Abstract
An epidemic of Chikungunya fever of unprecedented magnitude occurred in many parts of India in early 2006 after an interval of 33 years, and there has been a resurgence in some parts of South India since June 2007. The article highlights clinical manifestations of infection and various molecular tests that were used for diagnoses of Chikungunya virus infection. Of particular interest is the real-time loop-mediated isothermal amplification (RT LAMP) assay, which is rapid and cost-effective and can be adopted at ill-equipped laboratories. Clinical symptoms were characterized by a triad of fever, rash, and severe rheumatic manifestations. RT LAMP identified 20 additional Chikungunya virus—positive cases, compared with reverse-transcriptase polymerase chain reaction. Chikungunya virus was isolated from 20 randomly selected samples. Genotyping of the virus isolates revealed that the East Central South African genotype of Chikungunya virus was the etiologic agent of this epidemic. Molecular diagnosis is an important tool to identify such new vectorborne viral illnesses.
Emerging viral infections have become a serious problem in recent years. Emergence or reemergence of severe arboviral hemorrhagic fevers caused by mosquito-borne viruses, such as dengue virus and Chikungunya (CHIK) virus, have been frequently reported in the Indian subcontinent in the past few years. From the clinical perspective, these infections have similar clinical manifestations and are difficult to distinguish from one another. Because the outcomes of these infections vary on the basis of the infecting agent (dengue has a high mortality rate), they pose a diagnostic dilemma for the clinician. Therefore, there is a need for a means of definitive diagnosis and identification of the viral agent to prognosticate the outcome.
The causative agent CHIK virus, a single-stranded, positive sense RNA, enveloped virus, is a member of the genus Alphavirus of the Togaviridae family. It is generally transmitted from primates to humans via Aedes aegypti and Aedes albopictus mosquitoes [1, 2].
CHIK infection produces a self-limiting illness in humans that is often characterized by sudden onset of fever, headache, fatigue, nausea, vomiting, rash, myalgia, and severe and very painful polyarthralgia, which lasts for 1–10 days. However, arthralgia may persist for months to years [1, 3]. As is the case for most alphaviruses, detection of CHIK virus depends on isolation of the virus in blood specimens obtained from viremic patients or in infected tissue specimens obtained from blood-feeding arthropods, which are time-consuming. Molecular diagnostic tools, such as the conventional RT-PCR, are available for the study of CHIK virus replication in virus culture supernatants or clinical samples [4, 5].
We report clinical observations and laboratory investigations involving virus isolation methods and molecular assays performed for 296 clinically suspected cases of CHIK fever. Of particular interest was the applicability of a novel method of gene amplification called real-time loop-mediated isothermal amplification (RT- LAMP) as a rapid, sensitive, and specific real-time method to detect and quantify CHIK virus in the acute phase of the infection.
Materials and Methods
Clinical Samples
The study included 296 patients with a history of sudden onset of fever, headache, fatigue, nausea, vomiting, rash, myalgia, and severe and very painful polyarthralgia suggestive of CHIK infection. Patients either reported directly or were referred to Nizam's Institute of Medical Sciences (Hyderabad, India) for treatment from regions in and around Hyderabad, Andhra Pradesh, South India, during the period March–December 2006. There was no sampling bias or any attempt to specially recruit patients. The study was approved by the Institutional Ethics Committee of Nizam's Institute of Medical Sciences (EC/NIMS/675/2006). Written informed consent was obtained from each patient. Acute-phase serum samples were obtained during days 1–7 after the onset of symptoms.
Two sets of whole-blood specimens were collected from all 296 patients. One set was used for virus isolation, which was conducted using molecular assays with vacutainer EDTA tubes (BD Biosciences); the other set underwent ELISA with SST vacutainer tubes (BD Biosciences). Plasma and serum specimens were aliquoted in sterile vials and stored at −80°C at the Department of Microbiology Department at Nizam's Institute of Medical Sciences until testing. The samples were transported under cold chain to the virology laboratory at the Defense and Research Establishment (Gwalior, India), where all the assays and virus isolations were performed.
