Abstract
We observed that a number of patients with severe acute respiratory syndrome (SARS) developed affective psychosis during the acute phase of their illness. We reviewed all SARS-related psychiatric consultations in Hong Kong and investigated the risk factors for psychosis among patients with SARS in a matched case-control study. Patients with SARS-related psychosis received higher total doses of steroids and had higher rates of family history of psychiatric illness. The findings of the present study suggest that steroid toxicity, personal vulnerability, and, probably, psychosocial stressors jointly contributed to the development of psychosis in patients with SARS.
In February 2003, an outbreak of atypical pneumonia occurred in southern China. The infection, subsequently named “severe acute respiratory syndrome” (SARS), was spread by travelers to many parts of the world [1]. The outbreak was so rampant that the World Health Organization issued warnings about international travel. In Hong Kong, within the short span of 6 months, >3000 people were infected with SARS, and, of them, >300 died.
During the SARS outbreak, we observed that a small number of patients with SARS developed acute psychotic disorders while they were receiving inpatient treatment. Psychosis among patients with SARS posed great challenges and threats to the clinical team. The psychotic symptoms—such as elation, irritable mood, persecutory delusions, agitation, and hyperactivity—rendered affected patients uncooperative with the clinical management and with the infection-control measures. When the psychosis was severe and the patient displayed disruptive behavior that posed harm to himself or others, physical restraint and sedation were necessary. However, even when wearing protective apparel, the clinical team faced a substantial risk of contracting SARS during the course of action.
Many patients with SARS were given high doses of steroids—as much as 160 mg of methylprednisolone per day—to control virus-induced autoimmune lung damage. Because previous research has shown that patients who receive high doses of steroids can develop manic symptoms or manic syndrome [2, 3], it has been theorized that SARS-related psychosis was caused by steroid treatment. However, other etiologies should also be considered. SARS-associated coronavirus, the pathogen involved in SARS, has been detected in CSF samples obtained from patients with SARS [4]. In addition, patients with SARS are subject to many physical and psychological stresses. Hypoxia, concomitant sepsis, sleep deprivation, social isolation (as a result of being quarantined), fear of death, and concurrent SARS infections within the family are all profound stressors that can precipitate psychosis. Last, the fact that only a small number of these highly stressed patients with SARS developed acute psychosis suggests that personal vulnerability may be relevant.
Patients and methods. We studied the etiology of SARS-related psychosis retrospectively, using a case-control design. A total of 15 case patients with SARS-related psychosis and 30 nonpsychotic control subjects were compared.
All liaison psychiatrists in Hong Kong reviewed their SARS-related consultations that had occurred from 13 March through 31 May 2003. All patients who had received a recent diagnosis of a psychotic disorder after the onset of SARS were identified. Psychotic disorders were defined according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for diagnoses of Brief Psychotic Disorder, Delusional Disorder, Psychotic Disorder Due to a General Medical Condition, Manic Episode, Major Depressive Episode with Psychotic Features, Schizoaffective Disorder, Schizophrenia, Schizophreniform Disorder, Shared Psychotic Disorder, Substance-Induced Psychotic Disorder, and Psychotic Disorder Not Otherwise Specified.
For control comparison, we identified 30 age- and sex-matched patients with SARS who had no psychotic symptoms (twice the number of patients who had SARS-related psychosis), using the hospital's registry of patients with SARS. Two control subjects were matched, by age (±2 years) and sex, with each psychotic case patient. All control subjects had been assessed by a research psychiatrist (Y.K.W.), who used the Structured Clinical Interview for DSM-IV (SCID) to confirm the absence of psychotic symptoms [5]. The SCID comprises an hour-long, semistructured clinical interview that addresses every symptom domain, ensuring accurate and reliable psychiatric diagnosis [5].
The medical records of the case patients and control subjects were reviewed for information on sociodemographics, past medical history, and SARS development and treatment history. The ascertainment of risk factors was uniform between case patients and control subjects. We hypothesized that personal and family histories of psychiatric illness, intensive care unit admission, receipt of steroid treatment, steroid dosage, and SARS infection among family members would increase the risk of psychosis among patients with SARS.
Case patients and control subjects were compared using the χ2 test or Fisher's exact test, for categorical variables, and the Mann-Whitney U test, for continuous variables. ORs with 95% CIs were calculated.
Results. A total of 15 patients with psychotic disorders were identified among the 1744 patients with SARS who were identified during the study period, yielding an incidence rate of 0.9% (table 1). The clinical diagnoses made by the liaison psychiatrists were as follows: Steroid-Induced Manic Episode (n = 10), Steroid-Induced Psychotic Disorder (n = 3), Major Depressive Episode with Psychotic Features (n = 1), and Psychotic Disorder Not Otherwise Specified (n = 1). For 3 case patients (20%), the diagnosis of psychosis was determined only during the second assessment; the diagnosis of Adjustment Disorder was determined earlier during the study. All psychotic episodes met the DSM-IV criteria for “caseness,” and all case patients were receiving treatment for SARS when the psychotic episode began. None of the case patients or control subjects had a history of psychotic disorders.
Table 1.
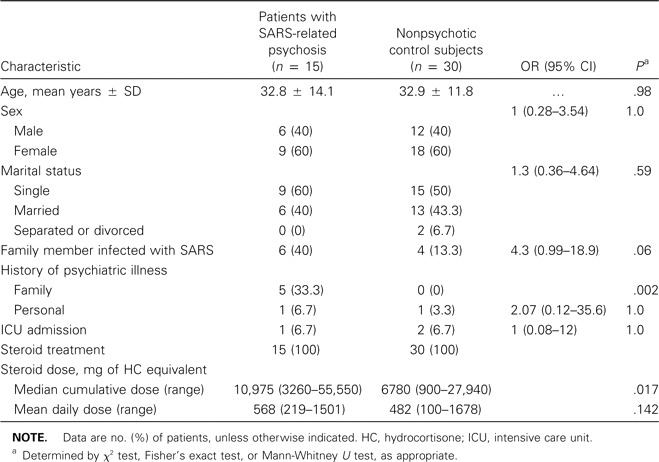
Characteristics assessed as potential risk factors for psychosis among patients with SARS.
