Abstract
Lassa fever is an acute viral hemorrhagic illness; the virus is endemic in West Africa and also of concern with regard to bioterrorism. Transmission of Lassa virus between humans may occur through direct contact with infected blood or bodily secretions. Oral administration of the antiviral drug ribavirin is often considered for postexposure prophylaxis, but no systematically collected data or uniform guidelines exist for this indication. Furthermore, the relatively low secondary attack rates for Lassa fever, the restriction of the area of endemicity to West Africa, and the infrequency of high-risk exposures make it unlikely that controlled prospective efficacy trials will ever be possible. Recommendations for postexposure use of ribavirin can therefore be made only on the basis of a thorough understanding and logical extrapolation of existing data. Here, we review the pertinent issues and propose guidelines based on extensive review of the literature, as well as our experience in this field. We recommend oral ribavirin postexposure prophylaxis for Lassa fever exclusively for definitive high-risk exposures. These guidelines may also serve for exposure to other hemorrhagic fever viruses susceptible to ribavirin.
Lassa fever is an acute and sometimes severe viral hemorrhagic illness caused by Lassa virus, a member of the virus family Arenaviridae. The virus is endemic in parts of West Africa, where an estimated 300,000–500,000 cases and 5000 related deaths occur yearly [1]. Lassa virus is also on the Centers for Disease Control and Prevention's list of category A Select Agents. Humans contract Lassa virus primarily through contact with excreta of the rodent Mastomys natalensis, which is the natural reservoir [1, 2]. Although uncommon, secondary transmission of Lassa virus between humans may occur through direct contact with infected blood or bodily secretions, such as saliva, vomit, stool, or urine [3]. Nosocomial transmission and outbreaks have been described in health care facilities in areas of endemicity [4–7]. Laboratory diagnosis for Lassa fever can be achieved by various methods, including enzyme-linked immunosorbent assay, polymerase chain reaction (PCR), and virus culture, but the reagents and assays are not widely available and do not detect infection during the incubation period [8, 9].
Ribavirin (1-β-d-ribofuranosy-l-1,2,4-triazole-3-carboxamide) is a guanosine analogue with broad-spectrum virustatic activity. The drug has been used, with varying degrees of proven clinical efficacy, for the treatment of Lassa fever and other arenavirus infections (Junin and Machupo viruses), hemorrhagic fever with renal syndrome, Crimean-Congo hemorrhagic fever, hepatitis C, respiratory syncytial virus pneumonia, La Crosse encephalitis, influenza, and adenovirus infection [3, 10–22]. Various mechanisms of action of ribavirin have been suggested, including direct inhibition of viral RNA-dependent RNA polymerases, inhibition of host inosine monophosphate dehydrogenase, modulation of the host immune response, inhibition of viral capping enzymes, and lethal mutagenesis [23–27]. The specific mechanism of action of ribavirin against Lassa virus has not been investigated. Administration of intravenous ribavirin within the first 6 days of illness has been shown to decrease the mortality of severe Lassa fever from 55% to 5% [10]. Oral ribavirin is also efficacious but less so than the intravenous form [10].
Ribavirin has been associated with a number of adverse effects, although most of them are mild, and all are reversible with cessation or dose reduction of the drug (Table 1) [10, 28, 30–39]. Although no deaths from the use of oral ribavirin are known to have occurred, chest pain thought to be due to coronary ischemia exacerbated by anemia has been reported [36]. Ribavirin is teratogenic and embryotoxic in rodents and is therefore contraindicated during pregnancy and while breastfeeding, although the high maternal and fetal mortality associated with Lassa fever during pregnancy may outweigh the risks [10, 40–42].
Table 1.

Adverse Effects Associated with Ribavirin Use in Humans
The demonstrated efficacy of intravenous ribavirin for Lassa fever, the communicable nature of Lassa virus, and the inability to diagnose Lassa fever during the incubation period frequently prompt consideration of oral ribavirin for postexposure prophylaxis (PEP), often in a setting of considerable anxiety when health care workers or family members are exposed to persons who subsequently receive a diagnosis of Lassa fever [10, 43–50]. However, no systematically collected data or uniform guidelines for use, dose, or duration of therapy exist for ribavirin for this indication, causing considerable confusion [29, 31, 45, 48, 50]. Furthermore, the relatively low secondary attack rates of Lassa fever, the restriction of the area of endemicity to West Africa, and the infrequency of imported cases and high-risk exposures in contacts (discussed below in “Mode of transmission and attack rate,” in the Literature Review and Pertinent Issues section) make it unlikely that controlled, prospective efficacy trials for ribavirin PEP for Lassa fever will ever be possible. In the absence of efficacy data, recommendations for the use of ribavirin for this indication can be made only on the basis of a thorough understanding and logical extrapolation of existing data. Here, we review the pertinent issues and propose guidelines for the use of ribavirin PEP for Lassa fever based on extensive review of the literature, as well as our considerable experience in this field.
