Abstract
We conducted a prospective pilot study over a 1-year period in New Caledonia in preparation for the Pneumonia Research for Child Health (PERCH) project. The pathogens associated with hospitalized lower respiratory infections in children were identified through the use of culture of induced sputum and blood, urinary antigen detection, polymerase chain reaction (PCR) on respiratory specimens, and serology on paired sera. Respiratory viruses were detected on respiratory specimens by immunofluorescence and PCR, and by serology on paired sera. Pathogens were detected in 87.9% of the 108 hospitalized cases. Viruses represented 81.6% of the 152 pathogens detected. Respiratory syncytial virus and rhinovirus were the most frequent, accounting for 32.2% and 24.3% of the pathogens identified, respectively. Only 26.3% of 99 induced sputum specimens collected were determined to be of good quality, which may be a consequence of the collection method used.
According to World Health Organization (WHO) estimates, infectious diseases and especially pneumonia were the leading causes of death globally in children younger than 5 years of age in 2008 [1]. Accurate and rapid diagnosis of the etiology of lower respiratory infections (LRI) is challenging, especially in children. Up-to-date LRI epidemiological data are therefore essential to determine first line antibiotic therapy that can be used in each country and to identify potential new pathogens to be targeted by future vaccines [2], which is the goal of the Pneumonia Etiology Research for Child Health (PERCH) project [3]. In order to gather data for the design of the PERCH study, a pilot study was undertaken in New Caledonia. Little is known about respiratory pathogens responsible for LRI in New Caledonia [4]. Chest physiotherapy is recommended for infants with bronchiolitis as a standard of care [5] and is also often used in patients with difficulty breathing due to pneumonia. Besides being a therapeutic procedure, chest physiotherapy can also be used to obtain induced sputum specimens. Recently, it has been established that induced sputum analysis could be relevant for the diagnosis of community-acquired pneumonia in children, and will be a key specimen collected for PERCH [6]. As a pilot site for the PERCH project, the objectives of this study were to (i) evaluate the relevance of induced sputum for the etiological diagnosis of LRI in children and (ii) evaluate the relevance of nasopharyngeal specimens collected from cases by comparing them to specimens collected from controls.
METHODS
Site Description
New Caledonia is a French archipelago located in the South Pacific with a population of 245 580 inhabitants (2009 census) [7]. One quarter of the population is under 15 years old and the ethnic distribution within this age group is as follows: Melanesian (44.1%), European (27.8%), Polynesian (10.9%), people of mixed ethnicity (13.8%), Indonesian (0.6%) and other ethnic groups (2.8%). This multicultural population is mainly urban; 33.3% have a rural lifestyle and 2.1% live in tribal villages. New Caledonia has two marked seasons: a warm and wet season (December–March) and a cooler one (June–September). The population has excellent access to the health care system, which possesses European standards. However, medical consultation is sometimes delayed for people living in tribal villages and/or for those who use traditional medicine as the first point of care.
Study Design
This was a case-control study conducted at the New Caledonia Hospital (Noumea) from January 2010 through January 2011.
Case Population
Hospitalized children (1 month–15 years) admitted with LRI (ie, pneumonia or bronchiolitis) were enrolled prospectively. Criteria described in Table 1 were used for patient enrollment. LRI was classified as not severe or severe using the WHO criteria [8].
Table 1.
Enrollment Criteria for Cases and Controls [8]
| Inclusion Criteria | Exclusion Criteria | |
| Case | ||
| Age | 1 month–15 years old | <1 month or >15 years old |
| Admission status | Hospitalized | Not hospitalized or the duration of hospitalization before enrollment was >48 hours |
| Clinical features [8] | ||
| Pneumonia | Cough with tachypneaa or difficulty breathing | Chronic respiratory disease with bacterial colonization of the respiratory tract (bronchiectasis, cystic fibrosis) |
| Severe or very severe pneumonia | Signs of pneumonia plus any of the following signs or symptoms: | |
| Lower chest wall indrawing | ||
| Nasal flaring | ||
| Grunting (in young infants) | ||
| Central cyanosis | ||
| Inability to breastfeed or drink | ||
| Vomiting everything | ||
| Convulsions, lethargy or unconsciousness | ||
| Severe respiratory distress (head nodding) | ||
| Bronchiolitis | Wheezing, resistant to rapid-acting bronchodilators | |
| Hyperinflation of the chest with increased resonance to percussion | ||
| Lower chest wall indrawing | ||
| Fine crackles or rhonchi on auscultation of the chest | ||
| Difficulty in feeding, breastfeeding or drinking due to respiratory distress | ||
| Severe bronchiolitis | Signs of bronchiolitis plus any of the following signs or symptoms: | |
| Central cyanosis | ||
| Difficulty in breastfeeding or drinking, or vomiting everything | ||
| Convulsions, lethargy or unconsciousness | ||
| Respiratory distress (obvious discomfort in breathing, difficulty in drinking, feeding or talking) | ||
| Control | ||
| Age | Matched by age to a study case: | |
| Same numerical age in years if the case is >1 year | ||
| Same age group strata (1–5 months or 6–11 months) if the case is <1 year | ||
| Admission status | Hospitalized or outpatient | >48 hours of hospitalization before enrollment |
| Date of enrollment | ≤31 days after case | |
| Clinical features | Children without respiratory symptoms | Signs of respiratory infection (cough, wheezing) |
| Invasive bacterial infection (septicemia, meningitis, osteomyelitis) | ||
| Asthma |
Respiratory rate of ≥60/min in children under 2 months, ≥50/min in children between 2 and 11 months, ≥40/min in children between 1 and 5 years, ≥30/min in children between 5 and 15 years.
Control Population
Children (1 month–15 years) consulting at the hospital (Emergency Department admission, hospitalization, or appointment at the outpatient ward) without respiratory symptoms were checked for matching eligibility with cases (Table 1).
