Abstract
Background. We report the temporal-spatial spread of severe acute respiratory syndrome (SARS) among inpatients in a hospital ward during a major nosocomial outbreak and discuss possible mechanisms for the outbreak.
Methods. All inpatients who had stayed in the same ward as the initial index case patient for any duration before isolation were recruited into a cohort and followed up to document the occurrence of SARS. The normalized concentration of virus-laden aerosols at different locations of the ward was estimated by use of computational fluid dynamics modeling. The attack rates in the various subgroups stratified by bed location were calculated. Multivariate Cox proportional hazards regression was used to document important risk factors.
Results. The overall attack rate of SARS was 41% (30 of 74 subjects). It was 65%, 52%, and 18% in the same bay, adjacent bay, and distant bays, respectively (P = .001). Computation fluid dynamics modeling indicated that the normalized concentration of virus-laden aerosols was highest in the same bay and lowest in the distant bays. Cox regression indicated that staying in the ward on 6 or 10 March entailed higher risk, as well as staying in the same or adjacent bays. The epidemic curve showed 2 peaks, and stratified analyses by bed location suggested >1 generation of spread.
Conclusions. The temporal-spatial spread of SARS in the ward was consistent with airborne transmission, as modeled by use of computational fluid dynamics. Infected health care workers likely acted as secondary sources in the latter phase of the outbreak.
Severe acute respiratory syndrome (SARS), which originated from the Guangdong Province of China, was first recognized in February 2003 [1]. A medical doctor from Guangzhou (Canton), who stayed in a Hong Kong hotel in February 2003, was identified as the index case patient for the international spread of SARS to at least 5 countries [2]. A young Hong Kong resident visited the hotel and contracted the disease, subsequently leading to a big outbreak in the Prince of Wales Hospital, where he was treated.
Hong Kong was one of the places hardest hit by SARS, with a total of 1755 cases and a death toll of 299. In the initial phase of the outbreak in the Prince of Wales Hospital alone, >100 persons were affected, including doctors, nurses, medical students, other hospital patients, visitors, and their relatives [3]. A government investigation reported that 1 of the patients with SARS subsequently spread the disease to >300 residents in a private housing estate (Amoy Gardens) [4]. It is generally believed that SARS is primarily transmitted by droplets or direct contacts [5]; however, probable airborne transmission during the Amoy Gardens outbreak has been reported by us [6].
A detailed epidemiological study of the outbreak in the Prince of Wales Hospital may shed light on the possible modes of transmission and help in the prevention of nosocomial spread in the future. Four groups of subjects could be identified in this outbreak: the health care workers (HCWs), including doctors and nurses, the medical students who were attending clinical teaching or examinations in the ward, other inpatients staying in the same ward, and visitors. The spread of SARS among the medical students and HCWs has been reported elsewhere [7, 8]. Here, we report the temporal-spatial spread of SARS to other inpatients staying in the same ward and explore the possible factors and mechanisms involved in the nosocomial outbreak.
Materials and Methods
A retrospective cohort study was conducted. The index case patient was first admitted into the male medical ward on 4 March 2003 and was put under isolation on 12 March. All inpatients who had stayed in the same ward as the index case patient for any duration during the period 4–12 March were recruited into the cohort. Baseline information for each subject was obtained from the computerized medical record system and included age, date of admission, bed number, comorbidity, drug treatment, and date of leaving the ward (discharge or transfer). All subjects were observed until the end of March 2003 (at least 22 days) to observe whether they developed SARS. SARS was diagnosed on the basis of the interim case definition at the time [9], and all cases were subsequently confirmed serologically by immunofluorescence assay [10] showing rising titer of IgG to the SARS-associated coronavirus. The dates of onset of fever for all patients with SARS were recorded.
The attack rates of SARS among all inpatients and in the subgroups were calculated. The relationships between SARS and bed location, date of exposure, duration of exposure, smoking habit, preexisting medical illness, drug treatments, and age were first examined by univariate analyses with Χ2 tests and Student's t test. Beds were divided into 3 groups according to their proximity to the bed of the index case patient: same bay, adjacent bay, and distant bays (figure 1). Multivariate analysis with Cox proportional hazards regression was used to identify important risk factors. Variables were entered by use of a forward stepwise strategy: location, presence in the ward on specific dates, age (continuous variable), smoking habit, steroid and antiviral treatments, and various chronic diseases. All statistical analyses were done with SPSS for Windows, version 11.0.1 (SPSS).