Diagnostic Assays
Serologic testing. Sixty-five of 296 patients reported for follow-up. Serum specimens obtained from these 65 patients were tested for the presence of CHIK virus—specific IgM and IgG antibodies using an in-house dipstick ELISA kit [6]. Because symptoms of CHIK fever mimic those of dengue fever, a panel of 107 of 296 serum samples obtained from patients with clinical features similar to those of CHIK or dengue fever was included in the study. In addition, a panel of 20 serum samples obtained from healthy individuals without any signs and symptoms of CHIK or dengue fever was included as a negative control.
Virus isolation. Virus isolation was attempted in C6/36 cell lines from 32 RT-PCR—positive plasma samples that were randomly selected during the outbreak. Virus isolation was performed using the virus adsorption technique [7]. In brief, a confluent monolayer of cells grown in a 25-cm2 culture flask was adsorbed with 0.5 mL of inoculum at 37°C for 2 h. After adsorption, the inoculum was replenished with 8 mL of maintenance medium supplemented with 2% fetal bovine serum. Suitable mock-infected cell controls were also incubated for comparison of cytopathic events. Cells were incubated at 37°C and were observed daily for cytopathic effects. After observation of 80%–100% cytopathic effects, the infected culture supernatant was clarified by light centrifugation at 2000 rpm for 10 min, which was further purified by sucrose gradient ultracentrifugation. The isolated virus was confirmed to be CHIK virus by RT-PCR.
Molecular assays. All 296 plasma samples were tested for the presence of CHIK virus—specific RNA by RT-PCR and RT-LAMP. Positive and negative controls were included in each run of the assays, and all precautions to prevent cross-contamination were observed.
For RT-PCR, RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen). One-step RT-PCR was performed using the Access quick RT-PCR Kit (Promega), in accordance with the manufacturer's protocol, employing primer pairs targeting the E1 gene designed from the nucleotide sequence of the reference S27 strain (GenBank accession number AF490259; CK 13, TTA CAT CAC GTG CGA TA C; CK-14, CTT TC TCT CAG GG TGC GAC TTT). The amplification was performed in a 50-µL total reaction volume with the Promega Access Quick One-Step RT-PCR kit, with 50 pmol of each forward and reverse primer and 2 µL of extracted viral RNA, in accordance with the manufacturer's instructions. The thermal profile of RT-PCR was 48°C for 45 min and 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 30 s and a final extension at 72°C for 10 min.
RT-LAMP was performed at a total 25-µL reaction volume using the Loopamp RNA amplification kit (Eiken Chemical). The real-time monitoring was accomplished by incubating at 63°C for 60 min in a Loopamp real-time turbidimeter (LA-200; Teramecs).
Real-time monitoring of the RT-LAMP amplification of CHIK virus template was observed through spectrophotometric analysis by recording the optical density at 400 nm every 6 s, with the aid of the Loopamp real-time turbidimeter (LA-200; Teramecs). The cutoff value for positivity for the real-time RT-LAMP assay was determined by taking into account the time of positivity (in min), at which point the turbidity increases to more than the threshold value (which was fixed at 0.1, which is 2 times more than the mean turbidity value for the negative controls of several replicates).
After incubation at 63°C for 60 min, 10-µL aliquots of RT-LAMP products were examined by electrophoresis on 3% NuSieve 3:1 agarose gel (BMA) in Tris-borate buffer, followed by staining with ethidium bromide and visualization on a UV transilluminator at 302 nm.
To facilitate the field application of the RT-LAMP assay, the monitoring of RT-LAMP amplification was also performed with naked-eye inspection. After amplification, tubes were inspected for white turbidity by the naked eye after a pulse spin to deposit the precipitate in the bottom of the tube. The inspection for amplification was also performed by observing a color change after the addition of 1 µL of SYBR Green I dye to the tube. Positive amplification is indicated by green fluorescence, which is permanent and which can be stored for recording purposes [7].
