Influenza
Unbeckoned Queen of Crusades past whose subjects told of swathes cut through the rich, the poor, the young and old; Your reign we've fought, your scourge we've sought to yet confine, and thus we've learned your mutable genomic spine.
But we have yet to learn your drummer's warning beat; We still don't know when you are close on silent feet, or if you've wakened from celestial slumberings and will descend abruptly on miasmic wings.— C.B.H.
Since ancient times, the abrupt appearance and spread of maladies has demanded and defied explanation by mankind. In 400 B.C., Hippocrates suggested that the environment, including water and air, were important, and in the second century A.D., Galen suggested that outbreaks of illnesses were caused by inhaled air. For centuries thereafter, the prime theories proposed that illnesses arose from mystical influences and noxious effluvia borne by air [1]. The widespread devastation inflicted by plagues was thought to result from inhaling the putrescent vapors of decaying corpses. This belief in “miasmas” (derived from the Greek word for pollution) engendered perhaps the first infection-control procedures. The unfortunate souls assigned to handle the corpses were gowned in long robes with hoods to which were attached beaks stuffed with herbs, which were intended to filter the miasmas from the air (figure 1).
Figure 1.

Protective clothes of a plague physician from the 17th Century. German engraving by Paul Furst Nuremberg, 1656.
Epidemics of influenza were similarly believed to erupt from the dispersion of mystical elements. Indeed, our current term “influenza” may be traced to the Italian “influenza coeli” or “influenza del diavolo” (i.e., influence of celestial bodies or of the devil). Even in the mid-1800s, when John Snow conclusively demonstrated, during his famous investigation of the 1848–1854 cholera epidemic, that the disease was spread by contaminated water, the English Board of Health officially decreed that the cause was noxious vapors arising from the Thames River. Not until 1910, when Charles Chapin challenged the dominance of the airborne route by proposing that most common infectious diseases were acquired by close contact with an infected individual, was the current alternative paradigm accepted that organisms may have >1 mechanism of spread [2, 3].
The Spread of Viruses Shed
At present, 3 major means of transmission of infectious organisms are generally recognized and form the basis of current recommendations for infection control (table 1) [4, 5]. These differing sojourns and the survival of particles in aerosols have been better elucidated recently by new technology and help explain their capricious nature as effective vectors of infectious agents [6,7,8–9]. of prime importance are the size of the particle, usually described as the particle's aerodynamic equivalent diameter (AED), and its settling velocity. Particles of large AED settle quickly and, thus, are hazards primarily to those in close vicinity to an infected person. On the other hand, small particles are likely to remain airborne and destined for further spread [7, 8]. For example, particles with AEDs of 100 have settling times of 6.7 s, compared with 18.5 h for particles with an AED of 1. Furthermore, the size of the particles may, to a large extent, dictate the likelihood of infection occurring and the site of infection [6, 7, 9]. Although the concentration of infectious particles in airborne aerosols usually is low, these small particles are likely to result in lower respiratory tract disease, whereas larger particles are more apt to settle in the upper respiratory tract, often resulting in milder disease and longer incubation periods.
Table 1.
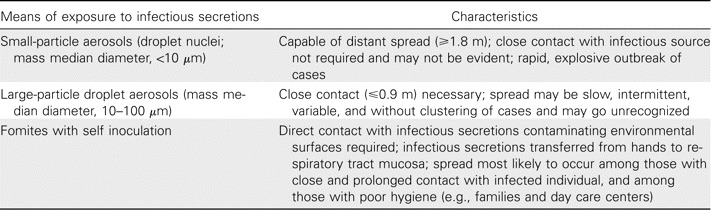
Major modes of spread of respiratory viruses and associated characteristics.
These properties of viral agents and environmental conditions are constantly changing, making determination of the primary mode of transmission at any one time difficult. However, they may explain the “celestial influences” that have produced the unpredictability of the disease and of which individuals will be stricken.