Ethics
The protocol was approved by the Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health (Baltimore, MD), and the Health Authorities in France (Agence Francaise de Sécurité Sanitaire des Aliments et des Produits de Santé, Comité de Protection des Personnes Sud-Ouest et Outre-Mer III) and in New Caledonia.
Signed written informed parental consent was obtained before enrollment.
Data and Specimen Collection
Upon enrollment, standardized questionnaires were used to record demographic and epidemiological data, clinical and radiological features and medical history for cases and controls. Cases were assessed after 48 hours and at hospital discharge for follow-up. A senior radiologist reviewed all the chest-X-rays for confirmation.
Specimens were collected shortly after admission. For cases, two different respiratory tract specimens were collected: (i) a nasopharyngeal specimen (NPS), which consisted of a nasopharyngeal aspiration (NPA) or 2 nasopharyngeal flocked swabs (NPFS) if parents or children refused the NPA, and (ii) induced sputum. Induced sputum was collected by a chest physiotherapist who cleared the upper airway and then used the increased exhalation technique (IET) to clear the distal airways, with assisted cough (AC) to facilitate large-airway clearance [4]. The manual compression of an infant’s thorax used in IET is aimed at achieving distal airway flow limitation at low lung volume to facilitate mucus clearance [9].
NPA and induced sputum specimens were divided into 2 parts: one in a sterile vial for bacterial culture and one in a viral transport medium (VTM) for virus detection. NPFS (Copan Diagnostics) were placed into STGG (skimmed milk/tryptone/glucose/glycerol) medium [10] and VTM.
Blood was collected for culture, serology (acute serum and convalescent serum 4–6 weeks after admission), antibacterial activity detection and procalcitonin measurement. Urine was collected for urinary antigen detection. For controls, one respiratory sample (NPA or 2 NPFS if parents or children refused the NPA) was collected and processed as described above. Acute and convalescent sera were also collected.
Diagnostic Methods
Tests were performed at the Institut Pasteur of New Caledonia (Noumea, New Caledonia), except for the serology tests to detect viruses and Chlamydophila pneumoniae, which were performed at Canterbury Health Laboratories (Christchurch, New Zealand).
Microbiological Laboratory Tests
Microbiological tests were carried out on respiratory specimens according to the French Society of Microbiology (SFM) criteria [11]. Induced sputum samples were Gram stained and leukocytes and epithelial cell counts recorded. Only good quality induced sputum (>25 granulocytes and <25 epithelial cells per low power field) were retained for culture [12]. Induced sputum and NPA specimens were digested by adding 0.1% dithiothreitol (Digest-EUR, Eurobio) in equal amount to the sample volume, which was then cultured at 37°C on blood agar with nalidixic acid, and on chocolate agar. For induced sputum, cultures were counted, with results expressed as colony-forming units per millilitre (CFU/mL) and interpreted using the SFM standards. NPFS were Gram stained and cultured at 37°C on the same agar media as induced sputum or NPA. Blood was cultured using the BacT/ALERT 3D system (bioMérieux) with pediatric bottles. Legionella pneumophila serogroup 1 antigens were detected in nonconcentrated urine using an immunochromatographic assay (Binax NOW, Binax Inc.).
Immunofluorescence Detection
Digested samples (obtained by induced sputum and NPA) or VTM (obtained by NPFS) were centrifuged. Epithelial cells were then spotted onto multiwell microscope slides, fixed and stained using a Monofluo Kit (Bio-Rad) for the detection of influenza A and B viruses, Parainfluenza Virus type 3 and Adenovirus (AdV); the cells were also subjected to a Monofluo Screen (Biorad) for Respiratory Syncytial Virus (RSV) detection.
Nucleic Acid Extraction and Molecular Analysis of Induced Sputum and NPS
RNA and DNA contained in induced sputum and NPS were extracted from the VTM on the EasyMag system (bioMérieux).
Real-time PCR was performed on both induced sputum and NPS for parallel detection of Chlamydophila pneumoniae and Mycoplasma pneumoniae [13] and for detection of Bordetella pertussis as previously described [14], using the LightCycler real-time PCR system (Roche Diagnostics).
A detection procedure for 18 respiratory viruses (influenza A, B and C viruses, PIV 1, 2, 3 and 4, human metapneumovirus [hMPV], RSV, human coronaviruses [hCoV] 229E, OC43, NL63, HKU1 and the severe acute respiratory syndrome-associated coronavirus [SARS-CoV], human rhinovirus [hRhV], human bocavirus [hBoV], AdV and enterovirus [EnV]) was performed on both induced sputum and NPS using a previously published multiplex RT-PCR protocol with slight modifications [15, 16].
Serology
Particle agglutination (Serodia MycoII, Fujirebio) was performed to detect immunoglobulin M (IgM) antibodies to Mycoplasma pneumoniae. We used ELISA immunoassays (Euroimmun) to detect antibodies to influenza A and B viruses, AdV, RSV and PIV1-3, and immunofluorescence (MRL Diagnostics, Cypress) to detect antibodies to C. pneumoniae.
Detection of Pre-Hospital Antibiotic Use
Acute serum was screened for antibacterial activity to detect prehospital antibiotic use as described elsewhere [17, 18].
Procalcitonin Measurement
Procalcitonin levels were measured using the Vidas B.R.A.H.M.S PCT assay (bioMérieux).
Criteria for Determination of Microbial Infection
In the case population, viral infection was confirmed by any one of these conditions: (i) the presence of a respiratory virus detected from induced sputum and/or NPS by immunofluorescence, real-time PCR or multiplex RT-PCR, (ii) a seroconversion (4-fold or greater rise) in reciprocal antibody titers to a respiratory virus on paired serum samples. Bacterial infection was confirmed by any one of these conditions: (i) the presence of a usual respiratory pathogen (Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis) cultured from blood, (ii) the presence of a usual respiratory pathogen cultured from good quality induced sputum, (iii) a urinary antigen tested positive for L. pneumophila, (iv) a PCR from induced sputum or NPS was positive for M. pneumoniae, C. pneumoniae or B. pertussis, (v) the presence of IgM reciprocal antibody titers ≥160 for M. pneumoniae in acute and/or convalescent serum, and (vi) a seroconversion (4-fold or greater rise) in reciprocal antibody titers to C. pneumoniae.