Genotyping
The RT-PCR—positive amplicons were subjected to double-stranded sequencing with Big Dye Terminator Cycle Sequencing Ready Reaction Kit on an ABI 310 sequencer (Applied Biosystems). The genotype of the CHIK virus, based on the partial E1 gene sequence, was determined by nucleotide sequencing and compared with 30 other globally diverse CHIK isolates. A dendrogram was constructed by pair-wise comparison of 340 nucleotide sequences of partial E1 gene (positions 10254-10503, with respect to the S27 genome), which classified all isolates into 3 different genotypes. The phylogenetic tree was constructed with the neighbor-joining method, with a bootstrap analysis of 1000 replicates, using MEGA software, version 2.1 [8].
Results
The CHIK fever epidemic affected male and female patients at a ratio of 1:1.6. The most affected age group was persons aged 31–40 years (figure 1). During the acute phase of infection, which lasted for 7–10 days, the most common symptoms among 296 patients were fever (temperature, 38.5°C–40°C) and severe arthralgia and arthritis, which affected the fingers, wrists, toes, ankles, and knee joints (table 1). The chronic phase of infection was characterized by severe joint pain, which severely limited the patients' ability to walk and perform everyday tasks. Almost 10% of cases reported experiencing prolonged arthralgia (duration, >3 weeks).
Figure 1.
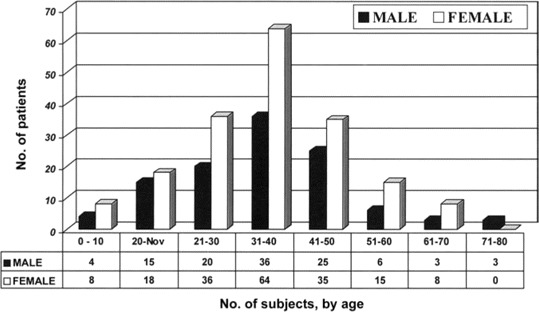
Age and sex distributions for patients with clinically suspected Chikungunya infection. The study included 112 male subjects and 184 female subjects (ratio of male to female subjects, 1:1.6).
Table 1.
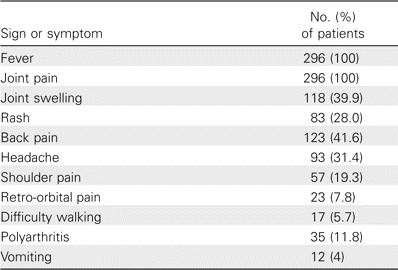
Signs and symptoms in 296 patients with clinically suspected Chikungunya fever.
A comparative analysis of blood specimens obtained from patients with suspected CHIK infection was conducted using RT-PCR, RT-LAMP, virus isolation, and IgM antibody detection; results are shown in table 2. Fifty-two (48.6%) of 107 samples tested positive for dengue virus IgM antibodies by ELISA.
Table 2.
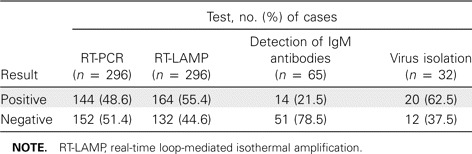
Comparative analysis of results tests for detection of Chikungunya virus in suspected cases of Chikungunya infection.
A total of 144 of 296 samples tested positive for the presence of CHIK virus RNA by RT-PCR. An additional 20 samples yielded positive results by RT-LAMP. The remaining 132 samples had negative results by both RT-PCR and RT-LAMP.
A search of the BLAST database revealed maximum homology (>99%) with CHIK virus isolates from a 2005–2006 outbreak of infection on Reunion Island. All of the isolates from the Reunion Island outbreak had 99.6%–100% sequence identity and were considered to be very closely related. The isolates also revealed a nucleotide sequence identity of 98% and 94.5% with African prototype CHIK virus (S27) and the first Indian CHIK virus (isolated from Calcutta in 1963), respectively. On the basis of the dendrogram, the Indian CHIK isolates from this epidemic belonged to the East Central South (ECS) African genotype (figure 2). Critical analysis of the branching pattern revealed that these new Indian isolates form a separate clade with other Indian Ocean island nations' isolates (from the 2005–2006 outbreaks) within the ECS Africa genotype. The older or earlier Indian CHIK virus isolates (from 1963–1973), however, belonged to the Asian genotype.