Viral and Environmental Factors
Nonenveloped viruses, such as picornaviruses, are usually hardier and able to survive longer than enveloped viruses, which are more susceptible to degradation because of their lipid envelopes. The envelope of Paramyxovirinae respiratory viruses, such as respiratory syncytial virus (RSV), parainfluenzavirus, and influenzaviruses, is studded with the major glycoproteins, which are integral for attachment and infection of the host cell. The cellular tropism of the virus is also instrumental with regard to whether infection occurs. Most common cold viruses, such as rhinovirus, parainfluenzavirus, influenzavirus, and RSV, readily infect the epithelium of the upper airway, thus allowing infection to occur via direct contact with infectious secretions by large droplets or contaminated hands. Some organisms, such as Mycobacterium tuberculosis, however, require settling in the lower respiratory tract for infection to occur [6, 8].
Environmental conditions also affect whether a virus, once propelled by coughing or sneezing, will remain stable and viable during its journey to a susceptible host. Viruses generally survive better on hard surfaces than on porous surfaces or hands. RSV remains infectious on counter tops for ⩽6 h, but it remains so for 20–30 min on gowns or paper tissues and for <t;20 min on skin; this is sufficient to cause infection when contaminated hands touch the eyes or nose [10]. Similar findings have been demonstrated for influenzaviruses [11]. Rhinoviruses and adenoviruses, however, are hardy survivors, and under varying conditions, they can be recovered from contaminated nonporous surfaces for days.
The relative humidity or dew point also affects the viability and dispersal of viruses in secretions. The optimal levels of relative humidity that prolong infectivity vary appreciably on the basis of the virus. Under experimental conditions, rhinoviruses, other picornaviruses, and adenoviruses tend to survive best at high relative humidities (approximately 70%–80%), whereas the viability of RSV, parainfluenzavirus, and influenzavirus A is better at the lower relative humidities (<t;30%), which are frequently present in hospital wards during winter respiratory seasons [12, 13].
Low levels of humidity, however, enhance evaporation and may cause the metamorphosis of large particles into droplet nuclei that become airborne with distant dispersal. Conversely, small particles may be humidified during inhalation and balloon into larger particles that settle in the upper respiratory tract [14]. Small particle aerosol dissemination is also highly affected by air movement, which may be affected by causes as mundane as opening and shutting doors or walking in and out of a room. Dispersal of aerosols may be uneven in the crannies and corners of a room and dependent on the location of the ventilation system [15]. Settled infectious particles ferried on “rafts” (i.e., shed flakes of skin) or dust may be recirculated by such movement and, if they reach sunlight, rendered less infectious.
Recent intriguing data suggest that individuals inherently differ in their ability to spread viral infectious agents. Sneezing and coughing have long been recognized as much more effective means of propelling secretions than are shouting and speaking loudly [14, 16]. More recently, however, even quiet breathing has been shown to generate small particle aerosols in quantities that vary among individuals [17]. Normal mouth breathing by some people may produce larger quantities of airborne droplets than nose breathing, talking, or even coughing [18, 19]. These droplets are primarily <t;1 µm in diameter, because larger droplets tend to be filtered out during expiration.
Some individuals are “super shedders”—the infectious “Pig Pens” of the Charlie Brown cartoon—who exhale such great quantities of aerosols that they continually are surrounded by clouds of respiratory secretions [17]. Among 11 subjects examined, the number of respiratory particles generated by quiet breathing varied bimodally and by individual. Over a 6-h period, 5 subjects produced only 14–71 particles per liter of expired air, whereas 6 volunteers exhaled 10 times that number (mean quantity, 500 particles per liter of expired air). One superlative shedder generated >3000 particles per liter of expired air during quiet breathing. Nebulized saline administered to these “high producers,” however, diminished their expelled bioaerosol load, suggesting that altered surface tensions of fluids in the airway may explain the variable generation of aerosols among individuals. These unknown individual differences and the inclusion of “super shedders” in studies that examine the transmission of viruses may account for the inconsistent and confounding results that are sometimes observed [20,21,22,23,24–25].