Figure 1.
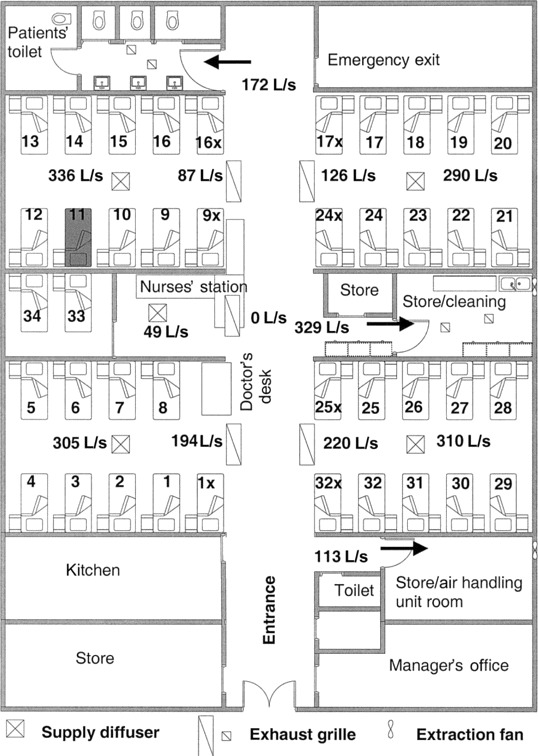
Layout of the ward where the index case patient with severe acute respiratory syndrome (SARS) was hospitalized, showing the location of beds, air supply diffusers, and exhaust grilles. Supply and exhaust flow rates were measured in liters per second). The index case patient stayed in bed 11 during the period 4–12 March. Same bay, beds 9–16, 9x, and 16x; adjacent bay, beds 17–24, 17x, and 24x; distant bays, all other beds.
To explore the possible roles of HCWs in the outbreak of infection, all nurses working on the ward during the study period were interviewed in person between 28 March and 8 April with a questionnaire to collect information on symptoms, contacts, and working practices.
Information about the ventilation system of the ward was collected, including the location and size of supply diffusers and exhaust grilles, supply air temperature, and the air-flow rate through each supply diffuser, exhaust grille, and exhaust fan. Dispersion of the hypothetical virus-laden aerosols, which originated from the index case patient's bed, through the entire ward was analyzed by computational fluid dynamics (CFD) method. CFD allows prediction of detailed 3-dimensional air-flow pattern as well as pollutant dispersion in the ward. The industry-standard CFD package Fluent [11] was used. The computational domain included the ward and the entrance.
Results
All 74 subjects who ever stayed in the ward during the study period were included into the cohort. Thirty-six subjects had their first exposures on 4 March, when the index case was admitted, and 38 new admissions took place during 5–9 March. All subjects were men, with a mean age of 66 years (range, 19–90 years); patients with SARS were slightly older than those without SARS (mean, 69 vs. 65 years; P > .05, by Student's t test). Sixteen (22%) were current smokers, and 25 (34%) were ex-smokers.
Thirty subjects developed SARS during the follow-up period, giving an overall attack rate of 40.5%. Twenty-three subjects died during the follow-up period (14 died of SARS, and the other 9 died of other unrelated causes).
Two peaks were present in the overall epidemic curve-11 March and 15 March (figure 2). Patients who stayed in the distant bays had, in general, a later onset of fever.
Figure 2.
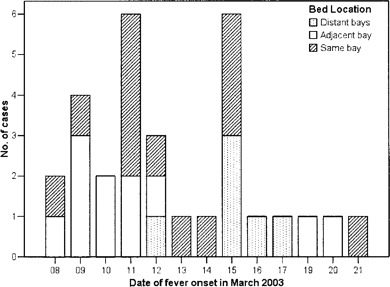
Date of fever onset for all patients with severe acute respiratory syndrome in the ward, stratified by bed location.
Spatial distribution of cases. Thirteen (65.0%) of the 20 subjects in the same bay developed SARS. The attack rate was 52.4% (11 of 21 subjects) in the adjacent bay and 18.2% (6 of 33) in the distant bays. The proportions infected in the 3 locations differed significantly (Χ2 = 13.03; P = .001).
Effects of exposure dates and duration. The date of presence in the ward was associated with SARS (table 1). Those who were not present in the ward on 6 March had a significantly lower attack rate (7 [21.2%] of 33 subjects) than did those who were present (23 [56.1%] of 41; P = .005). The duration of stay did not have any influence on SARS.
