Abstract
Background. In addition to the human coronaviruses (HCoVs) OC43 and 229E, which have been known for decades to cause infection in humans, 2 new members of this genus have recently been identified: HCoVs NL63 and HKU1. Their impact as a cause of respiratory tract disease in adults at risk for complications needs to be established.
Methods. We prospectively assessed the clinical impact of coronavirus infection (excluding cases of severe acute respiratory syndrome) among hospitalized adults. All patients with respiratory disease for whom bronchoalveolar lavage was performed were screened by reverse-transcriptase polymerase chain reaction for the presence of all 4 HCoVs.
Results. HCoV was identified in 29 (5.4%) of 540 bronchoalveolar lavage fluid specimens from 279 subjects (mean age, 51 years; 63% male). HCoV OC43 was identified most frequently (12 isolates), followed by 229E (7 isolates), NL63 (6 isolates), and HKU1 (4 isolates). In all, 372 (69%) of 540 bronchoalveolar lavage fluid specimens were negative for bacteria, and 2 persons were coinfected with other respiratory viruses. Transplantation was the most common underlying condition. Of the 29 patients who had HCoV identified in their bronchoalveolar lavage fluid specimens, 9 (31%) were hospitalized in the intensive care unit, 22 (76%) presented to the hospital with acute respiratory symptoms, 16 (55%) presented with cough and/or sputum, 13 (45%) presented with dyspnea, 16 (55%) had experienced prior respiratory infection, and 18 (62%) had a new infiltrate that was visible on chest radiograph. The most frequent final diagnosis was a lower respiratory tract infection.
Conclusions. The recently discovered HCoVs NL63 and HKU1 contribute significantly to the overall spectrum of coronavirus infection. Our study also suggests that coronaviruses contribute to respiratory symptoms in most cases.
Human coronaviruses (HCoVs) are recognized as one of the most frequent causes of common colds and upper respiratory tract infections, after rhinoviruses, in adults [1]. In immunocompetent adults, most of these illnesses are self-limiting; however, recent evidence suggests that these viruses are also associated with lower respiratory tract symptoms and protracted disease in subjects at risk for complications [2, 3]. Indeed, HCoVs have been recovered from the lower respiratory tract specimens of patients with pneumonia, young infants, and immunocompromised persons with lower respiratory tract complications [4]. However, most studies investigating the clinical impact of coronavirus infection have been limited by their retrospective design or by the study population. Another unexpected limitation has been the number of different HCoVs analyzed [5]. Until recently, only HCoV 229E and HCoV OC43 were known to infect humans; however, since the identification of the severe acute respiratory syndrome (SARS)—associated coronavirus (not considered in this study), 2 new coronaviruses have been identified: HCoV NL63 was identified in 2004 [6], and HCoV HKU1 was identified in 2005 [7, 8]. Several studies from different parts of the world have now established that HCoV NL63 can be recovered from a substantial number of respiratory specimens, mainly from specimens from children with respiratory symptoms [9–12] and, less frequently, from specimens from adults with respiratory symptoms [13, 14]. HCoV HKU1 was originally identified in a specimen obtained from an elderly man with pneumonia in Hong Kong [8]. Recent studies suggest that this virus might be distributed worldwide [15], although the reported number of cases of infection remains very limited. Few studies have attempted to prospectively establish the clinical impact of all 4 HCoVs as causes of infection in hospitalized adult patients and to describe the associated clinical features and the potential respiratory complications. In the present prospective study, we first systematically analyzed the presence of all 4 HCoVs in patients who required bronchoalveolar lavage (BAL) for an acute respiratory event and then analyzed the associated clinical conditions and outcome of infection.
