to be performed.
Diagnosis
Viral diagnostic methods include culture, rapid antigen detection, RT-PCR, and serologic testing, with availability and sensitivity varying on the basis of the specific virus. Respiratory secretions appropriate for testing include nasal swab or wash specimens, sputum specimens, and bronchoalveolar lavage fluid samples. Nasal wash samples, the preferred sample for children, are difficult to obtain in frail or cognitively impaired elderly persons. Although they are slightly less sensitive than washes, nasal swab samples are acceptable alternatives.
Viral culture remains the gold standard for diagnosis and is available in many clinical laboratories, but optimal results require prompt transportation of the specimen on ice for immediate inoculation. Influenza is relatively hardy and grows well in culture, but the 2–3 days required for detection is unsatisfactory when one must make decisions regarding antiviral therapy. Culture is insensitive for diagnosis of RSV infection, because RSV is labile, and adults typically shed low titers. PIVs and rhinoviruses can be detected by culture using standard techniques, but hMPV and coronaviruses have special growth requirements necessitating research facilities for isolation.
Rapid antigen testing is commercially available for influenza viruses A and B and RSV, offers immediate results, and (in some cases) can be done at the point of care. For diagnosis of influenza in adults, these tests offer a sensitivity of 50%–60% and specificity of ⩾90%. Many institutions screen patients with rapid tests and only perform culture if rapid testing is negative. This allows quick identification of many patients while not sacrificing the sensitivity of culture. Given the rate of false-negative results for rapid tests, patients for whom influenza is highly suspected during epidemics should be isolated and treated with antivirals, if appropriate, while awaiting culture results [5]. Rapid RSV antigen tests are useful in young children who shed high titers of virus, but results are disappointing in adults. Compared with serologic tests and RT-PCR, the sensitivity of commercially available tests is low (0%–20%).
RT-PCR is very sensitive and specific for the diagnosis of all the common respiratory viruses and has been pivotal in defining adult disease for RSV, hMPV, rhinoviruses, and coronaviruses. Although rapid, these assays are expensive and not widely available outside of major medical centers. However, a multiplex RT-PCR (Hexaplex; Prodes) is commercially available for the diagnosis of infection due to multiple common respiratory viruses.
Because infection with respiratory viruses (with the exception of SARS-CoV) represents reinfection in adults, the presence of preexisting antibody precludes a single serologic test to detect IgG antibody. IgM assays have generally not been useful. Serologic testing provides retrospective diagnosis when a ⩾4-fold increase in a specific antibody is detected by complement fixation or EIA. Because rhinovirus has >100 serotypes, serologic testing is impractical for diagnosis, whereas serologic tests for detection of hMPV and coronavirus are only available in research settings.
Because of overlapping clinical syndromes, diagnosis of viral pneumonia requires laboratory confirmation. This is particularly important for influenza, because treatment is available and infection-control issues arise in hospitals and nursing homes (figure 1). Therefore, during the winter months, we recommend screening by rapid antigen tests for patients who are admitted to the hospital and residents of long-term care facilities who have suspicious clinical syndromes. Once influenza is active in the community, we recommend screening all patients with pneumonia with rapid tests, followed by influenza culture if the results of the rapid test are negative. Full respiratory viral cultures and RT-PCR are desirable to identify other viral pathogens, but if resources are limited, these tests may be restricted to immunocompromised or severely ill patients, for whom use of unproven therapies is sometimes attempted. Because of its retrospective nature, serologic testing is more useful for investigation of nursing home outbreaks of respiratory disease rather than for individual patient care.
Figure 1.
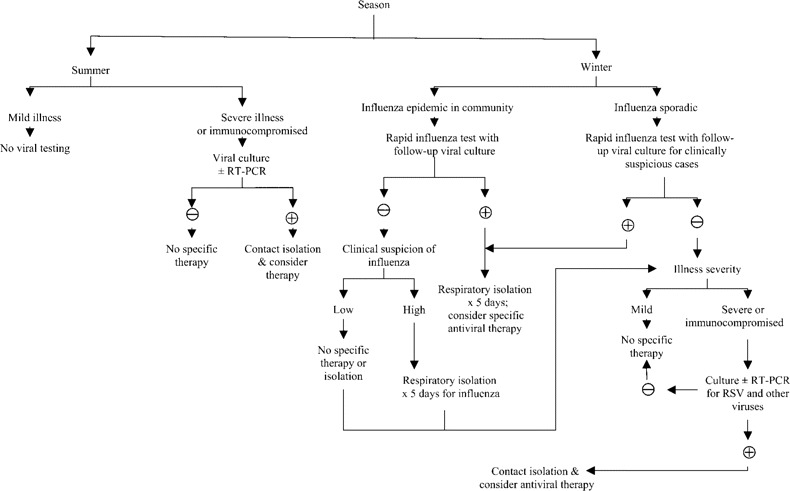
Diagnosis and treatment of older adults with pneumonia. RSV, respiratory syncytial virus; ±, with or without; +, positive, -, negative.