In the control population, infection was confirmed by any one of these conditions: (i) the presence of a usual respiratory pathogen detected in the NPS by culture, immunofluorescence, PCR or multiplex RT-PCR, (ii) a seroconversion or a 4-fold or greater rise in reciprocal antibody titers for respiratory viruses observed on paired serum samples, (iii) the presence of IgM reciprocal antibody titers ≥160 for M. pneumoniae in acute and/or convalescent serum, and (iv) a seroconversion (4-fold or greater rise) in reciprocal antibody titers to C. pneumoniae.
Statistics
We calculated medians and interquartile ranges (IQRs) for continuous variables, and absolute numbers and proportions for categorical variables. Comparisons between groups were conducted using Student’s t tests or the Wilcoxon rank-sum test for continuous variables, and chi-square or Fisher’s exact tests for categorical variables, as appropriate. All reported P values were 2-sided, and P < .05 was considered significant. A simple kappa coefficient was calculated to assess the agreement between the induced sputum and nasopharyngeal samples. Statistical analyses were performed using Stata 11.0 (StataCorp LP, College Station).
RESULTS
Patients
Of 251 children hospitalized with LRI during the study period, 108 (43%) were enrolled in the study (69 bronchiolitis and 39 pneumonia). The others were not screened, not eligible for the study or refused to consent.
Patient characteristics are summarized in Table 2. Patients with severe LRI (pneumonia and bronchiolitis) were younger (P = .036) and more likely not to live in tribal villages (n = 49 vs n = 20; P = .057). Melanesian patients were overrepresented in the study compared with the general population of New Caledonia (64.2% vs 44.1%, P < .001). There was no significant association between ethnic group and LRI severity. None of the patients died.
Table 2.
Main Characteristics of Cases Hospitalized With Lower Respiratory Infection in New Caledonia and Matched Controls
| All Cases |
Matched Cases and Controls |
||||||
| Characteristics | LRI (n = 108) | Bronchiolitis (n = 69) | Pneumonia (n = 39) | P a | Cases (n = 22) | Controls (n = 22) | P b |
| Age in monthsc, mean (range) | 18.1 (1–133) | 7.2 (1–44) | 37.5 (3–133) | <.001 | 19 (2–55) | 19.1 (3–53) | .98 |
| Age group in months | n = 108 | n = 69 | n = 39 | <.001 | n = 22 | n = 22 | |
| 0–5 | 41 (38%) | 37 (53.6%) | 4 (10.3%) | 4 (18.2%) | 4 (18.2%) | 1 | |
| 6–23 | 41 (38%) | 29 (42%) | 12 (30.8%) | 12 (54.5%) | 12 (54.5%) | 1 | |
| 24–59 | 19 (17.6%) | 3 (4.3%) | 16 (41%) | 6 (27.3%) | 6 (27.3%) | 1 | |
| ≥60 | 7 (6.5%) | 0 (0%) | 7 (17.9%) | 0 (0%) | 0 (0%) | NA | |
| Male gender | 69 (63.9%) | 47 (68.1%) | 22 (56.4%) | .22 | 15 (68.2) | 13 (59.1%) | .75 |
| Children diagnosed with severe infection | 69 (64.5%) | 48 (69.6%) | 21 (55.3%) | .10 | 17 (77.3%) | NA | NA |
| PCT level, ng/mL, median (IQR) | 0.155 (0.6) | 0.1 (0.24) | 0.64 (1.68) | .0001d | 0.17 (0.29) | NA | NA |
| Ethnicity | n = 106 | n = 68 | n = 38 | .6 | n = 22 | n = 22 | |
| Melanesian | 68 (64.1%) | 43 (63.2%) | 25 (65.8%) | 14 (63.6%) | 7 (31.8%) | .07 | |
| European | 8 (7.5%) | 7 (10.3%) | 1 (2.6%) | 2 (9.1%) | 6 (27.3%) | .24 | |
| Polynesian (Wallisian or Tahitian) | 21 (18.9%) | 12 (17.6%) | 9 (21%) | 4 (18.2%) | 3 (13.6%) | 1 | |
| People of mixed ethnicity | 7 (6.6%) | 5 (7.4%) | 2 (5.3%) | 1 (4.5%) | 6 (27.3%) | .09 | |
| Indonesian | 2 (2.8%) | 1 (1.5%) | 1 (5.3%) | 1 (4.5%) | 0 (0%) | 1 | |
| Lifestyle | n = 107 | n = 69 | n = 38 | .24 | n = 22 | n = 22 | |
| Urban | 55 (51.4%) | 38 (55.1%) | 17 (44.7%) | 12 (54.5%) | 16 (72.7%) | .35 | |
| Rural | 52 (48.6%) | 31 (44.9%) | 21 (55.3%) | 10 (45.4%) | 6 (27.3%) | .35 | |
| Life in a tribal village | 38/107 (35.5%) | 23/69 (33.3%) | 15/38 (39.5%) | .33 | 3/22 (13.6%) | 3/22 (13.6%) | 1 |
| Prior medical condition | n = 107 | n = 69 | n = 38 | .14 | n = 22 | n = 21 | |
| Asthma | 25 (23.4%) | 13 (18.8%) | 12 (31.6%) | 6 (27.3%) | 2 (9.5%) | .24 | |
| Prematurity | 16 (15%) | 12 (17.4%) | 4 (10.5%) | 1 (4.5%) | 2 (9.5%) | .61 | |