Methods
In the present investigation, patients were enrolled in a hospital-based, prospective cohort study. All patients who underwent BAL were eligible, irrespective of the reason leading to the procedure. For lung transplant recipients, the study was conducted at the 2 sites of our transplantation network, the University Hospitals of Geneva (Geneva, Switzerland) and the University Hospital of Lausanne (Lausanne, Switzerland). BAL was performed according to our guidelines for the following patients: immunocompromised patients who had persistent infiltrates visible on chest radiograph, despite large-spectrum antibiotic treatment; HIV-infected patients who had low CD4+ cell counts and interstitial infiltrates or suspected pulmonary opportunistic infections; patients with suspected pulmonary tuberculosis whose sputum analysis had negative findings; patients with persistent, diffuse interstitial infiltrates of unknown origin; and exacerbation of symptoms in patients with chronic interstitial diseases or nosocomial pneumonia who had exacerbation of symptoms (including patients who were intubated and treated with wide-spectrum antibiotics without response and who had tumor-like abnormalities). In lung transplant recipients, BAL was also performed as a follow-up or diagnostic procedure, either following transplantation when organ rejection is suspected or after antirejection treatment; thus, lung transplant recipients could have several BALs performed during the follow-up period. Patients were considered to be immunosuppressed when they were receiving treatment with antirejection drugs, were receiving chemotherapy (regardless of neutropenia), were receiving steroids (⩾10 mg per day) for at least 1 week before the BAL procedure, or were HIV infected with a CD4+ cell count <200 cells/mm3.
BAL fluid specimens from all patients were screened by RT-PCR for the presence of the 4 coronaviruses known to infect humans (HCoV OC43, HCoV 229E, HCoV NL63, and HCoV HKU1), as well as 10 additional respiratory viruses (influenza A and B viruses; respiratory syncytial viruses A and B; human parainfluenza viruses 1, 2, and 3; human rhinovirus; enterovirus; and human metapneumovirus). Presence of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila was also determined by PCR.
Shortly before the BAL procedure was performed, a baseline case report form was completed by a physician who was blinded to any microbiological test results. The information recorded included underlying diseases, presence of fever, type and duration of respiratory symptoms, response to any antimicrobial and/or antiviral therapy, reason(s) for the BAL procedure, and the presumed diagnosis (determined on the basis of the available evidence at the time). Three days after the BAL procedure, a follow-up case report form was completed to assess symptom evolution, response to treatment, new chest radiography findings (if available), and results of microbiological tests. Follow-up at 30 days was performed to assess the outcome and final diagnosis and to record data from any additional microbiological tests and/or pathological examinations, if available. For patients included in this particular analysis of coronavirus infection, we also performed an additional review of all medical files available to collect any additional information on the description, evolution, and cause of clinical symptoms.
BAL was performed in accordance with standardized protocol: 30–50 mL of sterile saline solution were instilled 2–4 times into the distal bronchial tree, either at the radiographic site of the chest abnormality or in the middle lobe. All BAL fluid specimens were initially pooled and then separated into aliquots and processed similarly for subsequent analysis. Gram, acridine orange, Truant auramine-rhodamine, and Giemsa staining were performed for direct identification of bacteria, mycobacteria, fungi, and parasites, respectively. Cultures for bacterial identification were inoculated under standard aerobic conditions onto 4 different media, as well as onto specific media for mycobacterium detection, when indicated by the clinical conditions. Pulmonary bacterial infection was considered to be proven when quantitative culture for a given bacteria yielded >103 colony-forming units per mL of specimen, in association with clinical and radiological symptoms; if these conditions were not met, the culture was considered to indicate colonization.
BAL fluid specimens were inoculated on 4 different cell lines for viral detection (human embryonic fibroblasts and A549, MDCK, and LLC-MK2 cells). An aliquot was immediately frozen at -80°C and used for duplicate RT-PCR and PCR assays. Real-time TaqMan RT-PCR assays for the detection of influenza A and B viruses, respiratory syncytial viruses A and B, human parainfluenza viruses 1, 2, and 3, human rhinovirus, enterovirus, and human metapneumovirus were performed as described elsewhere [16, 17]. In brief, RNA was extracted from 200 µL of each BAL fluid specimen (HCV Amplicor Specimen Preparation Kit; Roche Diagnostics), and retrotranscription was performed using the SuperscriptTM II RNase H–Reverse Transcriptase (Invitrogen Life Technologies) and the random primer pd(N)6 (Roche Diagnostics GmbH). Amplification and detection were performed using an ABI Prism 7900 HT (Applied Biosystems). All of the different steps of the extraction and retrotranscription were conducted in parallel with a known positive supernatant of human rhinovirus 2-infected cells and appropriate negative controls.