Concern over the “airborne spread of noxious elements” has reemerged with recognition of new pathogens, such as the severe acute respiratory syndrome (SARS) coronavirus and the avian influenzavirus. Yet the importance of the airborne route in spreading these agents remains difficult to estimate and control, and for influenza, it remains controversial. Animals and volunteer experimental studies have revealed that influenzavirus may be transmitted by droplet nuclei [14, 26,27–28]. Some clinical studies also have indicated airborne spread by a subsequent abrupt outbreak of infection, but others have not [29,30,31–32].
Considering these conflicting and limited data, deciding which infection-control procedures should be recommended during the respiratory infection season, especially for pediatric wards, is problematic. Should the routine precautions be effective for both influenza and RSV infection, being that outbreaks of these infections almost always overlap? Should they be effective for both close contact and airborne spread? If so, is the observed benefit enough to balance the considerable added cost, effort, and risk of noncompliance?
Nosocomial Influenza on Infant Wards
Experimental studies have defined the possible modes of spread of influenza, but they do not provide pragmatic answers to the aforementioned questions. Thus, I and our staff conducted a study that examined the transmission of nosocomial influenza on our infant ward as part of our ongoing viral nosocomial studies during 2 respiratory infection seasons (November–April). The aim was to determine whether nosocomial influenza resulted primarily from close contact or airborne spread in the “real world” of a ward housing influenza-naive infants, who would be at high risk for nosocomial infection during the busy respiratory season.
Study design. In the first year, the outbreak of influenza, which was caused by influenzavirus A/H3N2, began in mid-February and lasted for 5 weeks. The following year, an outbreak of influenza due to influenzaviruses B and A/H3N2 began in mid-January and lasted for 8 weeks.
The ward had 18 patient rooms; 6 contained 2 (or sometimes 3) cribs, and 12 had 1 crib (Figure 2). Each room had a sink and its own bathroom. The ventilation system was designed to maintain pressures that were equal between the inside of the room and the rest of the ward. However, when tested, the differential between the inside and outside pressures of the individual rooms varied considerably, especially when doors were opened and closed and when people entered or exited the room. The doors of most rooms were left open for appreciable periods during the day.
Figure 2.
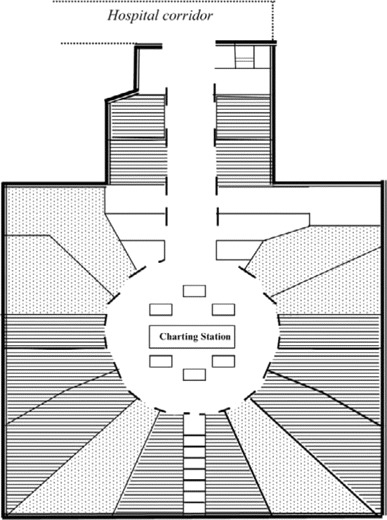
Diagram of an infant ward showing rooms with 1 bed (horizontal lines) and 2–3 beds (dots).
The infants studied were ⩽1 year of age and, thus, had not been exposed previously to an influenza outbreak. All of the infants were in cribs and did not require assisted ventilation. The dates of hospitalization, room location, and number of roommates were recorded for each infant. Nasal aspirate specimens were obtained for viral isolation from all children every 2–3 days.
The infection-control procedures were those routinely instituted on the pediatric ward during the respiratory season. In addition to standard precautions, these included admission to a single-bed room for children with signs of acute respiratory illness of unidentified etiology. Cohorting was used when a specific virus had been identified by rapid screening tests or culture. If a child developed fever or acute respiratory signs after admission to the pediatric ward, the child was moved to a separate room as soon as possible. Nursing personnel were requested to be immunized and, as feasible, did not simultaneously care for infants with and infants without acute respiratory infection. Visits were discouraged, and family members were screened for signs of acute illness. Young siblings were allowed to visit only with permission and were confined to the patient's room. For close contact with infants with acute respiratory symptoms, personnel wore gowns but not gloves. Surgical masks that covered the mouth and nose were not routinely recommended, but they were used when the child was known or suspected to have influenza.