Table 1.
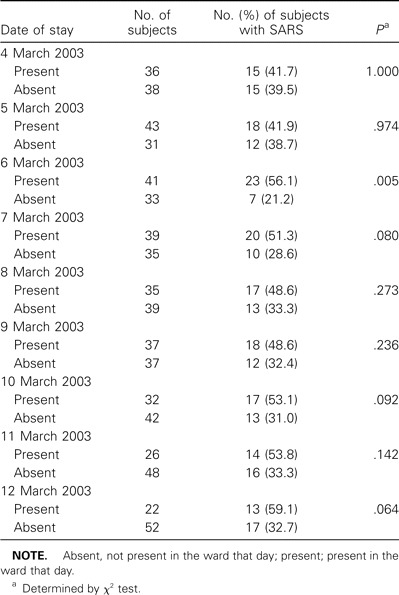
Attack rates of severe acute respiratory syndrome (SARS) for inpatients according to the dates of presence in the hospital ward during the outbreak in March 2003.
Preexisting medical diseases and treatment. All subjects had ⩾1 chronic disease, including cardiovascular disease (61% of subjects), pulmonary disease (41%), liver disease (22%), diabetes mellitus (19%), blood disease (19%), and renal disease (12%). Preexisting chronic diseases were not associated with SARS. Fifteen subjects were taking steroids for other diseases, and they had a slightly lower attack rate (33.3%), but the difference was statistically insignificant. Stratified analysis by location did not support any protective effects. of the 3 subjects receiving antiviral treatment for viral hepatitis (2 receiving lamivudine and 1 receiving oseltamivir), 2 subsequently developed SARS.
Multivariate analysis. The results of the Cox regression model are given in table 2. Presence in the ward on either 6 or 10 March was associated with a significantly higher risk of SARS. Subjects in the same bay or adjacent bay also had significantly higher risks. Age, smoking, preexisting diseases, and drugs received were not significant. The SARS-free survival curves in the 3 locations are shown in figure 3. The log rank test result was highly statistically significant (P < .001).
Table 2.
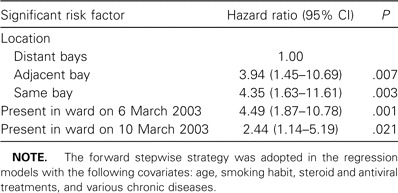
Risk factors for severe acute respiratory syndrome (SARS) among inpatients in the hospital ward during the outbreak in March 2003 identified by Cox regression.
Figure 3.
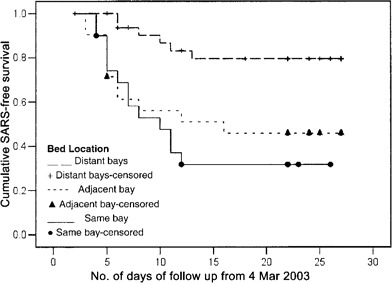
Severe acute respiratory syndrome (SARS)-free survival curves for inpatients in the 3 bed locations in the ward.
Survey among nurses. All but 1 of the 15 nurses were infected with the agent of SARS. The fever onset dates are given in figure 4A. All reported having direct contacts with the index case patient except the ward manager, who also developed SARS during this outbreak. Most subject reported washing their hands after caring for the index case patient.
Figure 4.
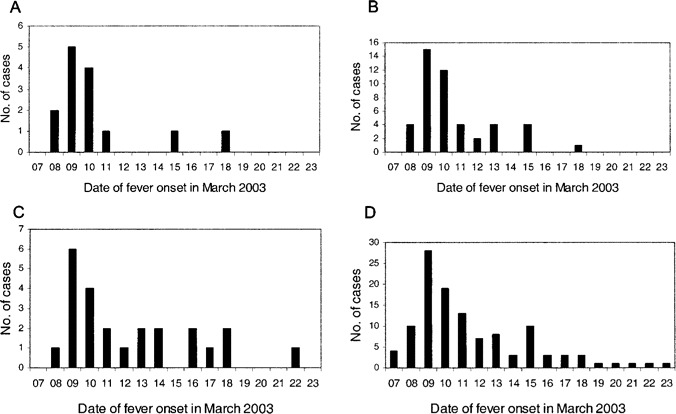
Dates of fever onset for nurses (A), all health care and supporting workers (B), visitors (C), and all patients combined (D) involved in the outbreak of severe acute respiratory syndrome.