The forward primers, reverse primers, and probes that were used for the detection of coronaviruses are described elsewhere [17]. We completed the analysis with an RT-PCR targeting HCoV HKU1 using similar PCR conditions. As a control for this assay, we used a 506-base pair PCR product (GenBank accession number AY59704). The forward primer was 5′-GAA TTT TGT TGT TCA CAT GGT GAT AGA-3′, the reverse primer used was 5′-GCA ACC GCC ACA CAT AAC TAT TT-3′, and the probe was 5′-FAM TTT ATC GCC TTG CGA ATG AAT GTG CTC TAMRA-3′. Validation experiments using the above-mentioned PCR product as our PCR target showed that the analytical sensitivity of our PCR assay was <18 copies/µL.
This study was approved by the ethics committees of University Hospitals of Geneva (Geneva, Switzerland) and the University Hospital of Lausanne (Lausanne, Switzerland). Informed, signed consent forms were obtained from all participants.
Results
During a 20-month period, we analyzed 540 BAL fluid specimens obtained from 279 patients who were enrolled in the study (median length of enrollment, 1 month; range, 1–20 months). The mean age was 51 years, and 177 patients (63.4%) were male. Among all BAL fluid specimens that were examined, 307 (57%) of 540 were from lung transplant recipients, 39 (14%) of 279 were from patients treated with immunosuppressive therapy, 19 (7%) of 279 were from HIV-infected patients, and 8 (3%) of 279 were from patients with other immunosuppressive conditions. HCoV was identified in 29 (5.4%) of 540 BAL fluid specimens, whereas 57 (11%) of 511 BAL fluid specimens that had results negative for HCoV had results positive for other respiratory viruses. Overall, 86 (16%) of 540 specimens had test results positive for any respiratory virus. Among HCoV-positive specimens, HCoV OC43 was the most frequent species isolated (12 [41.4%] of 29 specimens), followed by 229E (7 [24.1%] of 29 specimens), NL63 (6 [20.7%] of 29 specimens), and HKU1 (4 [13.8%] of 29 specimens). The median age of the patients from which the HCoV-positive specimens were obtained was 46.6 years (range, 13–77 years), and 20 (69%) of 29 were male. Transplantation was the most frequent underlying condition; 12 patients (49%) were lung transplant recipients, and 2 (16.7%) of those 12 also received a liver transplant. Only 8 (28%) of 29 patients were not considered to be immunosuppressed. Other baseline characteristics and comorbidities are described in table 1. BAL was performed for only 2 (0.7%) of 279 ambulatory patients; all others (277 [99.3%]) were hospitalized for a median duration of 48 days (range, 2–245 days). At the time of the procedure, 22 (76%) of the 29 patients had received antibiotic treatment. Nine (31%) of the 29 patients were hospitalized in the intensive care unit at the time of the BAL procedure (of these, 8 were receiving mechanical ventilation). The reasons leading to the performance of the BAL procedure are given in table 2.
Table 1.
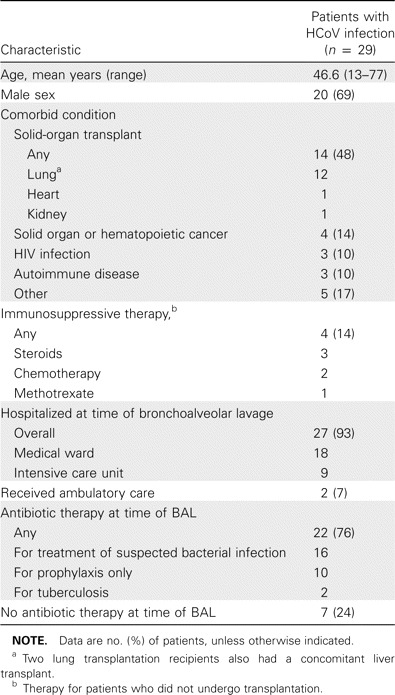
Baseline characteristics and comorbidities of patients with human coronavirus (HCoV) infection.
Table 2.