Figure 2.
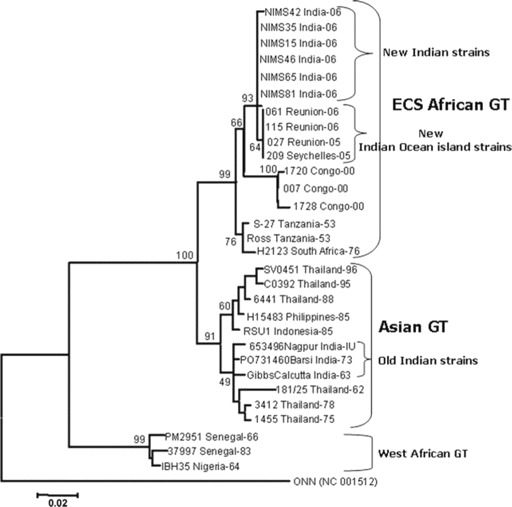
Phylogenetic analysis of Chikungunya isolates in this study. GT, genotype.
Discussion
CHIK infection has an important economic impact in many tropical countries, and because of the lack of specific symptoms, the infection cannot be differentiated from dengue or yellow fever [1, 9]. A CHIK fever epidemic is characterized by its sudden disappearance for a considerably long period from a particular geographic area before its resurgence. This has been well documented in the Republic of the Congo and Indonesia. However, in early 2005, a major epidemic of CHIK fever started in many Indian Ocean island nations, and in late 2005, it started spreading to several parts of India, after a hiatus of epidemic activity of nearly 32 years. This recent outbreak of CHIK infection in many parts of southern India is a point of major concern. This was the largest and most severe epidemic, affecting >1,000,000 persons in Andhra Pradesh, Maharastra, and Karnataka states of southern India and spreading to several new areas, with huge public health and administrative concerns to control the epidemic [10]. Although the resurgence of CHIK fever was anticipated, this epidemic is considered to be unprecedented, owing to the magnitude of morbidity and geographical distribution. In several villages, >90% of inhabitants were found to be affected [7], with an estimated figure of 1.3 million cases to date [11]. In 2006, outbreaks of CHIK fever were reported from 194 districts in 12 states across India. The clinical manifestations of cases in the current outbreak match the known description of the disease. Analysis of the 2006 outbreak suggested that the increased severity of disease may have been associated with a change in the genetic sequence, altering the virus coat protein, which potentially allowed the virus to multiply more easily in mosquito cells [12]. Laboratory diagnosis is critical to establish the diagnosis and to initiate a specific public health response.
The alphavirus species can be characterized by hemagglutination inhibition, ELISA, complement fixation, and neutralization of viral infectivity using reference serum samples [1, 9]. Serodiagnosis rests on demonstrating a 4-fold increase in CHIK virus IgG antibody titer between the acute- and convalescent-phase serum samples. Because obtainment of paired serum samples is usually not practical, the demonstration of IgM antibodies specific for CHIK virus in acute-phase serum specimens is done. A CHIK-positive viral culture, coupled with neutralization by reference serum, is taken to be definitive proof of the presence of CHIK virus. PCR results for E1 and C genome either singly or together constitute a positive result for CHIK virus [13].
In the present study, the detection of CHIK virus RNA in 48.6% of samples by RT-PCR and in 55.4% of samples by RT-LAMP, as well as the detection of IgM antibodies in 21.5% of samples, confirmed that the causative agent of this epidemic was CHIK virus. All 132 patients who had clinically suspected CHIK virus but whose RT-PCR and RT-LAMP results were negative presented >7 days after the onset of fever; this may be the reason for the negative test results. The peak number of positive PCR results occurred on day 2 of illness (figure 3).
Figure 3.