| Other | 8 (7.5%) | 4 (5.8%) | 4 (10.5%) | 2 (9.1%) | 1 (4.8%) | 1 | |
| Passive smoking | 57/102 (55.9%) | 36/66 (54.5%) | 21/36 (58.3%) | .5 | 14/22 (63.6)% | 5/19 (26.3%) | .03 |
| Number of children living under the same roof | n = 98 | n = 62 | n = 36 | .14 | n = 21 | n = 20 | |
| 1 | 32 (32.7%) | 22 (35.5%) | 10 (27.7%) | 9 (42.9%) | 7 (35%) | .75 | |
| 2 | 20 (20.4%) | 16 (25.8%) | 4 (11.1%) | 5 (23.8%) | 7 (35%) | .51 | |
| 3 | 21 (21.4%) | 10 (16.1%) | 11 (30.6%) | 4 (19%) | 4 (20%) | 1 | |
| ≥4 | 25 (25.5%) | 14 (22.6%) | 11 (30.6%) | 3 (14.3%) | 2 (10%) | 1 | |
| Vaccinations in children aged 6 mo–5 y | n = 60 | n = 32 | n = 28 | n = 17 | n = 16 | ||
| Children given 3 doses of PCV7 | 47 (78.3%) | 25 (78.1%) | 22 (78.6%) | .39 | 12 (70.6%) | 13 (81.2%) | .67 |
| Children given 3 doses of Hib vaccine | 55 (91.7%) | 29 (90.6%) | 26 (92.9%) | .56 | 16 (94.1%) | 15 (93.7%) | 1 |
| Vaccinations in children aged 2 y–5 y | n = 18 | n = 2 | n = 16 | n = 6 | n = 5 | ||
| Children given 4 doses of PCV7 | 12 (66.7%) | 2 (100%) | 10 (62.5%) | .53 | 3 (50%) | 4 (80%) | .55 |
| Children given 4 doses of Hib vaccine | 16 (88.9%) | 2 (100%) | 14 (87.5%) | .16 | 6 (100%) | 4 (80%) | .45 |
| Specimens collected | |||||||
| Acute serum | 94/108 (87%) | 61/69 (88.4%) | 33/39 (84.6%) | .58 | 18/22 (81.8%) | 17/22 (77.3%) | 1 |
| Convalescent serum | 50/108 (46.3%) | 29/69 (42%) | 21/39 (53.8%) | .24 | 11/22 (50%) | 6/22 (27.3%) | .22 |
| NPA/NPFS | 106/108 (98.1%) | 68/69 (98.5%) | 38/39 (97.4%) | .68 | 22/22 (100%) | 21/22 (95.5%) | 1 |
| IS | 99/108 (91.7%) | 65/69 (94.2%) | 34/39 (87.2%) | .18 | 21/22 (95.5%) | NA | NA |
| Good quality IS | 26/99 (26.3%) | 17/65 (26.1%) | 9/34 (26.5%) | .97 | 4/21 (19%) | NA | NA |
| Blood culture | 107/108 (99.1%) | 69/69 (100%) | 38/39 (97.4%) | .36 | 21/22 (95.5%) | NA | NA |
| Urine | 93/108 (86.1%) | 58/69 (84%) | 35/39 (89.7%) | .41 | 20/22 (90.9%) | NA | NA |
Data are no. (%) of patients, unless otherwise indicated.
Abbreviations: LRI, lower respiratory infection; IQR, interquartile range; NA, not applicable, PCV7, 7-valent pneumococcal conjugate vaccine; Hib, Haemophilus influenzae type b; NPA, naso-pharyngeal aspiration, NPFS, naso-pharyngeal flocked swabs; IS, induced sputum.
P value (χ2 or Fisher’s exact test) is given for comparison between bronchiolitis and pneumonia.
P value (Fisher’s exact test) is given for comparison between bronchiolitis and pneumonia.
The median age of the patients was 8.5 mo.
Wilcoxon test.
Induced sputum was obtained from 99 (91.6%) cases, of which 97 (97.9%) also had an NPS collected. Twenty six (26.3%) induced sputum samples were of good quality and 25 had a matching NPS. Patients were tested for the presence of antibiotics in the body before hospital admittance in 94 (87%) cases, and antibacterial activity was found in 26 sera (27.7%).
Pathogen Detection
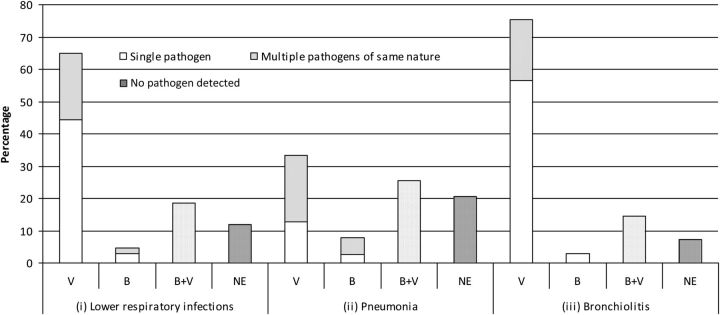
Of the 108 cases enrolled, a pathogen was detected in 95 (87.9%) (Figure 1). The yield was higher in cases with bronchiolitis than in those with pneumonia (92.7% vs 79.5%, P = .04). A viral pathogen was found in 90 (83.3%) cases and a bacterial pathogen in 25 (23.1%). We identified 51 monomicrobial infections and 44 polymicrobial infections (Table 3). Most of the bacteria were detected by culture (1 blood culture tested positive for M. catarrhalis and 13 cultures from good-quality induced sputum samples tested positive for various bacteria), 11 were detected by serology and 3 by PCR. No urinary samples tested positive for L. pneumophila serogroup 1.
Figure 1.
Distribution of lower respiratory infections in hospitalized children in New Caledonia. Distribution among (i) 108 cases with lower respiratory infections, (ii) 39 cases with pneumonia (iii) 69 cases with bronchiolitis. Abbreviations: B, Bacterial infections; V, Viral infection; B + V, Mixed bacterial and viral infection; NE, No pathogen detected; Single pathogen, one virus or one bacterium in the V or B categories respectively; Multiple pathogens of the same nature, several viruses or bacteria in the V or B categories, respectively.