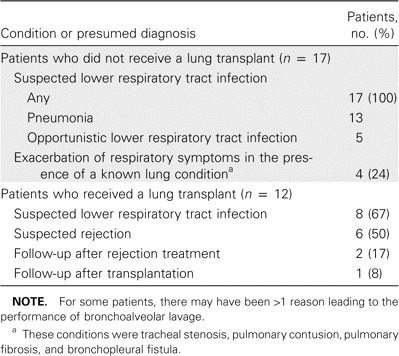
Condition and/or the presumed diagnosis leading to performance of bronchoalveolar lavage for 29 patients with human coronavirus infection.
At the time of BAL, acute respiratory symptoms and/or new chest abnormalities visible on radiograph were present in all patients with HCoV infection. Of the 29 patients, 22 (76%) presented with acute respiratory symptoms, 16 (55%) presented with cough and/or sputum, and 13 (45%) presented with dyspnea. New abnormalities visible on chest radiograph were documented in 18 patients (62%). Abnormalities included interstitial infiltrates (10 patients [55.6%]), alveolar or localized infiltrates (5 [27.8%]), pleural effusions (2 [11.1%]), and pneumothorax (1 [5.6%]). The mean cell count in the BAL fluid specimens was 24 × 104 cells/mL (range, 4–102 × 104 cells/mL), with 18.3% neutrophils (range, 1%–71%) and 14.4% lymphocytes (range, 2%–37%).
Sixteen patients (55%) were already receiving treatment for a respiratory infection at the time of BAL, and in 12 patients (41%), antibiotic therapy was modified during the follow-up period. In 7 other patients (24%), antibiotic therapy was started immediately following BAL. Most patients (52%) received a diagnosis of acute lower respiratory tract infection at hospital discharge (table 3).
Table 3.
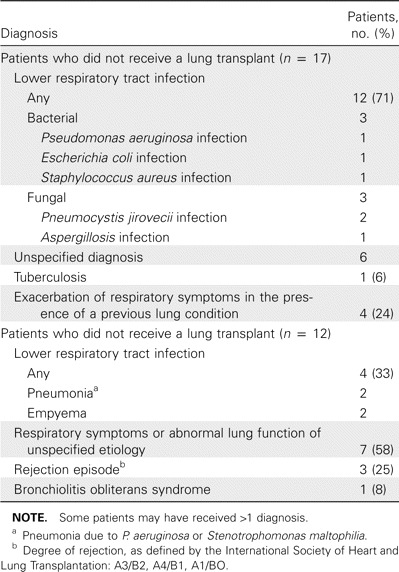
Final diagnosis received by 29 patients with human coronavirus infection.
In 20 patients (69%), the microbiological test results of the BAL specimens were negative for typical bacteria (tested for by standard culture) and atypical bacteria (tested for by PCR), as well as for other respiratory viruses and herpesviruses. Of note, 15 (75%) of these patients were receiving antibiotic treatment at the time that they provided BAL fluid specimens.
In the remaining 9 patients (31%), microbiological test results were positive for typical or atypical bacteria or other respiratory viruses or herpesviruses, and 5 (55.6%) of these patients were receiving antibiotic treatment (OR, 0.412; 95% CI, 0.0793–2.190). A bacterium likely to be a cause of infection was isolated from samples obtained from 5 patients, and 2 patients received a diagnosis of Pneumocystis jirovecii pneumonia. Herpes simplex virus and cytomegalovirus were isolated from samples from 1 patient each but were not considered to be the cause of the respiratory symptoms. Screening for other respiratory viruses had positive results in 2 patients, 1 of whom was coinfected with human rhinovirus and 1 of whom had respiratory syncytial virus coinfeciton.
For 3 (10.3%) of 29 patients, the 30-day outcome was not available: 1 patient (3.4%) was lost to follow-up, and 2 (6.9%) were transferred to hospitals outside Switzerland on days 9 and 12, respectively. The overall mortality rate at day 30 was 10% (3 deaths). Two patients with chronic pulmonary disease (pulmonary fibrosis and lung cancer) died of respiratory failure on days 2 and 10, respectively, and 1 patient died of heart failure on day 30. One of these patients was infected with HCoV 229E, and 2 were infected with HCoV OC43.