Spatial distribution of the hypothetical virus-laden aerosols. The airflow pattern in the ward was very unsteady because of the interactions of various thermal buoyancy and momentum forces as well as movements of people. The ward was air-conditioned by a fan coil system with a separate fresh air supply. The exhaust air from this ward was discharged to the outside and not recirculated to other wards. A steady-state normalized concentration contour of virus-laden aerosols at a height of 1.1 m above ground is shown in figure 5. The concentration was between 0.015 and 1 in the same bay, between 0.005 and 0.008 in the adjacent bay, and between 0.0015 and 0.005 in the distant bays.
Figure 5.
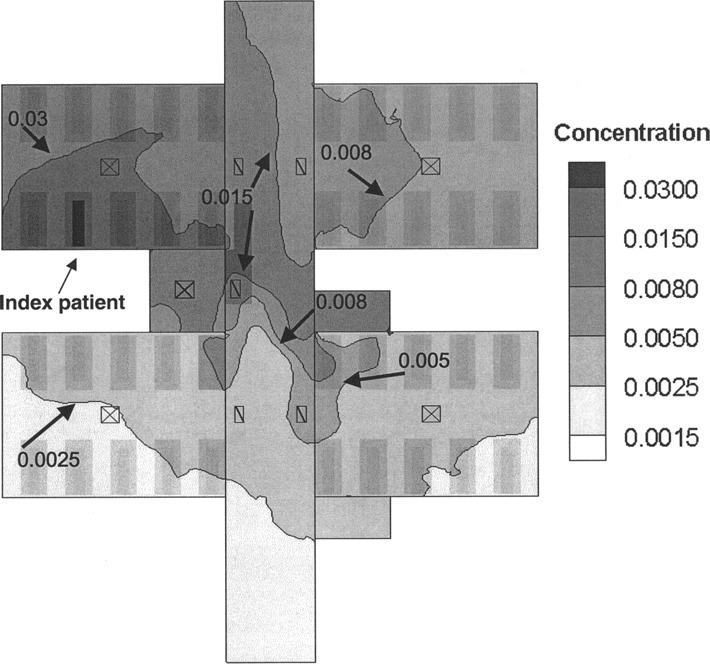
Steady-state normalized concentration contours of virus-laden aerosols in the ward at a height of 1.1 m
Discussion
In this analysis of inpatients in the medical ward with the largest nosocomial outbreak during the SARS epidemic in 2003, we included all eligible subjects into the cohort, and there was no selection bias. The data used for analysis were collected at the time of occurrence and did not depend on any recall and, thus, should be objective and unbiased. This cohort of subjects provided a good opportunity to explore the risk factors associated with nosocomial infections with the SARS agent.
The attack rate was very high, with 41% of subjects infected within a period of 8–9 days. The risk of infection was significantly associated with the bed location and the dates of presence on the ward. The duration of exposure and personal factors were not significant predictors of SARS in this cohort.
The spatial distribution of the infected inpatients was not random. The probability of such a distribution occurring by chance was only 1 in 1000. The division of bed location, based on proximity to the index case, into high-exposure (same bay), middle-exposure (adjacent bay), and low-exposure (distant bays) areas corresponded well with the normalized concentrations of the virus-laden aerosols predicted by CFD modeling. The attack rate was highest in the same bay (65%), slightly lower in the adjacent bay (52%), and much lower in the distant bays (18%), suggesting the possibility of a nonlinear relationship between concentration of virus and risk of infection. The SARS-free survival curves also indicated clearly that those staying in the distant bays were at lower risk. The relationship between proximity to the index case patient and risk of infection suggested that airborne transmission probably played an important role in this nosocomial outbreak.
Were there any alternative explanations for the particular pattern of distribution? Clustering of susceptible subjects in certain bays could theoretically lead to different attack rates. However, no particular high-risk personal characteristics or comorbidities have been identified thus far. Movements of the index case patient around the ward could have spread the infection to the other bays through direct contacts or droplets, but he was very ill during his first week of hospitalization and was bed-bound in the same bed [8]. Some inpatients in the ward were ambulatory, but it was most unlikely that many inpatients visited the index case patient and contracted the disease directly from him. They could also have acquired the infection in common areas that had been contaminated, such as the toilets, but because inpatients in the different bays were not particularly bed-bound or ambulatory, their probability of contracting the infection should not differ. We checked the bed movements (transfers) of the inpatients during the study period and found that none of the inpatients with SARS were moved to other beds after the onset of fever and, thus, could not have played a role in the spread of the infection to other bays.