Treatment
Four antivirals (amantadine, rimantadine, zanamivir, and oseltamavir) have been approved for the treatment of influenza (table 4). Amantadine and rimantadine are active against influenza A, whereas zanamivir and oseltamavir are active against both influenza A and B. These agents are 70%–90% effective for prophylaxis and also reduce illness severity, duration of symptoms, and viral shedding when given ⩽48 h after symptom onset in uncomplicated influenza. CNS adverse effects limit the utility of amantadine in the elderly population, as does the issue of resistance, which develops quickly for both amantadine and rimantadine. Zanamivir and oseltamivir are associated with fewer adverse effects, although zanamivir should be avoided in patients with chronic obstructive pulmonary disease and those with asthma because of bronchospasm [5]. The benefit of antiviral agents for influenza pneumonia is unknown, because placebo-controlled trials for pneumonia have not been conducted. Although definitive data are lacking, most authorities feel that it is reasonable to administer an antiviral agent to elderly patients who are hospitalized with influenza pneumonia.
Table 4.
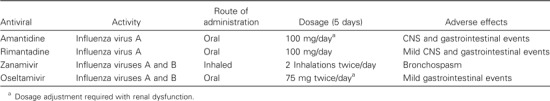
Administration characteristics of influenza antivirals and associated adverse effects.
The treatment of RSV pneumonia in elderly adults is largely supportive. Antiviral therapy with aerosolized ribavirin and RSV-specific immunoglobulin have been approved for high-risk infants, but no controlled data are available in adults [11, 12]. Although the general use of ribavirin cannot be recommended, use of this agent may be considered in selected cases, such as those involving immunocompromised patients. Anecdotal experience suggests that, for adults who are not undergoing intubation, high-dose, short-duration therapy (60 mg/mL for 2 h given by mask 3 times a day) is better tolerated than tent treatment. Ribavirin has in vitro activity against PIV and hMPV, but there are no clinical data involving humans. No antivirals are approved for the treatment of coronavirus and rhinovirus infections.
Prevention
Immunization remains the most effective means of reducing morbidity and mortality associated with influenza in the elderly population. Currently, 2 vaccines are available: standard trivalent inactivated (killed) virus vaccine and the recently introduced live, attenuated virus vaccine [5]. Most studies have shown a 30%–50% reduction in influenza hospitalization rates when there is a good antigenic match between the vaccine and circulating strain [33]. In a study of nearly 300,000 community-living elderly subjects, administration of trivalent inactivated virus vaccine was associated with 48%–50% reduction in the rate of deaths due to all causes and with significant reductions in rates of hospitalization for pneumonia and influenza, cardiac disease, and stroke, compared with rates among nonimmunized persons [34]. In contrast, a recent analysis found no decrease in morbidity and mortality rates among older persons, even as vaccine uptake increased from 20% in 1980 to 65% in 2001, suggesting that better influenza vaccines are still needed [35]. Although live, attenuated virus vaccine is efficacious in young children and adults aged <49 years, the vaccine has not been studied in older persons [5]. Currently, there are no licensed RSV vaccines for children or adults.
Transmission of influenza may occur via small particle aerosols, and thus, respiratory isolation of patients with documented or suspected influenza during periods of high influenza activity is appropriate. Hand washing is also very important, especially for patients who are receiving amantadine or rimantadine, to prevent nosocomial spread of drug-resistant virus. The other major respiratory viruses are spread via fomites and large particle droplets; therefore, respiratory isolation is not required.
In summary, viruses account for a substantial portion of cases of respiratory illness and pneumonia in the elderly population. As diagnostic tests continue to improve, new agents and the relative importance of known agents may change. Presently, influenza virus A H3N2 and RSV are the most-commonly identified viral pathogens in older adults with viral pneumonia. Because clinical features and periods of peak activity overlap during the winter months, laboratory confirmation of influenza is recommended for seriously ill or institutionalized patients.
Acknowledgments
Potential conflicts of interest. A.R.F. and E.E.W.: no conflicts.