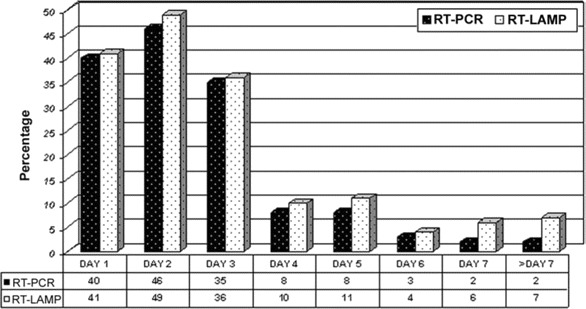
Day of illness and results for RT-PCR versus real-time loop-mediated isothermal amplification (RT-LAMP). A total of 144 of 296 samples tested positive for the presence of Chikungunya virus RNA by RT-PCR, compared with 164 of 296 samples to RT-LAMP.
The RT LAMP assay is a novel nucleic acid amplification method developed by Eiken Chemical that has the potential to replace PCR because of its simplicity, rapidity, specificity, and cost-effectiveness [14–16]. The RT-LAMP assay has emerged as a powerful gene amplification tool for rapid identification of microbial infections and is being increasingly used by various investigators for rapid detection and typing of emerging viruses, such as the West Nile, severe acute respiratory syndrome, dengue, and Japanese encephalitis viruses [17–19]. The LAMP method is cost-effective, because it requires only 1 type of DNA polymerase with strand displacement activity. In the present study, the 1-step, single-tube, real-time accelerated RT-LAMP assay was standardized by targeting the immunodominant E1 gene for rapid and real-time detection of CHIK virus. In our study, the RT-LAMP assay uncovered 20 additional cases of infection, which were detected by naked eye or with a UV lamp. These samples had negative RT-PCR results, thereby proving the high sensitivity of RT-LAMP. There were a few mismatches in the RT-PCR primers (i.e., 1 base in the forward primer and 2 bases in the reverse primer), but they did not affect the test's sensitivity [20]. All of the RT-PCR—positive samples also yielded positive RT-LAMP results. The RT-LAMP allows rapid, real-time detection of CHIK virus in acute-phase serum samples, without requiring sophisticated equipment, and has potential usefulness for clinical diagnosis and surveillance of CHIK virus in developing countries. Real-time quantification of the CHIK virus level can also be performed by RT—LAMP with use of a loop turbidimeter. Isolation of CHIK virus from 20 of 32 RT-PCR—positive clinical samples further confirmed that the infection was due to CHIK virus.
Previous phylogenetic studies showed that strains of CHIK virus were clustered into 3 distinct groups on the basis of origin: West Africa, Central/South Africa, and Asia [21]. The sequence of CHIK virus was directly determined from clinical samples without risk of altering the genome by in vitro passaging. Molecular phylogenetic analysis revealed that all of these new Indian CHIK virus strains were very closely related to analogous strains from Indian Ocean island nations and that they formed a distinct clade in ECS African genotype. These new strains are reported to harbor many unique molecular features, making them more virulent and evolutionarily more competent [22]. The clustering of older Indian isolates (including the last outbreak isolates) into the Asian genotype indicates that the cause of this outbreak was the sudden introduction of the ECS genotype into the Telangana region of Andhra Pradesh in southern India, rather than in situ evolution of existing strains. Because the period of our study was March–December 2006, which is later than the period studied by Prasanna et al. [23] and Abu Barkar et al. [24], to the best of our knowledge (after an extensive search of the Pubmed database), we submit that this is the first report of ECS African genotype of CHIK virus as the causative agent of an unprecedented epidemic.
A clustering of cases among members of the same family (40%) was observed in our study, which is in concordance with the finding that direct human-to-human transmission can occur as a consequence of high viral loads in patients, as was demonstrated in southern France [21]. Although the viremia is transient, the concentration of virus is sufficient to infect feeding vector mosquitoes [25]. That the RT-PCR and RT-LAMP results were positive during the period of peak viral load (i.e., days 1 and 2 of fever) also confirms this fact.
CHIK infection is a self-limited illness, with joint symptoms and signs usually lasting for months and occasionally for ⩾1 year [25]. However, deaths due to CHIK infection are rare [25]. Indiscriminate use of antibiotics and nonsteroidal anti-inflammatory drugs (especially aspirin) can contribute to thrombocytopenia, gastrointestinal bleeding, and vomiting. This may lead to prerenal acute renal failure and dehydration. These can indirectly contribute to mortality due to CHIK fever [26]. A high morbidity rate with no mortality was noted in our study. This is in contrast to the findings from Kerala, southern India, where the cause of death for 3 patients with CHIK fever was probably an underlying illness and was not related to CHIK infection [27].