Results. During the 2 respiratory infection seasons, 225 eligible children were admitted to the ward, and influenzavirus was isolated from the nasal aspirate specimens obtained from 45 children. Twenty-six of these children were admitted with laboratory-proven influenza, leaving 199 children who could have potentially acquired influenza nosocomially. of these 199 infants whose specimens initially tested negative for influenzavirus, 19 (9.5%) subsequently acquired influenza nosocomially (table 2). During the study, other respiratory viruses also were identified (primarily RSV), and 3 children had influenza and RSV coinfection.
Table 2.
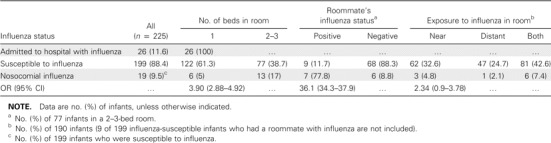
Proportion of 225 infants (age, ⩽1 year) hospitalized during 2 respiratory seasons (November–April) who acquired influenza nosocomially, according to the number of beds in the room and the distance from exposure to a laboratory-confirmed case of influenza.
We examined the risk that a child would acquire influenza nosocomially in relation to the number of roommates in the child's room, in relation to whether the child had a roommate who subsequently developed laboratory-confirmed influenza, and in relation to the distance of the infant's room from a room that housed a child with proven influenza (table 2). Seventy-seven infants were admitted to rooms that housed 1 or 2 infant roommates, and 148 were housed in a single room, including the 26 infants who were admitted with influenza. Thus, 122 infants in single rooms were potentially susceptible to influenza, and of these, 6 (5%) acquired influenza nosocomially. In comparison, 13 (17%) of the 77 infants in a multiple-crib room became infected. Thus, compared with those in a single-crib bedroom, children with 1 or 2 roommates were ∼3 times more likely to acquire influenza nosocomially (OR, 3.90; 95% CI, 2.88–4.92). of the 77 children with roommates, 9 had a roommate with subsequently proven influenza, and 7 (77.8%) acquired influenza nosocomially, compared with 6 (8.8%) of the 68 children whose roommates remained uninfected (OR, 36.1; 95% CI, 34.3–37.9). The rate at which infants acquired influenza among those whose only laboratory-proven exposure to a child with influenza during hospitalization was from a distant room across the ward was 4.8%, compared with 2% for children whose only known exposure involved a person with influenza within 2 doors of the child's room (OR, 2.37; 95% CI, 0.9–3.78) (table 2).
These findings suggest that airborne droplets were not the major mode of transmission of influenzavirus on this infants' ward. However, as mentioned previously, the prominent mode may change transiently with fluctuating environmental conditions. Although none of the surveillance specimens obtained from working personnel tested positive for influenza, other sources of infection (e.g., visitors) may not have been detected, even though we screened them for illness. of note is that, during each of the 2 respiratory infection seasons studied, 1–3 separated influenza cases occurred before peak influenza activity began, none of which were followed by an abrupt outbreak of influenza illnesses on the ward, as would be expected with airborne droplet spread from a single source.
Infection-Control Procedures During the Viral Respiratory Season
In view of these often conflicting experimental and clinical observations [24, 25], which infection-control procedures during the respiratory season should be recommended that would be feasible? Those most commonly recommended procedures have aimed primarily at interrupting spread among close contacts from large droplets and from secretions that contaminate fomites, and precautions preventing airborne transmission are applied only to rooms that house patients whose illness is proven or suspected to be influenza [4, 33,34–35] (table 3).
Table 3.