References
- 1.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 2.Weksler ME. Aging and the immune system. Infect Dis Clin Practice. 1995;3:464–7. [Google Scholar]
- 3.Effros RB. Long-term immunological memory against viruses. Mech Ageing Dev. 2000;121:161–71. doi: 10.1016/s0047-6374(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 4.File TM., Jr Community-acquired pneumonia. Lancet. 2003;362:1991–2001. doi: 10.1016/S0140-6736(03)15021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prevention and control of influenza: recommendations of the Advisory Committee of Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2005;54:1–41. [Google Scholar]
- 6.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 7.Govaert TME, Dinant GJ, Aretz K, Knottnerus JA. The predictive value of influenza symptomatology in elderly people. Fam Pract. 1998;15:16–22. doi: 10.1093/fampra/15.1.16. [DOI] [PubMed] [Google Scholar]
- 8.Walsh EE, Cox C, Falsey AR. Clinical features of influenza A virus infection in elderly hospitalized persons. J Am Geriatr Soc. 2002;50:1498–503. doi: 10.1046/j.1532-5415.2002.50404.x. [DOI] [PubMed] [Google Scholar]
- 9.Louria DE, Blumenfeld HL, Ellis JT, Kilbourne ED, Rogers DE. Studies on influenza in the pandemic of 1957–1958. II. Pulmonary complications of influenza. J Clin Invest. 1959;38:213–65. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 11.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–28. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 12.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13:371–84. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 14.Dowell SF, Anderson LJ, Gary HE, Jr, et al. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174:456–62. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 15.Wald TG, Miller BA, Shult P, Drinka P, Langer L, Gravenstein S. Can respiratory syncytial virus and influenza A be distinguished clinically in institutionalized older persons? J Am Geriatr Soc. 1995;43:170–4. doi: 10.1111/j.1532-5415.1995.tb06384.x. [DOI] [PubMed] [Google Scholar]
- 16.van den Hoogen BG, DeJong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nature Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 18.Hamelin ME, Abed Y, Boivin G. Human metapneumovirus: a new player among respiratory viruses. Clin Infect Dis. 2004;38:983–90. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boivin G, Abed Y, Pelletier G, et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–4. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 20.Marx A, Gary HE, Martston BJ, et al. Parainfluenza virus infection among adults hospitalized for lower respiratory tract infection. Clin Infect Dis. 1999;29:134–40. doi: 10.1086/520142. [DOI] [PubMed] [Google Scholar]
- 21.Fransen H, Heigl Z, Wolontis S, Forsgren M, Svedmyr A. Infections with viruses in patients hospitalized with acute respiratory illness, Stockholm 1963–1967. Scand J Infect Dis. 1969;1:127–36. doi: 10.3109/inf.1969.1.issue-2.09. [DOI] [PubMed] [Google Scholar]
- 22.Public Health Laboratory Service Communicable Disease Surveillance Centre Parainfluenza infections in the elderly 1976–82. Br Med J (Clin Res Ed) 1983;287:1619. doi: 10.1136/bmj.287.6405.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIntosh K, Kapikian AZ, Turner HC, Hartley JW, Parrott RH, Chanock RM. Seroepidemiologic studies of coronavirus infection in adults and children. Am J Epidemiol. 1970;91:585–92. doi: 10.1093/oxfordjournals.aje.a121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fouchier RA, Hartwig NG, Bestebroer TM, et al. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci U S A. 2004;101:6212–6. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo PCY, Lau SKP, Chu C, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–95. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntosh K, Anderson LJ. Coronaviruses. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practices of infectious diseases. 6th edition. Philadelphia: Elsevier Churchill Livingston; 2005. pp. 1990–8. [Google Scholar]
- 27.El-Sahly HM, Atmar RL, Glezen WP, Greenberg SB. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graat JM, Schouten EG, Heijnen MLA, et al. A prospective, community-based study on virologic assessment among elderly people with and without symptoms of acute respiratory infection. J Clin Epidemiol. 2003;56:1218–23. doi: 10.1016/S0895-4356(03)00171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson KG, Kent J, Hammersley V, Esperanza C. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315:1060–4. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wald TG, Shult P, Krause P, Miller BA, Drinka P, Gravenstein S. A rhinovirus outbreak among residents of a long-term care facility. Ann Intern Med. 1995;123:588–93. doi: 10.7326/0003-4819-123-8-199510150-00004. [DOI] [PubMed] [Google Scholar]
- 31.Louie JK, Yagi S, Nelson FA, et al. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis. 2005;41:262–5. doi: 10.1086/430915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–84. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 33.Govaert TME, Thijs CTMCN, Masurel N, Sprenger MJW, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals- A randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–5. [PubMed] [Google Scholar]
- 34.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. New Engl J Med. 2003;348:1322–32. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 35.Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165:265–72. doi: 10.1001/archinte.165.3.265. [DOI] [PubMed] [Google Scholar]