Young adults (age, 21–40 years) were the most affected persons, and there was a preponderance of female patients in our study (P<.001, by Fisher's exact test); these findings are in concordance with the findings of the study from Reunion Island [28]. Some patients (4.7%) were so disabled that they required hospitalization, but the number of such cases was negligible, and such cases mostly involved elderly patients. The chronic phase of disease resolved over a few months, extending up to 6 months. Some patients improved after receiving short-term steroid treatment. Because no specific antiviral therapy exists for CHIK infection, treatment consists of supportive care, including administration of analgesics and anti-inflammatory medication for joint symptoms. Persons with febrile illness that is suspected to be due to CHIK virus should avoid mosquito exposure for at least 7 days after the onset of illness, to reduce the likelihood of transmitting CHIK virus to local mosquitoes, which might then transmit the virus to other humans [24]. Although the number of cases being reported has decreased, this epidemic may still be continuing and spreading. Therefore, continuous surveillance is warranted to monitor the spread of infection and to track the possible evolution of the virus during the epidemic.
The natural history of CHIK fever is not fully understood. Although mortality is rare or infrequent, early diagnosis and vector control will play an important role in preventing the outbreak of epidemics in the future. Intraoutbreak studies point toward recent changes in the viral genome that have facilitated the rapid spread and enhanced pathogenecity of infection [29]. The lack of herd immunity, as evidenced from available studies [30, 31], appears to be the simplest attributable factor. Also, the reasons for the current outbreak and the causes behind reemergence of the virus in India have to be further assessed. Molecular diagnosis is an important tool to identify new vectorborne viral illnesses, such as CHIK fever, at an early stage. Public health measures need to be improved to prevent such epidemics in the future. There is also an immediate need for an effective vaccine for CHIK infection.
Acknowledgments
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Jupp PG, McIntosh BM. Chikungunya virus disease. In: Monath TP, ed. The arboviruses: epidemiology and ecology. Boca Raton, FL: CRC Press. 1988. pp. 137–57. [Google Scholar]
- 2.Khan AH, Morita K, Mdel MD, et al. Complete nucleotide sequence of Chikungunya virus and evidence for an internal polyadenylation site. J Gen Virol. 2002;83:3075–84. doi: 10.1099/0022-1317-83-12-3075. [DOI] [PubMed] [Google Scholar]
- 3.Monath TP, Hinz FX. The alpha viruses. In: Fields BN, Knipe DM, Howley PM. Fields virology. 3rd ed. Philadelphia: Lippincott-Raven. 1996. pp. 843–98. [Google Scholar]
- 4.Hasebe F, Parquet MC, Pandey BD, et al. Combined detection and genotyping of Chikungunya virus by specific reverse transcription-polymerase chain reaction. J Med Virol. 2002;67:370–4. doi: 10.1002/jmv.10085. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi A, Tanaka M, Morita K, et al. Detection of West Nile and Japanese encephalitis viral genome sequences in cerebrospinal fluid from acute encephalitis cases in Karachi, Pakistan. Microbiol Immunol. 1994;38:827–30. doi: 10.1111/j.1348-0421.1994.tb01866.x. [DOI] [PubMed] [Google Scholar]
- 6.Schuffenecker I, Iteman I, Michault A, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parida MM, Santhosh SR, Dash PK, et al. Rapid and real-time detection of Chikungunya virus by reverse transcription loop-mediated isothermal amplification assay. J Clin Microbiol. 2007;45:351–7. doi: 10.1128/JCM.01734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics (MEGA) software, version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 9.Hundekar SL, Thakare JP, Gokhale MD, Barde PV, Argade SV, Mourya DT. Development of monoclonal antibody based antigen capture ELISA to detect Chikungunya virus antigen in mosquitoes. Indian J Med Res. 2002;115:144–8. [PubMed] [Google Scholar]