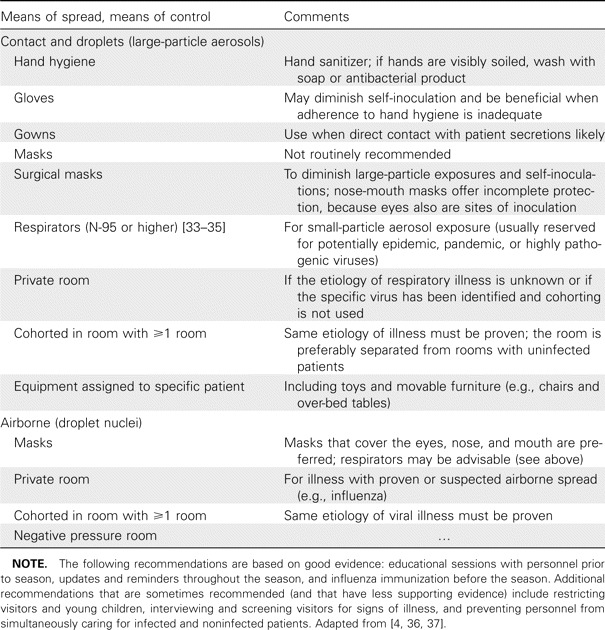
Infection-control procedures for prevention of spread of common respiratory viruses.
Among the many proposed procedures for infection control, hand cleansing has been perhaps the only one that is not controversial. Recognition of the pivotal role of clean hands for the control of infections may be credited to Ignaz Semmelweis. In the 1840s, he observed that fewer puerperal infections occurred in his obstetrical clinic in Vienna when he required physicians to clean their hands with a chlorine-containing solution between visits with patients. Despite the recognized importance of hand cleansing in diminishing nosocomial infections, compliance with hand hygiene procedures by health care personnel is singularly poor [36,37–38]. This situation may be improved by the recent recommendation for the preferential use of alcohol-based hand-sanitizing products [36]. This recommendation is based on the fact that their efficacy is greater than that for soap-and-water washing in reducing the number of organisms on the skin, on the low occurrence of adverse effects, and on the resulting increased compliance by health care workers. Although use of hand sanitizers has been correlated with diminished occurrence of nosocomial infections in general, evidence that they decrease the spread of and illness associated with specific viruses is limited. Alcohol-containing products have been demonstrated in vitro to have greater efficacy against enveloped respiratory viruses than against nonenveloped viruses, including picornaviruses, parechoviruses, adenoviruses, and rotavirus. Inactivation of these latter, more-stubborn viral agents may be enhanced by higher concentrations of alcohol or by increasing the time and thoroughness of the scrubbing during hand washing.
The evidence that specific measures (other than hand hygiene) effect a significant reduction in nosocomial viral infections is sparse. Disinfection of environmental surfaces has been commonly incorporated into infection-control programs. The goal is not to eradicate organisms but to diminish their viability sufficiently such that, when transferred to hands or fomites, infection will not result. A review of 236 articles about the practice of using chemical disinfectants in health care facilities, however, concluded that use of these products was no more beneficial in reducing nosocomial infections than was usual cleansing with soap or detergent [39]. Some disinfecting agents also have potentially adverse or toxic effects, and their routine use in patient areas is not recommended [40].
Short wavelengths (100–280 nm) of UV light (UVC) or germicidal UV radiation have also been used in hospitals to inactivate organisms that contaminate environmental surfaces, as well as airborne organisms, especially M. tuberculosis [41]. Respiratory viruses have been shown to be inactivated by UV light experimentally and in an epidemiologic study from the 1950s that suggested that a hospital wing with UV lights had experienced a diminished spread of influenzavirus, compared with a wing that lacked UV lights [42]. More recent experimental studies have demonstrated that UV inactivation of viruses is affected by multiple factors, including varying sensitivity of viruses, the location of UV light fixtures, wattage, and relative humidities >60% [41]. UV light fixtures have been used in some hospitals in open patient areas, such as clinics, and in ventilation ducts and air conditioning systems, rather than in patient rooms, because of safety concerns. However, whether UV light reduces nosocomial viral infections on patient wards remains unclear.