Family history of psychiatric illness was significantly more common among patients with SARS-related psychosis than among control subjects (33% vs. 0%; P = .002; table 1). Among the patients with SARS-related psychosis, there was also a higher rate of family members contracting SARS, but the trend was marginally insignificant (40% vs. 13%; OR, 4.3; 95% CI, 0.99–18.9; P = .06). We found that personal history of psychiatric illness and intensive care unit admission were not associated with psychosis among patients with SARS (table 1).
All case patients and control subjects had received steroid treatment for SARS. The patients with SARS-related psychosis received significantly higher cumulative doses of steroids during their inpatient hospital stays than did the control subjects (median dose of hydrocortisone equivalent, 10,975 mg vs. 6780 mg; P = .017). The mean daily steroid dose for the cohort of patients with SARS-related psychosis was also higher than that for the control subjects, but the trend was not statistically significant (568 mg vs. 482 mg of hydrocortisone equivalent per day; P = .142; table 1). Multivariate analysis was attempted, but no explanatory variable could be consistently identified in the logistic regression modeling.
Discussion. All except 2 of the case patients with SARS-related psychosis were given a diagnosis of Steroid-Induced Mania Episode or Psychotic Disorder. These diagnoses were based on the clinical observation of the length of the interval between the introduction of steroid treatment and the onset of the manic or psychotic symptoms. Besides, the disturbance could not have been better attributed to a non—substance-induced psychotic disorder. That most of the case patients were manic at presentation also supported the relevance of receipt of steroids in the etiology [2, 3]. Analysis of steroid dosage also suggested that the patients with SARS-related psychosis had received higher cumulative doses of steroids during their inpatient hospital stays.
However, data from the present study argued against simple, direct steroid toxicity as the etiology of psychosis among patients with SARS. An unusually high rate of family history of psychiatric illness was observed in the cohort of patients with SARS-related psychosis, which suggested that personal vulnerability is also relevant in determining which patients with SARS would experience a psychotic episode. The study also showed that psychosocial stressors, such as concurrent SARS infection in another family member, may also be relevant, but a study with a larger sample is needed to confirm this observed trend.
We would like to point out that acute psychosis is not the only form of mental illness that has been observed among patients with SARS. Another study from Hong Kong had reported that ∼40% of 148 patients with SARS had a been given a diagnosis of 1 or more DSM-IV-defined disorders during the acute and/or convalescent phases of their illness (Y.K.W., H.C.M.L., and I. Kam, unpublished data). The study also showed that ∼11% of patients with SARS who were treated with steroids displayed some manic symptoms during the acute phase of the illness.
The present study had several limitations that warrant Discussion. First, because of the small sample size and the methodological limitations, the study findings—both positive and negative ones—should be interpreted with caution. Second, we were not able to ascertain the nature of psychiatric illnesses within the patients' families, because the details were not always recorded in the case notes. Also, that none of the control subjects had a family history of psychiatric illness could well be a chance finding. Third, the patients with SARS-related psychosis were evaluated by clinical assessment rather than by a standardized research interview, such as the SCID. Last, the retrospective nature of this study is a key limitation. However, amidst the confusion, shock, panic, and fear associated with the SARS outbreak, it was difficult to design and launch a prospective study quickly enough to match the rapid course of the epidemic. Hence, we have had to resort to a retrospective study design, which limits our examination to variables that had been regularly recorded in the case notes.
A prospective study with detailed recording of the phenomenology of the outbreak would allow a researcher to ascertain whether the psychotic episodes observed had clinical features that distinguished them from the typical steroid-induced psychoses seen in other contexts. Likewise, routine MRI examination of each psychotic case patient would allow for the evaluation of direct CNS involvement of the SARS virus. Future studies should preferably employ 1 or 2 psychiatrists to collect clinical data in a systematic manner.
Although the rate of psychotic complications in the SARS population does not appear to be high, it is important to warn clinicians about the possibility of psychosis among patients during the management of this highly contagious disease. Before additional data are available, we believe that it would be advisable to routinely ascertain the family histories of psychiatric illness and, probably, the well-being of other family members during the clinical assessment of patients with SARS. This is especially important if high-dose or intravenously administered steroids are to be prescribed.
Acknowledgments
We thank the other members of the Hong Kong SARS Psychosis Study: I. Kam, D. Nguyen, W. N. Tang, W. H. Leung, M. Tay, and V. Wong. We also thank Mandy Yu, Fiona Lee, Sarah Chia, Edwina Chan, Kathy Chan, and Tony Leung, for their assistance.
Conflict of interest. All authors: No conflict.
References
- 1.Cheng CC, Hung FN, Tang SF, et al. Viral replication in the nasopharynx is associated with diarrhea in patients with severe acute respiratory syndrome. Clin Infect Dis. 2004;38:467–75. doi: 10.1086/382681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days' corticosteroid treatment: a prospective study. Psychoneuroendocrinology. 1996;21:25–31. doi: 10.1016/0306-4530(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 3.Boston Collaborative Drug Surveillance Program Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13:694–8. doi: 10.1002/cpt1972135part1694. [DOI] [PubMed] [Google Scholar]
- 4.Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342–4. doi: 10.3201/eid1002.030638. Available at: http://www.cdc.gov/ncidod/EID/vol10no2/03-0638.htm. Accessed 19 February 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2001. [Google Scholar]