- 10.Ravi V. Re-emergence of Chikungunya virus in India. Indian J Med Microbiol. 2006;24:83–4. doi: 10.4103/0255-0857.25175. [DOI] [PubMed] [Google Scholar]
- 11.Mudur G. Failure to control mosquitoes has led to two fever epidemics in India. BMJ. 2006;333:773–773. doi: 10.1136/bmj.333.7572.773-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chikungunya Doctor NDTV health Information on Chikungunya.htm. 6 July 2006. Available at: http://www.doctorndtv.com/faq/faq.asp. [Google Scholar]
- 13.World Health Organization. Communicable Diseases Branch. Chikungunya fever, laboratory diagnosis of Chikungunya fevers. Geneva: World Health Organization. 2007. [Google Scholar]
- 14.Mori Y, Nagamine K, Tomita N, Notomi T. Detection of Loop mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Com. 2001;289:150–4. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- 15.Nagamine K, Hase T, Notomi T. Accelerated reaction by loop mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16:223–9. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]
- 16.Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong TCT, Mai QL, Cuong DV, et al. Development and evaluation of a novel Loop mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome corona virus. J Clin Microbiol. 2004;42:1956–61. doi: 10.1128/JCM.42.5.1956-1961.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parida MM, Guillermo P, Inoue S, Hasebe F, Morita K. Real-time reverse transcription loop mediated isothermal amplification for rapid detection of West Nile virus. J Clin Microbiol. 2004;42:257–63. doi: 10.1128/JCM.42.1.257-263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parida MM, Upadhyay C, Saxena P, et al. Evaluation of a dipstick ELISA and a rapid immunochromatographic test for diagnosis of dengue virus infection. Acta Virologica. 2001;45:299–304. [PubMed] [Google Scholar]
- 20.Dash PK, Parida MM, Santhosh SR, et al. East Central South African genotype as the causative agent in reemergence of Chikungunya outbreak in India. Vector Borne Zoonotic Dis. 2007;7:519–27. doi: 10.1089/vbz.2007.7272. [DOI] [PubMed] [Google Scholar]
- 21.Parola P, de Lamballerie X, Jourdan J, et al. Novel Chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–9. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuffenecker I, Iteman I. Genome microevolution of Chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yergolkar PN, Tandale BV, Arankalle VA, et al. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–3. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu Bakar S, Sam IC, Wong PF, MatRahim N, Hooi PS, Roslan N. Reemergence of endemic Chikungunya, Malaysia. Emerg Infect Dis. 2007;13:147–9. doi: 10.3201/eid1301.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Update: Chikungunya fever diagnosed among international travelers—United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:276–7. [PubMed] [Google Scholar]
- 26.Mohan A. Chikungunya fever: clinical manifestation and management. Indian J Med Res. 2006;124:471–4. [PubMed] [Google Scholar]
- 27.Das P. Infectious diseases surveillance update. Lancet Infect Dis. 2006;6:765. doi: 10.1016/S1473-3099(01)00060-3. [DOI] [PubMed] [Google Scholar]
- 28.Paquet C, Quatresous I, Solet JL. Chikungunya outbreak in Reunion: epidemiology and surveillance 2005 to early January 2006. Euro Surviell. 2006;11:E060202.3. doi: 10.2807/esw.11.05.02891-en. [DOI] [PubMed] [Google Scholar]
- 29.Lahariya C, Pradhan SK. Emergence of Chikungunya virus in Indian subcontinent after 32 years: a review. J Vector Borne Dis. 2006;43:151–60. [PubMed] [Google Scholar]
- 30.Dandawate CN, Thiruvengadam KV, Kalyanasundaram V, Rajagopal J, Rao TR. Serological survey in Madras city with special reference to chikungunya. Indian J Med Res. 1965;53:707–14. [PubMed] [Google Scholar]
- 31.Neogi DK, Bhattacharya N, Mukherjee KK, et al. Serosurvey of chikungunya antibody in Calcutta metropolis. J Commun Dis. 1995;27:19–22. [PubMed] [Google Scholar]