The use of rapid diagnostic tests, primarily for RSV infection and influenza, to aid in decisions regarding isolation procedures has appeared to be beneficial in some studies [43,44,45–46]. The recent guidelines for the management of bronchiolitis from the American Academy of Pediatrics, however, do not recommend the routine use of laboratory tests to determine a specific viral etiology, because testing “rarely alters the management decisions or outcomes for the vast majority of children with clinically diagnosed bronchiolitis” [47, p. 6], and most respond to supportive care. The guidelines, however, do note “virologic testing may be useful when cohorting of patients is feasible” (p. 6).
The limitations of the use of rapid antigen tests, nevertheless, should be recognized. Their sensitivity and specificity vary according to the adequacy of the specimen and, in particular, to the prevalence of the disease in the community. False-positive test results occur more frequently at the beginning or end of an outbreak, when viral activity in the community is low.
In summary, these data illustrate the complex nature of the spread of viral infection. Experimental and clinical observations may not always concur. Thus, development of infection-control policies that are strictly evidence based is difficult, if not impossible. However, a practical and tenable conclusion is that an effective infection-control program depends not as much on the inclusion of procedures tailored to specific pathogens as on the incorporation of procedures that enhance compliance and awareness of the risks of nosocomial infection for both patients and personnel. Recommended infection-control procedures should be convenient, consistent, pragmatic, and publicized.
Acknowledgments
Potential conflicts of interest. C.B.H.: no conflicts.
References
- 1.Riley RL. Prevention control of airborne infection in the community. Ann NY Acad Sci. 1980;353:331–9. doi: 10.1111/j.1749-6632.1980.tb18936.x. [DOI] [PubMed] [Google Scholar]
- 2.Chapin CV. Infection by air: sources modes of infection. New York: John Wiley; 1910. [Google Scholar]
- 3.Langmuir AD. Changing concepts of airborne infection of acute contagious diseases: a reconsideration of classic epidemiologic theories. Ann NY Acad Sci. 1980;353:35–44. doi: 10.1111/j.1749-6632.1980.tb18903.x. [DOI] [PubMed] [Google Scholar]
- 4.Garner JS. Guidelines for isolation precautions in hospitals. The Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1996;17:53–80. doi: 10.1086/647190. [DOI] [PubMed] [Google Scholar]
- 5.Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in healthcare settings. Clin Infect Dis. 2003;37:1094–101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- 6.Roy CJ, Milton DK. Airborne transmission of communicable infection—the elusive pathway. N Engl J Med. 2004;350:1710–2. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- 7.Fabian MP, McDevitt J, Milton DK. Modes of transmission of respiratory viral infections. In: Johnston S, O'Byrne P, editors. Exacerbations of asthma. Boston: Exposure, Epidemiology Risk Program, Department of Environmental Health Harvard School of Public Health; 2005. pp. 1–2. [Google Scholar]
- 8.Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2:143–54. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–62. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall CB, Geiman JM, Douglas RG., Jr Possible transmission by fomites of respiratory syncytial virus. J Infect Dis. 1980;141:98–102. doi: 10.1093/infdis/141.1.98. [DOI] [PubMed] [Google Scholar]
- 11.Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, Balfour HH., Jr Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982;146:47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Karim YG, Ijaz MK, Sattar SA, Johnson-Lussenburg CM. Effect of relative humidity on the airborne survival of rhinovirus-14. Can J Microbiol. 1985;31:1058–61. doi: 10.1139/m85-199. [DOI] [PubMed] [Google Scholar]