The SARS coronavirus could have been spread from the index case patient to other inpatients indirectly through fomites or HCWs as mechanical carriers. A similar pattern of nosocomial infection has been reported for vancomycin-resistant enterococci and Clostridium difficile, and contaminated hands of HCWs were believed to be responsible for the contact spread [12, 13]. However, nursing duties in our ward were assigned by function and not by patient groups or geographical areas in the ward, as commonly practiced in other places. Our interviews confirmed that all nurses serving in the ward cared for inpatients in all 4 bays during each shift. All physicians in the ward also had to visit different inpatients in all 4 bays. The HCWs could potentially spread the virus to other inpatients through their unwashed hands after making contacts with the index case. Because every nurse had a chance of contacting different inpatients in the different bays, the risk of infection should be distributed more evenly among the different bays. It was still possible that some HCWs washed hands between servicing the next bay but did not wash hands between inpatients in the same bay. This would contribute substantially to within-bay spread but could not explain the relatively high attack rate in the adjacent bay.
The sixth and tenth of March were the 2 important dates in the multivariate analysis, with hazard ratios of 4.5 and 2.4, respectively. These dates corresponded very well with the 2 peaks observed in the epidemic curve on 11 and 15 March, suggesting a modal incubation period of ∼5 days from exposure to fever onset, which was very similar to that of the largest community outbreak in Amoy Gardens [6].
The epidemic curve for the distant bays started later, on 12 March, and peaked on 15 March, suggesting that there might be a secondary source of SARS in addition to the index case. Stratified Cox regression analysis by bed location (results not shown) revealed that presence on 6 March was the only significant factor in the same and adjacent bays, whereas presence on 10 March was significant in the distant bays.
The index case patient had symptom onset on 24 February, and the virus load would be at its maximum around 6 March, which was 10 days after onset of symptoms [14]. In fact, the patient's cough was most severe during 4–7 March, and his condition deteriorated on 6 March; therefore, nebulizer use was started in that afternoon and maintained until 12 March. Clinical details of the index case have been described in 2 earlier reports [8, 15]. The release of a large amount of virus into the air by the index case patient through coughing around 6 March probably caused the first wave of infections, which mainly affected inpatients staying in the same and adjacent bays. Spread by direct contacts and droplets could explain only a small number of the infections, because droplet spread is believed to be effective only within a distance of ∼1 m. Airborne spread through virus-laden aerosols was probably responsible for spreading the infection to beds outside the same bay.
The epidemic curves of nurses, all staff (including nurses, doctors, and other service workers), and visitors supported point source infection (see figure 4). There was much overlapping of the dates of onset of fever for all groups, including the inpatients (figure 2), suggesting that the infections likely came from a common source, at least in the first phase up to 12 March. Unfortunately, proper spatial analysis of SARS among the non-inpatient groups was not possible. All HCWs visited and worked in all bays during their routine work in the ward, and all but 1 had direct contacts with the index case patient. We did not have a register to provide reliable denominator data for calculating the attack rates in the different locations for visitors. However, quite a number of the infected visitors only visited inpatients staying in the adjacent bay and did not get anywhere near the index case patient. A substantial number of infected visitors experienced onset of fever earlier than did the inpatients' relatives who they visited and, thus, could not have contracted the infection from them. This offers additional indirect support for a possible airborne spread of the virus from the index case patient.
The second wave of infections could not be adequately explained by the spread from the index case patient alone. The analysis of SARS among medical students suggested that the index case patient became less infectious on 7 March [8]. Although it was possible that he became more infectious again around 10 March, this was not very likely, because his fever and chest condition gradually improved starting on 9 March [15]; other explanations should be considered. We have discussed above that bed movements of inpatients were unlikely to be responsible for the spread. We noted that a number of HCWs infected with SARS during the earlier phase of the outbreak developed fever and other symptoms on 8 and 9 March but continued working in the ward until 10 March. One or several of them most likely spread their infections to other inpatients through direct contacts or droplets and caused the second wave of the outbreak.