We had the opportunity to analyze 34 BAL fluid specimens obtained from 10 (34.5%) of the 29 patients with HCoV infection (9 of whom were lung transplant recipients) after the patients were known to have HCoV infection (median of 3 BAL fluid specimens per patient; range, 1–10 specimens), with a median follow-up duration of 32 weeks (range, 15–65 weeks). The screening for all 4 coronaviruses had negative results in all but 1 patient. This patient had HIV infection (CD4+ cell count, 80 cells/mm3) and developed P. jirovecii pneumonia with persistent abnormalities visible on chest radiographs, despite 3 weeks of treatment with sulfamethoxazole. Results of cultures of BAL fluid samples obtained after 3 weeks were still positive for HCoV OC43.
Of the 540 BAL procedures conducted, 303 (56%) were performed during the cold season (autumn and winter), and 237 (44%) were performed during the rest of the year. The seasonal distribution for cases of HCoV infection was significantly higher during the cold season (20 cases [67%]), compared with the rest of the year (9 cases [31%]; P =.01).
Discussion
We analyzed the results of all cultures of BAL fluid specimens obtained from patients with acute respiratory tract illnesses in a prospective, hospital-based cohort of adult patients. Of the 540 specimens analyzed, we identified 29 (5.4%) that demonstrated HCoV infection. Interestingly, all 4 species of HCoV were present: the classic OC43 and 229E types comprised 65% of isolates, and the new NL63 and HKU1 types comprised 35%. Infections due to NL63 and HKU1 illustrate the potential role and impact of these newly discovered virus types and highlight the need to continuously adapt diagnostic tools to new species. In most cases of illness, the presence of clinical signs or abnormalities visible on chest radiographs (mainly interstitial infiltrates) that were compatible with a diagnosis of acute viral respiratory tract infection prompted the performance of BAL. Antibiotics given to patients before performance of BAL had a limited effect, and clinical failure prompted a switch of antibiotic treatment in >40% of patients. In addition, HCoV was the only infectious agent identified in most cases; the results of all other microbiological testing were negative. Similarly, bacteriological test results were also negative in 45% of patients who were not treated with antibiotics before they underwent BAL, which suggests that, in these patients, HCoV infection was the leading cause of symptoms that led to performance of BAL. Serious associated comorbidities and/or immunocompromised conditions that could promote lower respiratory viral complications were also present in all patients. Finally, the study design provided the opportunity to analyze additional BAL samples obtained before and/or after illness due to HCoV infection in 10 (34.5%) of the 29 enrolled patients. All but 1 sample were negative for coronavirus, which demonstrates that the virus was present during the acute phase of respiratory symptoms that led to BAL and was not asymptomatic colonization. These observations strongly suggest that, in a substantial number of our patients, HCoV played a role in and/or promoted the lower respiratory diseases observed.
Coronaviruses have been shown to be associated with lower respiratory tract complications, but such complications mostly affect children. To our knowledge, no study has either prospectively analyzed a large number of adult patients or analyzed all 4 HCoVs as causes of infection in patients at high risk of complications. One study, performed retrospectively and involving bone marrow transplant recipients, failed to detect HCoV in stored BAL fluid specimens [18]. This difference with our results might be explained by several factors. Our patients were selected only on the basis of the need for BAL—not on the basis of predefined clinical criteria. We did not focus on selected subgroups of patients, 2 winter seasons were covered, and sensitive, real-time RT-PCR assays targeting the 4 HCoVs were performed. The prospective design of our investigation has, therefore, avoided many of the limitations observed in retrospective studies and gives an estimate of the impact of coronaviruses among patients hospitalized with serious chronic and/or immunosuppressive conditions.