Literature Review and Pertinent Issues
A decision to use PEP for any given exposure is logically based on a risk-benefit analysis taking into account aspects of the disease (mode of transmission, attack rate, incubation period, pathogenesis, and mortality), the drug (efficacy, adverse effects, ease of administration, and cost), and the patient (willingness or anxiety regarding PEP, premorbid conditions, and concomitant medications). Key elements of these aspects are reviewed below, although many remain poorly characterized for the use of ribavirin PEP for Lassa fever.
Mode of transmission and attack rate. Although aerosol transmission has been postulated, clinical and epidemiological observation during the past 40 years provides convincing evidence that direct exposure to blood or bodily secretions is usually required for transmission of Lassa virus between humans [3, 6]. The secondary attack rate for Lassa fever in nosocomial settings appears to be quite low, as long as adequate barrier nursing are maintained, as is the norm in most industrialized countries [51, 52]. Among >25 imported cases with at least 1500 cumulative identified contacts reported since 1969, only a single putative and asymptomatic instance of secondary transmission of Lassa virus has been noted [31] (A. Sanzone, personal communication).
Incubation period and pathogenesis. The incubation period for Lassa fever is usually µ10 days, with a range of 3–21 days [3]. The onset is typically insidious and difficult to diagnose by clinical means alone [3, 53–55]. Macrophages and dendritic cells are the initial targets of infection, followed by hematogenous seeding of a wide variety of cells and organs, along with the production of a host of inflammatory and vasoactive mediators that play a major role in disease [56, 57].
Mortality. Contrary to public perception, the overall mortality from Lassa fever appears to be low (<5%) [1]. However, mortality data are derived primarily from infections with the Josiah strain or close variants circulating in Sierra Leone, Liberia, or Guinea [54, 55, 58–60]. Strains from Nigeria are often considered to be more virulent [53, 61]. Severe and fatal disease may occur with all strains of Lassa virus, but, other than during the third trimester of pregnancy, in which maternal and fetal mortality are elevated, no prognostic indicators are known that would identify, prior to disease onset, patients who will ultimately develop severe Lassa fever and for whom PEP would logically be indicated [41]. Anecdotally, morbidity and mortality seem to be increased in expatriates relative to native Africans (D.G.B., unpublished observation). Whether this represents a genetic difference in susceptibility or the consequence of partial immunity from previous infection in populations indigenous to areas of endemicity is unknown.
Efficacy. Although the drug has frequently been given after potential exposure to Lassa virus, no data exist on the efficacy of ribavirin PEP for Lassa fever in humans, and anecdotal observations are difficult to interpret because of the low attack rate [28, 29, 31]. None of 16 contacts prescribed oral ribavirin PEP (10 mg/kg 4 times a day for 5–8 days) after what was considered high-risk exposure to an imported case of Lassa fever in Germany became sick, although Lassa virus-specific immunoglobulin G (IgG), but not IgM, antibodies were detected in one person who started ribavirin 36 h after exposure [31]. The person had never traveled to an area where Lassa fever is endemic, and no pre-exposure serum sample was available to determine whether seroconversion had occurred. Adherence to the prescribed regimen and adverse effects were not reported.
Studies of ribavirin PEP in animal models of Lassa fever show efficacy, but only intravenous and intramuscular routes of administration have been tested; ribavirin given intravenously (30–50 mg/kg loading dose followed by 10 mg/kg every 8 h) and intramuscularly (50 mg/kg loading dose followed by 10 mg/kg 3 times a day) have been shown to protect nonhuman primates from fatal Lassa fever when started up to 5 days after infection [62–64]. Protection was enhanced when ribavirin was given in combination with immune serum [63]. Intravenous ribavirin PEP and intramuscular ribavirin PEP have also been shown to be efficacious in nonhuman primate models of Junin virus infection when given up to 6 days after infection [38, 65, 66]. However, the intravenous and intramuscular routes used in these studies produce serum levels of ribavirin significantly above those achieved with the oral administration usually used for PEP.