- 13.Miller WS, Artenstein MS. Aerosol stability of three acute respiratory disease viruses. Proc Soc Exp Biol Med. 1967;125:222–7. doi: 10.3181/00379727-125-32054. [DOI] [PubMed] [Google Scholar]
- 14.Knight V. Airborne transmission pulmonary deposition of respiratory viruses. In: Knight V, editor. Viral mycoplasmal infections of the respiratory tract. Philadelphia: Lea & Febiger; 1973. p. 1. [Google Scholar]
- 15.Kao PH, Yang RJ. Virus diffusion in isolation rooms. J Hosp Infect. 2006;62:338–45. doi: 10.1016/j.jhin.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox CS. The aerobiological pathway of microorganisms. Chichester, UK: Wiley; 1987. [Google Scholar]
- 17.Edwards DA, Man JC, Brand P, et al. Inhaling to mitigate exhaled bioaerosols. Proc Natl Acad Sci U S A. 2004;101:17383–8. doi: 10.1073/pnas.0408159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fairchild CI, Stampfer JF. Particle concentration in exhaled breath. Am Ind Hyg Assoc J. 1987;48:948–9. doi: 10.1080/15298668791385868. [DOI] [PubMed] [Google Scholar]
- 19.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10:105–16. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 20.Goldmann DA. Transmission of infectious diseases in children. Pediatr Rev. 1992;13:283–94. doi: 10.1542/pir.13-8-283. [DOI] [PubMed] [Google Scholar]
- 21.Jennings LC, Dick EC. Transmission control of rhinovirus colds. Eur J Epidemiol. 1987;3:327–35. doi: 10.1007/BF00145641. [DOI] [PubMed] [Google Scholar]
- 22.Hendley JO, Wenzel RP, Gwaltney JM., Jr Transmission of rhinovirus colds by self-inoculation. N Engl J Med. 1973;288:1361–4. doi: 10.1056/NEJM197306282882601. [DOI] [PubMed] [Google Scholar]
- 23.Hendley JO, Gwaltney JM., Jr Mechanisms of transmission of rhinovirus infections. Epidemiol Rev. 1988;10:243–58. [PubMed] [Google Scholar]
- 24.Tellier R. Questioning aerosol transmission of influenza: in response. Emerg Infect Dis. 2007;13:174. doi: 10.3201/eid1301.061202. Available at: http://www.cdc.gov/ncidod/EID/13/1/173_174.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemieux C, Brankston G, Gitterman L, Hirji Z, Gardam M. Questioning aerosol transmission of influenza. Emerg Infect Dis. 2007;13:173–4. doi: 10.3201/eid1301.061202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alford RH, Kasel JA, Gerone PJ, Knight V. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med. 1966;122:800–4. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 27.Loosli CG, Hertweck MS, Hockwald RS. Airborne influenza PR8-A virus infections in actively immunized mice. Arch Environ Health. 1970;21:332–46. doi: 10.1080/00039896.1970.10667248. [DOI] [PubMed] [Google Scholar]
- 28.Snyder MH, Stephenson EH, Young H, et al. Infectivity antigenicity of live avian-human influenza A reassortant virus: comparison of intranasal aerosol routes in squirrel monkeys. J Infect Dis. 1986;154:709–11. doi: 10.1093/infdis/154.4.709. [DOI] [PubMed] [Google Scholar]
- 29.Moser MR, Bender TR, Margolis HS, Noble GR, Kendal AP, Ritter DG. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110:1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 30.Blumenfeld HL, Kilbourne ED, Louria DB, Rogers DE. Studies on influenza in the pandemic of 1957–1958. I. An epidemiologic, clinicalserologic investigation of an intrahospital epidemic, with a note on vaccination efficacy. J Clin Invest. 1959;38:199–212. doi: 10.1172/JCI103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salgado CD, Farr BM, Hall KK, Hayden FG. Influenza in the acute hospital setting. Lancet Infect Dis. 2002;2:145–55. doi: 10.1016/s1473-3099(02)00221-9. [DOI] [PubMed] [Google Scholar]