Since the first well-defined cases of HCoV infection were documented [6, 7], the incidence of infection due to and the respective roles of HCoV NL63 and HCoV HKU1 among an adult population have not been assessed in a systematic manner. HCoV NL63 and similar viruses [25, 26], as causes of respiratory disease, have been studied mostly in retrospective series or in analyses of stored specimens collected from children [12]. In these large series, the prevalence of HCoV NL63 infection ranged from 2.2% to 9.3% [10, 11, 13, 14, 19–21]. One of the largest studies [14] retrospectively tested 840 nasopharyngeal aspirates in a hospital-based pediatric population with acute respiratory disease and found 16 children with HCoV NL63 infection. Most of these children presented with lower respiratory tract symptoms requiring hospitalization. Another study [25] of 1265 specimens from 895 children with symptoms of lower and/or upper respiratory infection identified 79 specimens positive for HCoV (designated New Haven) that is closely related to NL63. Most children presented with infiltrates visible on chest radiographs, and a substantial number of these children were hospitalized in critical condition. Taken together, these data strongly suggest an association between HCoV NL63 and lower respiratory tract diseases in children. Since its first description in an elderly man with pneumonia [7], 2 other adults cases of infection due to HCoV HKU1 have been reported among a rescreened population [8, 22], and 2 cases were diagnosed by screening respiratory samples sent for viral analysis [15, 23]. In another study, by Woo et al. [24], conducted during the SARS outbreak in Hong Kong, nasopharyngeal aspirates were collected from 418 patients with community-acquired pneumonia (all of whom had abnormalities visible on chest radiographs) who were subsequently screened for HKU1. Woo and colleagues found 10 cases of HCoV infection, 9 of which occurred in adults. A review of patient medical charts showed that approximately one-half of the patients had chronic lung disease. Hospitalization was required for most patients, and 2 patients died. Our findings are consistent with those of these previous investigations; we demonstrate that HCoV NL63 and HKU1 can also be detected in lower respiratory tract specimens obtained from adults and provide a comparison of their respective roles in causing illness. During the 2 seasons during which our study was conducted, NL63 and HKU1 were less frequently identified than the classic OC43 and 229E species, but they nevertheless contributed significantly to the overall spectrum of coronavirus-associated diseases among hospitalized adult patients.
A large subgroup of patients that emerged in our analysis is lung transplant recipients. Recent reports regarding this population have suggested a link between viral respiratory tract infections and graft dysfunction or bronchiolitis obliterans syndrome [27–33]. However, in these studies, coronaviruses were not investigated because of the need to use in-house and updated PCR assays. For most of our patients, the main final diagnosis was a lower respiratory tract infection and/or acute respiratory symptoms of unspecified etiology, despite extensive routine investigations. This supports the hypothesis that coronavirus infection promoted the lower respiratory tract disease observed in these patients.
In conclusion, in the present prospective study, we provide a comprehensive analysis of the potential impact of all 4 HCoVs as causes of infection among hospital-based patients. Overall, coronaviruses were recovered in 5.4% of all BAL fluid specimens analyzed, and HCoV infection was most commonly observed in patients at high risk of respiratory complications, including lung transplant recipients. The recently discovered species HCoV NL63 and HCoV HKU1 contributed significantly to the spectrum of HCoV-associated disease in our study.
Acknowledgments
We thank L. van der Hoek from the Department of Human Retrovirology, University of Amsterdam (Amsterdam, The Netherlands), and K.-Y. Yuen from the Department of Microbiology, the University of Hong Kong (Hong Kong, People's Republic of China), for providing materials to establish the PCR assays. We are also grateful to Sabine Nobs and Delphine Garcia, for their excellent technical assistance.