Table 3.
Pathogens Detected in 108 Hospitalized Children with Lower Respiratory Infections
| All Cases |
Matched Cases and Controls |
|||||
| Pathogens | LRI (n = 108) | Bronchiolitis (n = 69) | Pneumonia (n = 39) | Cases (n = 22) | Controls (n = 22) | P a |
| Any pathogensb | 152 (100%) | 98 (100%) | 54 (100%) | 24 (100%) | 18 (100%) | |
| Bacteria | 28 (18.4%) | 12 (12.2%) | 16 (29.6%) | 7 (29.2%) | 8 (44.4%) | .35 |
| Mycoplasma pneumoniae | 12 (7.9%) | 3 (3.1%) | 9 (16.6%) | 3 (12.5%) | 0 (0%) | .25 |
| Moraxella catarrhalis | 6 (3.9%) | 4 (4.1%) | 2 (3.7%) | 1 (4.2%) | 3 (16.6%) | .30 |
| Haemophilus influenzae | 6 (3.9%) | 3 (3.1%) | 3 (5.5%) | 2 (8.3%) | 0 (0%) | .5 |
| Chlamydophila pneumoniae | 2 (1.3%) | 0 (0%) | 2 (3.7%) | 0 (0%) | 0 (0%) | NA |
| Streptococcus pneumoniae | 2 (1.3%) | 2 (2%) | 0 (0%) | 1 (4.2%) | 3 (16.6%) | .30 |
| Staphylococcus aureus | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (11.1%) | .18 |
| Viruses | 124 (81.6%) | 86 (87.7%) | 38 (70.4%) | 17 (70.8%) | 10 (55.5%) | .35 |
| Respiratory syncytial virus | 49 (32.2%) | 39 (39.8%) | 10 (18.5%) | 8 (33.3%) | 3 (16.6%) | .30 |
| Rhinovirus | 37 (24.3%) | 27 (27.5%) | 10 (18.5%) | 5 (20.8%) | 5 (27.7%) | .72 |
| Human metapneumovirus | 3 (2%) | 2 (2%) | 1 (1.8%) | 0 (0%) | 1 (5.5%) | .43 |
| Enterovirus | 5 (3.3%) | 4 (4.1%) | 1 (1.8%) | 1 (4.2%) | 0 (0%) | 1 |
| Influenza B virus | 3 (2%) | 2 (2%) | 1 (1.8%) | 0 (0%) | 0 (0%) | NA |
| Influenza C virus | 1 (0.6%) | 0 (0%) | 1 (1.8%) | 0 (0%) | 0 (0%) | NA |
| Adenovirus | 11 (7.2%) | 3 (3.1%) | 8 (14.8%) | 3 (12.5%) | 0 (0%) | .25 |
| Parainfluenza virus type 1 | 1 (0.6%) | 1 (1%) | 0 (0%) | 0 (0%) | 1 (5.5%) | .43 |
| Parainfluenza virus type 3 | 3 (2%) | 1 (1%) | 2 (3.7%) | 0 (0%) | 0 (0%) | NA |
| Parainfluenza virus type 4 | 3 (2%) | 1 (1%) | 2 (3.7%) | 0 (0%) | 0 (0%) | NA |
| Coronavirus OC43 | 2 (1.3%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | NA |
| Coronavirus NL63 | 2 (1.3%) | 1 (1%) | 1 (1.8%) | 0 (0%) | 0 (0%) | NA |
| Bocavirus | 4 (2.6%) | 3 (3.1%) | 1 (1.8%) | 0 (0%) | 0 (0%) | NA |
| Patients with 1 pathogen detected | 51/108 (47.2%) | 41/69 (59.4%) | 10/39 (25.6%) | 11/22 (50%) | 10/22 (45.4%) | 1 |
| Patients with 2 pathogens detected | 34/108 (31.5%) | 15/69 (21.7%) | 19/39 (48.7%) | 5/22 (22.7%) | 1/22 (4.5%) | .19 |
| Patients with ≥3 pathogens detected | 10/108 (9.2%) | 8/69 (11.6%) | 2/39 (5.1%) | 1/22 (4.5%) | 2/22 (9.1%) | 1 |
Abbreviations: LRI, lower respiratory infection; NA, not applicable.
P value (Fisher’s exact test) is given for comparison between matched cases and controls.
For cases: pathogens detected in nasopharyngeal specimens, induced sputum, blood, or urine; for controls: pathogens detected in nasopharyngeal specimens or blood.
For viruses, 42 were detected by immunofluorescence, 108 by PCR and 11 by serology. There were 99 (64.3%) pathogens (97 viruses and 2 bacteria) that were detected on induced sputum samples by PCR.
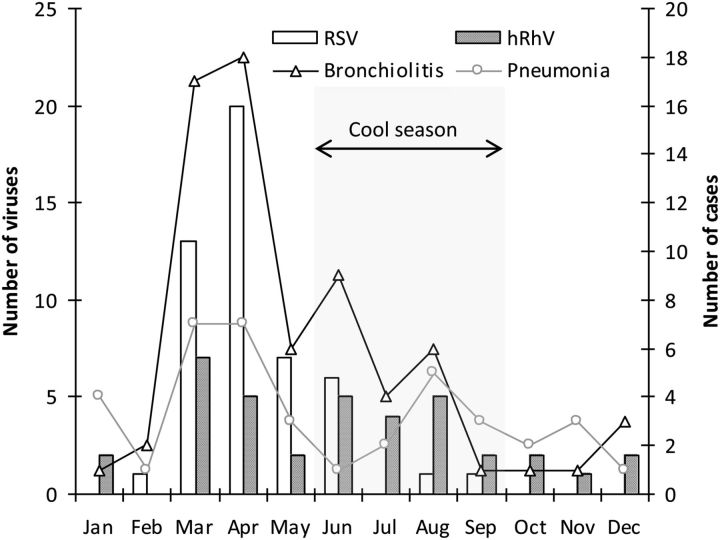
The seasonal distribution of the two most frequently detected viruses, RSV and hRhV, is shown in Figure 2. The incidence of LRI, especially bronchiolitis, was higher from March to June, and was associated with the seasonal distribution of RSV. Four influenza viruses were detected during the southern winter (August and September).