- 32.Drinka PJ, Krause P, Nest L, Tyndall D. Report of an outbreak: nursing home architecture influenza-A attack rates: update. J Am Geriatr Soc. 2004;52:847–8. doi: 10.1111/j.1532-5415.2004.52230_6.x. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control Prevention . Interim recommendations for infection control in health-care facilities caring for patients with known or suspected avian influenza. 2004. Available at: http://www.cdc.gov/flu/avian/professional/infect-control.htm. Accessed November 200. [Google Scholar]
- 34.Qian Y, Willeke K, Grinshpun SA, Donnelly J, Coffey CC. Performance of N95 respirators: filtration efficiency for airborne microbial inert particles. Am Ind Hyg Assoc J. 1998;59:128–32. doi: 10.1080/15428119891010389. [DOI] [PubMed] [Google Scholar]
- 35.Wiwanitkit V. N-95 face mask for prevention of bird flu virus: an appraisal of nanostructure implication for infectious control. Lung. 2006;184:373–4. doi: 10.1007/s00408-006-0021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyce JM, Pittet D. Healthcare Infection Control Practices Advisory Committee: Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23:3–40. doi: 10.1086/503164. [DOI] [PubMed] [Google Scholar]
- 37.Hall CB. Nosocomial respiratory syncytial virus infections: the “cold war” has not ended. Clin Infect Dis. 2000;31:590–6. doi: 10.1086/313960. [DOI] [PubMed] [Google Scholar]
- 38.Vernon MO, Trick WE, Welbel SF, Peterson BJ, Weinstein RA. Adherence with hand hygiene: does number of sinks matter? Infect Control Hosp Epidemiol. 2003;24:224–5. doi: 10.1086/502193. [DOI] [PubMed] [Google Scholar]
- 39.Dettenkofer M, Wenzler S, Amthor S, Antes G, Motschall E, Daschner FD. Does disinfection of environmental surfaces influence nosocomial infection rates?. A systematic review. Am J Infect Control. 2004;32:84–9. doi: 10.1016/j.ajic.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Sehulster L, Chinn RY Centers for Disease Control Prevention, Healthcare Infection Control Practices Advisory Committee. Guidelines for environmental infection control in health-care facilities: recommendations of Centers for Disease Control Prevention the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52:1–42. [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association CDC. MMWR Recomm Rep. 2005;54:1–47. [PubMed] [Google Scholar]
- 42.McLean RL. The effect of ultraviolet radiation upon the transmission of epidemic influenza in long-term hospital patients. Am Rev Respir Dis. 1961;83:36–8. [Google Scholar]
- 43.Abels S, Nadal D, Stroehle A, Bossart W. Reliable detection of respiratory syncytial virus infection in children for adequate hospital infection control management. J Clin Microbiol. 2001;39:3135–9. doi: 10.1128/JCM.39.9.3135-3139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doherty JA, Brookfield DSK, Gray J, McEwan RA. Cohorting of infants with respiratory syncytial virus. J Hosp Infect. 1998;38:203–6. doi: 10.1016/s0195-6701(98)90275-4. [DOI] [PubMed] [Google Scholar]
- 45.Krasinski K, LaCouture R, Holzman RS, Waithe E, Bonk S, Hanna B. Screening for respiratory syncytial virus assignment to a cohort at admission to reduce nosocomial transmission. J Pediatr. 1990;116:894–8. doi: 10.1016/s0022-3476(05)80646-8. [DOI] [PubMed] [Google Scholar]
- 46.Serwint JR, Miller RM. Why do we need to diagnose influenza infections in hospitalized pediatric patients? Pediatr Infect Dis J. 1993;12:200–4. doi: 10.1097/00006454-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 47.American Academy of Pediatrics Subcommittee on Diagnosis Management of Bronchiolitis. Diagnosis management of bronchiolitis. Pediatrics. 2006;118:1774–93. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]