Financial support. Swiss National Foundation (grant 320080-101670 to L.K.).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–42. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, McCann RM, Hall WJ, et al. The “common cold” in frail older persons: impact of rhinovirus and coronavirus in a senior daycare center. J Am Geriatr Soc. 1997;45:706–11. doi: 10.1111/j.1532-5415.1997.tb01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J Infect Dis. 2002;185:1338–41. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagneur A, Sizun J, Vallet S, Legr MC, Picard B, Talbot PJ. Coronavirus-related nosocomial viral respiratory infections in a neonatal and paediatric intensive care unit: a prospective study. J Hosp Infect. 2002;51:59–64. doi: 10.1053/jhin.2002.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Elden LJ, van Loon AM, van Alphen F, et al. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. J Infect Dis. 2004;189:652–7. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–73. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–95. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo PC, Lau SK, Huang Y, Tsoi HW, Chan KH, Yuen KY. Phylogenetic and recombination analysis of coronavirus HKU1, a novel coronavirus from patients with pneumonia. Arch Virol. 2005;11:2299–311. doi: 10.1007/s00705-005-0573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–9. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moes E, Vijgen L, Keyaerts E, et al. A novel pancoronavirus RT-PCR assay: frequent detection of human coronavirus NL63 in children hospitalized with respiratory tract infections in Belgium. BMC Infect Dis. 2005;5:6. doi: 10.1186/1471-2334-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebihara T, Endo R, Ma X, Ishiguro N, Kikuta H. Detection of human coronavirus NL63 in young children with bronchiolitis. J Med Virol. 2005;75:463–5. doi: 10.1002/jmv.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boivin G, Baz M, Cote S, et al. Infections by human coronavirus-NL in hospitalized children. Pediatr Infect Dis J. 2005;24:1045–8. doi: 10.1097/01.inf.0000183743.68569.c7. [DOI] [PubMed] [Google Scholar]
- 13.Bastien N, Anderson K, Hart L, et al. Human coronavirus NL63 infection in Canada. J Infect Dis. 2005;191:503–6. doi: 10.1086/426869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arden KE, Nissen MD, Sloots TP, Mackay IM. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia. J Med Virol. 2005;75:455–62. doi: 10.1002/jmv.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloots TP, McErlean P, Speicher DJ, Arden KE, Nissen MD, Mackay IM. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol. 2006;35:99–102. doi: 10.1016/j.jcv.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deffernez C, Wunderli W, Thomas Y, Yerly S, Perrin L, Kaiser L. Amplicon sequencing and improved detection of human rhinovirus in respiratory samples. J Clin Microbiol. 2004;42:3212–8. doi: 10.1128/JCM.42.7.3212-3218.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garbino J, Gerbase MW, Wunderli W, et al. Lower respiratory viral illnesses: improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med. 2004;170:1197–203. doi: 10.1164/rccm.200406-781OC. [DOI] [PubMed] [Google Scholar]
- 18.Ison MG, Hayden FG, Kaiser L, Corey L, Boeckh M. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin Infect Dis. 2003;36:1139–43. doi: 10.1086/374340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vabret A, Mourez T, Dina J, et al. Human coronavirus NL63, France. Emerg Infect Dis. 2005;11:1225–9. doi: 10.3201/eid1108.050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastien N, Robinson JL, Tse A, Lee BE, Hart L, Li Y. Human coronavirus NL-63 infections in children: a 1-year study. J Clin Microbiol. 2005;43:4567–73. doi: 10.1128/JCM.43.9.4567-4573.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102:12891–6. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vabret A, Dina J, Gouarin S, Petitjean J, Corbet S, Freymuth F. Detection of the new human coronavirus HKU1: a report of 6 cases. Clin Infect Dis. 2006;42:634–9. doi: 10.1086/500136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo PC, Lau SK, Tsoi HW, et al. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis. 2005;192:1898–907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. 2005;191:492–8. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fouchier RA, Hartwig NG, Bestebroer TM, et al. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci U S A. 2004;101:6212–6. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar D, Erdman D, Keshavjee S, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5:2031–6. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilchez R, McCurry K, Dauber J, et al. Influenza and parainfluenza respiratory viral infection requiring admission in adult lung transplant recipients. Transplantation. 2002;73:1075–8. doi: 10.1097/00007890-200204150-00010. [DOI] [PubMed] [Google Scholar]
- 29.Chakinala MM, Walter MJ. Community acquired respiratory viral infections after lung transplantation: clinical features and long-term consequences. Semin Thorac Cardiovasc Surg. 2004;16:342–9. doi: 10.1053/j.semtcvs.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170:181–7. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 31.Gerna G, Vitulo P, Rovida F, et al. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol. 2006;78:408–16. doi: 10.1002/jmv.20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garbino J, Gerbase-Weinbach M, Wunderli, et al. Respiratory viruses and severe Lower respiratory tract complications in hospitalized patients. Chest. 2004;125:1033–9. doi: 10.1378/chest.125.3.1033. [DOI] [PubMed] [Google Scholar]
- 33.Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. J Heart Lung Transplant. 2002;5:559–66. doi: 10.1016/s1053-2498(01)00405-3. [DOI] [PubMed] [Google Scholar]