Figure 2.
Seasonal distribution of respiratory syncytial virus (RSV) and human rhinovirus (hRhV) in New Caledonian children hospitalized with lower respiratory infections.
Findings in Controls
For each case, we aimed to enroll one control, matched for the age and date of admission. Control enrollment proved challenging due to the difficulty in obtaining informed consent from the children without LRI and the novelty of conducting research in this pediatric department. Twenty-two controls were enrolled, of which 13 (59.1%) had at least one pathogen detected. Bacteria were detected in 5 (22.7%) controls, viruses in 6 (27.3%) and both bacteria and viruses in 2 (9.1%). Cultures of NPS yielded 8 bacteria in 7 controls. We detected 10 viruses in 8 controls (8 by PCR, 1 by serology, 1 by PCR and immunofluorescence).
Comparison of Results in Paired Induced Sputum and Nasopharyngeal Specimen Samples
For commonly found viruses (RSV and hRhV), the agreement between induced sputum and NPS samples was high (kappa > 0.9; Table 4); the numbers were too small for reliable determination of agreement for other viruses detected, but the agreement for any virus detected was high (kappa = 0.89). For bacteria, the agreement between the samples was lower when all induced sputum were compared regardless of quality (kappa = 0.38) for any bacterial pathogen, and also when subsets of pairs that consisted of good-quality induced sputum samples (kappa = 0.43) were compared.
Table 4.
Pathogens Detected in Paired Induced Sputum and Nasopharyngeal Specimen Samples
| Pathogen Found in: | |||||
| Pathogens | Induced Sputum Only | Naso-Pharyngeal Specimen Only | Induced Sputum and Naso-Pharyngeal Specimen | Simple Kappa Coefficient | CI 95% |
| Bacteriaa | |||||
| Streptococcus pneumoniae | 5 | 12 | 6 | 0.32 | 0.07–0.56 |
| Haemophilus influenzae | 5 | 12 | 10 | 0.44 | 0.22–0.66 |
| Moraxella catarrhalis | 5 | 8 | 3 | NI | NI |
| Staphylococcus aureus | 0 | 3 | 0 | NI | NI |
| Escherichia coli | 1 | 0 | 0 | NI | NI |
| Proteus mirabilis | 1 | 0 | 0 | NI | NI |
| Klebsiella oxytoca | 0 | 1 | 0 | NI | NI |
| Pseudomonas aeruginosa | 0 | 1 | 0 | NI | NI |
| Bordetella spp. | 0 | 2 | 0 | NI | NI |
| Mycoplasma pneumoniae | 1 | 0 | 0 | NI | NI |
| Chlamydophila pneumoniae | 0 | 0 | 1 | NI | NI |
| Total | 18 | 39 | 20 | 0.39 | 0.26–0.51 |
| Bacteriab | |||||
| Streptococcus pneumoniae | 0 | 3 | 2 | 0.52 | 0.07–0.97 |
| Haemophilus influenzae | 3 | 0 | 3 | 0.60 | 0.22–0.99 |
| Moraxella catarrhalis | 4 | 1 | 1 | NI | NI |
| Total | 7 | 4 | 6 | 0.43 | 0.15–0.71 |
| Virusesa | |||||
| Respiratory syncytial virus | 1 | 3 | 38 | 0.91 | 0.83–0.99 |
| Rhinovirus | 2 | 1 | 32 | 0.93 | 0.85–1 |
| Human metapneumovirus | 0 | 1 | 1 | 0.66 | 0.04–1 |
| Human bocavirus | 1 | 0 | 3 | 0.85 | 0.57–1 |
| Enterovirus | 1 | 1 | 2 | 0.66 | 0.21–1 |
| Influenza viruses | 0 | 0 | 3 | NI | NI |
| Coronaviruses | 0 | 2 | 1 | NI | NI |
| Adenovirus | 1 | 3 | 3 | 0.58 | 0.21–0.95 |
| Parainfluenza virus type 1 | 0 | 0 | 1 | NI | NI |
| Parainfluenza virus type 3 | 1 | 0 | 2 | 0.79 | 0.40–1 |
| Parainfluenza virus type 4 | 0 | 0 | 3 | NI | NI |
| Total | 7 | 11 | 89 | 0.90 | 0.85–0.94 |
Abbreviations: NI, not informative; CI 95%, 95% confidence interval.
Results are given for all paired induced sputum specimens and nasopharyngeal specimens (n = 97), regardless of the quality of induced sputum.
Results are given for good-quality induced sputum specimens matched with nasopharyngeal specimens (n = 25).
DISCUSSION
This study is the first description of pathogens associated with LRI in hospitalized children in New Caledonia. In 87.9% of cases, a pathogen was detected, which is consistent with published results in other settings [6, 19] and is mostly due to the elevated number of viral pathogens detected, as 83.3% of cases had evidence of viral infection. Bacteria represented 18.4% of the pathogens detected. However, we did not use PCR to detect classical bacteria, so the low bacterial detection could be the consequence of the use of less sensitive tests. It may also result from the fact that 64% of cases enrolled had a clinical diagnosis of bronchiolitis, which is more likely to be of viral origin.