The analysis of the temporal-spatial spread of SARS from the index case patient to other inpatients in the ward suggested that airborne spread through virus-laden aerosols possibly played an important role. Unlike other reports of airborne outbreaks, we were unable to document the existence of the infective agent in aerosols. Such documentation was simply impossible in early March 2003, when the infective agent was yet to be identified. SARS was unlikely a communicable disease with obligate airborne transmission, such as tuberculosis, but there was evidence to suggest that SARS could have at least opportunistic airborne transmission under special circumstances when virus-laden aerosols could be generated [16]. The virus-laden aerosols could come from the nebulizer [17] or the nuclei resulting from the rapid evaporation of the droplets produced by coughing in a relatively dry, air-conditioned environment. The use of the nebulizer appeared not to be essential for the outbreak, because medical students who were present in the ward only before the use of the nebulizer also became infected [8]. Infected HCWs who continued to work after the onset of symptoms were most likely responsible for propagating the infection in the later phase of the outbreak.
The findings of this study have implications for future infection control inside hospitals. Any patients in whom any SARS-like infection is suspected should be adequately isolated, or at least segregated, to prevent the spread of infections. The bays in a large ward should be adequately partitioned structurally and provided with separate ventilation to reduce the mixing of air between bays. Personal protective equipment for preventing contact and airborne spread should be provided to HCWs, together with a proper program of equipment testing, training, and maintenance. HCWs with infections or carrying infective agents should refrain from working in areas or situations that favor the transmission of infections from them to the patients.
Acknowledgments
We gratefully acknowledge the assistance of Xinghua Huang (Shanghai Jiao Tong University, Shanghai) and Hua Qian (University of Hong Kong, Hong Kong SAR) with CFD simulations and field measurements.
Financial support. Research Grants Council of the Hong Kong Special Administrative Region, China (project no. HKU 7020/02E to Y.L.).
Conflicts of interest. All authors: no conflicts.
References
- 1.World Health Organization . Severe acute respiratory syndrome (SARS): multi-country outbreak—update, March 16, 2003. Available at: http://www.who.int/csr/don/2003_03_16/en. Accessed 25 October 2004. [Google Scholar]
- 2.Update: outbreak of severe acute respiratory syndrome—worldwide, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:241–8. [PubMed] [Google Scholar]
- 3.Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–94. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health, Hong Kong Special Administrative Region . Outbreak of severe acute respiratory syndrome (SARS) at Amoy Gardens, Kowloon Bay, Hong Kong—main findings of investigation, 17 April 2003. Available at: http://www.info.gov.hk/info/ap/pdf/amoy_e.pdf. Accessed 25 October 2004. [Google Scholar]
- 5.Seto WH, Tsang D, Yung RWH, et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–20. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu ITS, Li YG, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–9. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 7.Wong TW. An outbreak of SARS among health care workers. Occup Environ Med. 2003;60:528. doi: 10.1136/oem.60.7.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong TW, Lee CK, Tam W, et al. A cluster of severe acute respiratory syndrome among medical students exposed to a single patient in Hong Kong. Emerg Infect Dis. 2004;10:269–76. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention . Severe acute respiratory syndrome (SARS) updated interim case definition. 29 March. 2003. Available at: http://www.cdc.gov/ncidod/sars/casedefinition.htm. Accessed 22 April 2003. [Google Scholar]
- 10.Chan PKS, Ip M, Ng KC, et al. Severe acute respiratory syndrome—associated coronavirus infection. Emerg Infect Dis. 2003;9:1453–4. doi: 10.3201/eid0911.030421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fluent, version 6.1 (computer software) Lebanon, NH: Fluent; 2003. Available at: http://www.fluent.com. [Google Scholar]
- 12.Hayden MK. Insights into the epidemiology and control of infection with vancomycin-resistant enterococci. Clin Infect Dis. 2000;31:1058–65. doi: 10.1086/318126. [DOI] [PubMed] [Google Scholar]
- 13.Barbut F, Petit JC. Epidemiology of Clostridium difficile—associated infections. Clin Microbiol Infect. 2001;7:405–10. doi: 10.1046/j.1198-743x.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 14.Peiris JSM, Chu CM, Cheng VCC, et al. Clinical progress and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–72. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong RSM, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10:339–41. doi: 10.3201/eid1002.030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy CJ, Milton DK. Airborne transmission of communicable infection—the elusive pathway. N Engl J Med. 2004;350:1710–2. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson B, Cockram C. SARS: experience at Prince of Wales Hospital, Hong Kong. Lancet. 2003;361:1486–7. doi: 10.1016/S0140-6736(03)13218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