This study produced four main findings regarding LRI of viral origin in children in New Caledonia. First, molecular tools enabled us to detect newer viruses including hMPV, hCoV and hBoV, which had been previously described in respiratory infections but never before identified in our setting. Second, RSV infections were clearly seasonal and peaked at the beginning of the cool season, likely promoted by community life (the beginning of the school year). Third, the seasonal distribution of influenza in children appeared to be concordant with previous findings in adults, in which the majority of cases occurred between June and September [4]. However, our ability to assess flu seasonality was limited because the total number of influenza cases observed during our study period was surprisingly small and all were associated with viral types B and C. None of these cases was accompanied by a pneumococcal superinfection, which differs from other descriptions [4, 20], but the absence of this infection may be due to our small numbers. This finding is important, as it emphasizes the value of long-term continuous surveillance to monitor trends in influenza and other respiratory viruses over time. Finally, hRhV was detected throughout the year and was as common in cases as in controls, calling into question its role as a LRI pathogen.
We most likely missed detecting many bacterial pathogens due to several important factors. First, only a small minority of pneumonia cases are bacteremic, even in the absence of prior antibiotics. Second, we obtained very few good-quality induced sputum specimens appropriate for microbiological investigation despite our study’s emphasis on collecting these specimens. Third, culture-based methods can be affected by prior antibiotic administration, and 28% of our patients had evidence of antibiotic use before hospital admission.
In our study, the yield from induced sputum was not as high as previously described [6]. We obtained specimens from >90% of cases with LRI but only about one quarter of the samples from children with pneumonia were of good quality. This compares with 75.2% of good quality samples in a study from Finland and 76.5% in the Kilifi, Kenya, PERCH pilot study [6, 21]. The different methods used for sputum induction (IET vs hypertonic saline nebulization technique) and the frequent turnover of the physiotherapists in our study are likely to be at least partially responsible for discrepancies observed between studies. In the PERCH study, sputum will be collected by the hypertonic saline nebulization method used in the Kenyan pilot study and training in specimen collection will be standardized across all sites in an effort to obtain quality specimens from all children.
Overall agreement between induced sputum and NPS samples from the same patients was high for viral pathogens. However, nearly one third of the controls without respiratory infection had at least one virus present in their upper-respiratory airways, suggesting that the presence of a virus in NPS may not be sufficient to ascribe etiology. Even for RSV, the difference in viral prevalence between cases and controls was not statistically significant (P = .30), although the number of RSV detected was small. Seven viruses were found only in induced sputum, suggesting that induced sputum analysis could be of relevance for viral detection in some cases. Cultures of good-quality induced sputum yielded only 13 bacteria of 3 different species (S. pneumoniae, H. influenzae, M. catarrhalis); of note, all of these bacteria are also known to be involved in nasopharyngeal colonization in healthy children and in children with upper respiratory tract infection [22, 23], as confirmed in our study by bacterial colonization among 31.8% of the controls. The fact that 4 of the bacteria isolated from induced sputum were also cultured from the matched NPS indicates that we cannot exclude the possibility of contamination by upper respiratory secretions. Therefore, it would be interesting to determine if a pathogen isolated from a good-quality induced sputum sample is more likely to be responsible for the respiratory infection if absent from the NPS collected simultaneously. In order to progress in the interpretation of findings from paired samples of induced sputum and NPS, a comparison with a gold standard method (eg, transthoracic needle aspiration) is needed. Alternatively, assessing differences in the bacterial load at each site may help distinguish colonization from infection. Both approaches are planned in the PERCH study.
Although we have emphasized the limitations of pathogen detection to ascribe an etiological diagnosis, our findings may nevertheless reflect a true predominance of viruses among cases of hospitalized LRI. In other settings with good primary care, high H. influenzae type b (Hib) and pneumococcal vaccine coverage and high access to antibiotics, most pediatric respiratory illness of bacterial origin will be prevented or treated in the outpatient setting and never require hospitalization. In a trial of Hib vaccine in Lombok, Indonesia, which used active surveillance to identify sick children at home and treat them in village clinics early in the course of illness, the vaccine showed no protective effect against hospitalized LRI, suggesting that the early treatment of Hib cases prevented the progression to severe disease [24]. Similar results were observed for protection against hospitalized LRI by pneumococcal conjugate vaccine in a trial among Native Americans (K. O’Brien, personal communication). Together, these results indicate that viruses cause the majority of severe, hospitalized cases of LRI in locations with good access to outpatient antibiotic treatment and support the etiological findings from our study. Related to this point, our study also likely differs from the PERCH study because the majority of enrolled patients had a diagnosis of bronchiolitis, rather than severe pneumonia. The PERCH study will enroll hospitalized cases of severe and very severe pneumonia as defined by WHO. Because this definition is nonspecific, it will likely capture some cases of bronchiolitis as well as pneumonia. The PERCH study will attempt to exclude children with bronchiolitis by giving children a bronchodilator challenge [25]. Children with an indrawn lower chest wall that resolves with this therapy, regardless of its effect on wheeze, will be considered to likely have bronchiolitis, and will be excluded from the main PERCH study.
In conclusion, the high rates of viral pathogen detection were attributed to newly developed techniques (eg, PCR) as described elsewhere [26]. Bacterial detection remained challenging and induced sputum analysis was hampered by difficulties in obtaining good quality specimens, which may have been a consequence of the collection method. Further studies are needed in order to properly distinguish between infection and colonization and will consist of comparing findings in NPS and induced sputum with other reference standards. This pilot study allowed us to improve the study design of the multi-site PERCH study in the following respects: (i) emphasizing the importance of good induced sputum collection technique using a method shown to yield high quality specimens [21, 27] (ii) highlighting potential barriers to control enrollment.
Notes
Acknowledgments.
We thank the Pôle Intégré de Recherche Clinique (Institut Pasteur), Allan Massie, Dr Paul Martin, Laeticia Faust, Dr Yann Barguil, Dr Elisabeth Lhote, the medical and technical staff from Institut Pasteur of New Caledonia for their assistance and support, and the serology staff from Canterbury Health Laboratories.
Supplement sponsorship.
This article was published as part of a supplement entitled “Pneumonia Etiology Research for Child Health,” sponsored by a grant from The Bill & Melinda Gates Foundation to the PERCH Project of Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Black RE, Cousens S, Johnson HL, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Scott JA, Brooks WA, Peiris JS, Holtzman D, Mulholland EK. Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest. 2008;118:1291–300. doi: 10.1172/JCI33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine OS, O’Brien KL, Knoll MD, et al. PERCH: a 21st century childhood pneumonia etiology study. Clin Infect Dis. 2012;54(Suppl 2):S93–101. doi: 10.1093/cid/cir1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mermond S, Berlioz-Arthaud A, Estivals M, Baumann F, Levenes H, Martin PM. Aetiology of community-acquired pneumonia in hospitalized adult patients in New Caledonia. Trop Med Int Health. 2010;15:1517–24. doi: 10.1111/j.1365-3156.2010.02653.x. [DOI] [PubMed] [Google Scholar]
- 5.Agence Nationale d’Accréditation et d’Evaluation en Santé. Consensus conference on the management of bronchiolitis in infants. Arch Pediatr. 2001;8(suppl 1):1s–196s. Available at: http://www.has-sante.fr/portail/upload/docs/application/pdf/Bronchiolitis.pdf. Accessed 7 April 2010. [PubMed] [Google Scholar]
- 6.Lahti E, Peltola V, Waris M, et al. Induced sputum in the diagnosis of childhood community-acquired pneumonia. Thorax. 2009;64:252–7. doi: 10.1136/thx.2008.099051. [DOI] [PubMed] [Google Scholar]
- 7.Institut de la Statistique et des Etudes Economiques. Recensement de la population. 2009. Available at: http://www.isee.nc/population/population.html. Accessed 12 April 2010. [Google Scholar]
- 8.World Health Organization. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. 2005 Available at: http://whqlibdoc.who.int/publications/2005/9241546700.pdf. Accessed 7 April 2010. [PubMed] [Google Scholar]
- 9.Gajdos V, Katsahian S, Beydon N, et al. Effectiveness of chest physiotherapy in infants hospitalized with acute bronchiolitis: a multicenter, randomized, controlled trial. PLoS Med. 2010;7:e1000345. doi: 10.1371/journal.pmed.1000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien KL, Nohynek H World Health Organization Pneumococcal Vaccine Trials Carriage Working Group. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:e1–11. doi: 10.1097/01.inf.0000049347.42983.77. [DOI] [PubMed] [Google Scholar]
- 11.Société Française de Microbiologie. REMIC. Paris, France: Société Française de Microbiologie; 2010. Diagnostic microbiologique des infections broncho-pulmonaires; pp. 93–8. [Google Scholar]
- 12.Geckler RW, Gremillion DH, McAllister CK, Ellenbogen C. Microscopic and bacteriological comparison of paired sputa and transtracheal aspirates. J Clin Microbiol. 1977;6:396–9. doi: 10.1128/jcm.6.4.396-399.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raggam RB, Leitner E, Berg J, Mühlbauer G, Marth E, Kessler HH. Single-run, parallel detection of DNA from three pneumonia-producing bacteria by real-time polymerase chain reaction. J Mol Diagn. 2005;7:133–8. doi: 10.1016/S1525-1578(10)60019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ménard A, Lehours P, Sarlangue J, Bébéar C, Mégraud F, de Barbeyrac B. Development of a real-time PCR for the identification of Bordetella pertussis and Bordetella parapertussis. Clin Microbiol Infect. 2007;13:419–23. doi: 10.1111/j.1469-0691.2006.01659.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Ren P, Sheng J, et al. Simultaneous detection of respiratory viruses in children with acute respiratory infection using two different multiplex reverse transcription-PCR assays. J Virol Methods. 2009;162:40–5. doi: 10.1016/j.jviromet.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buecher C, Mardy S, Wang W, et al. Use of a multiplex PCR/RT-PCR approach to assess the viral causes of influenza-like illnesses in Cambodia during three consecutive dry seasons. J Med Virol. 2010;82:1762–2. doi: 10.1002/jmv.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berkley JA, Loew BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes J, Hyder JA, Peruski LF, et al. Antibiotic use in Thailand: quantifying impact on blood culture yield and estimates of pneumococcal bacteremia incidence. Am J Trop Med Hyg. 2010;83:301–6. doi: 10.4269/ajtmh.2010.09-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–7. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien KL, Walters MI, Sellman J, et al. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin Infect Dis. 2000;30:784–9. doi: 10.1086/313772. [DOI] [PubMed] [Google Scholar]
- 21.Hammitt L, Kazungu S, Morpeth SC, et al. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis. 2012 doi: 10.1093/cid/cir1071. ; This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison LM, Morris JA, Telford DR, Brown SM, Jones K. The nasopharyngeal bacterial flora in infancy: effects of age, gender, season, viral upper respiratory tract infection and sleeping position. FEMS Immunol Med Microbiol. 1999;25:19–28. doi: 10.1111/j.1574-695X.1999.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 23.Charvériat MA, Chomarat M, Watson M, Garin B. Nasopharyngeal carriage of Streptococcus pneumoniae in healthy children, 2 to 24 months of age, in New-Caledonia. Med Mal Infect. 2005;35:500–6. doi: 10.1016/j.medmal.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Gessner BD, Sutanto A, Linehan M, et al. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet. 2005;365:43–52. doi: 10.1016/s0140-6736(04)17664-2. [DOI] [PubMed] [Google Scholar]
- 25.Scott JA, Wonodi C, Moisi JC, et al. The Pneumonia Methods Working Group. The definition of pneumonia, the assessment of severity and clinical standardization. Clin Infect Dis. 2012;54(Suppl 2):S109–16. doi: 10.1093/cid/cir1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011;377:1264–75. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant L, Hammitt LL, Murdoch DR, O’Brien KL, Scott JA. Procedures for the collection of induced sputum specimens from children. Clin Infect Dis. 2012;54(Suppl 2):S140–5. doi: 10.1093/cid/cir1069. [DOI] [PMC free article] [PubMed] [Google Scholar]