Extensive pharmacological testing of ribavirin for Lassa fever has not been performed. In cell culture experiments, ribavirin has been reported to have a minimum inhibitory concentration (MIC) for Lassa virus in the range of 4–40 µmol/L. The median inhibitory concentration (IC50), reported either as IC50 or effective dose (ED50), ranges from 37 µmol/L to 82 µmol/L [43, 62, 67, 68]. IC50 and ED50 have also been reported for various other arenaviruses, including Junin virus (IC50, 4.5–13 µmol/L; ED50, 90 µmol/L), Machupo virus (ED50, 131 µmol/L), Tacaribe virus (IC50, 4.5 µmol/L), and Pichinde virus (ED50, 246 µmol/L) [43, 69–71]. Ribavirin concentrations in the range of the MIC or IC50 for Lassa virus can be achieved relatively easily with intravenous administration, in which single doses of 600–2400 mg produced peak serum concentrations of 47–161 µmol/L [72]. Ribavirin in patients with Lassa fever produced mean peak serum levels of 32.1–94 µmol/L after 4 days of 4000 mg administered intravenously daily (1000 mg every 6 h) and 68 µmol/L after continued treatment with 500 mg administered intravenously every 8 h for 6 days [68, 73]. In the single study in which the drug's efficacy for treatment of Lassa fever in humans was assessed, the dose of intravenous ribavirin used was a 2-g loading dose, followed by 1 g every 6 h for 4 days, followed by 500 mg every 8 h for 6 days (total course, 10 days) [10].
Reaching the target MIC or IC50 with oral ribavirin is less certain. Steady-state levels with oral ribavirin occur only after 2–4 weeks of administration [74–76]. Despite rapid and extensive absorption in the proximal small bowel, first-pass hepatic metabolism results in an oral bioavailability of µ50%, with peak plasma levels typically an order of magnitude less than that seen with intravenous administration [72, 77, 78]. Oral administration of single doses of 600–2400 mg produced peak plasma levels of only 5–12 µmol/L [72]. In patients with Lassa fever taking oral ribavirin at 1000 mg/day in 3 divided doses for 10 days, the mean peak plasma concentration was 3.1 µmol/L (range, 1.2–9.6 µmol/L) [68, 73]. These borderline serum levels probably explain the decreased efficacy of oral ribavirin, compared with intravenous ribavirin, for treatment of Lassa fever [10].
Adverse effects and ease of administration. Adverse effects, either biological or anxiety induced, are frequent with oral ribavirin PEP and often lead to decreased adherence [28, 36]. For example, a host of minor adverse effects was noted in 7 persons given oral ribavirin PEP (600 mg 4 times a day for 10 days) after possible exposure to an imported case of Lassa fever in London in 2000, prompting 2 of the 7 persons to stop taking the drug and another person to decrease the dose [28]. Similar problems were reported after use of ribavirin PEP following an imported case of Lassa fever in South Africa (L. Blumberg, personal communication). Despite the frequency of minor adverse effects, the risks from the short-term administration (7–10 days) required for PEP for Lassa fever appear to be minimal, although special considerations may be required regarding ribavirin use in populations with other comorbid conditions, especially anemia, human immunodeficiency virus (HIV) infection, and renal insufficiency, all of which are more common in West Africa, where Lassa fever is endemic.
Cost. Ribavirin capsules or tablets are available from a variety of pharmaceutical companies (primarily in the United States, China, and Switzerland), with the cost of a 200-mg dose ranging from ∼10 cents to >$12 (Table 2).
Table 2.
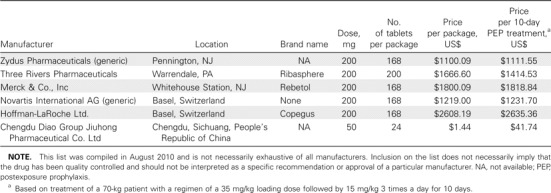
Manufacturers of Oral Ribavirin
Willingness or anxiety regarding PEP, premorbid conditions, and concomitant medications. PEP of any sort is obviously indicated only if the patient is predisposed to adhere to the regimen. Concomitant medications and premorbid conditions, especially those that might limit gut absorption or exacerbate anemia, would be major considerations in withholding PEP.
Proposed Guidelines
Although the safety and cost profile of ribavirin PEP for Lassa fever are favorable, we believe that the overall data argue against liberal use of the drug for this indication, for the following reasons: (1) the secondary attack rate for Lassa fever in most circumstances is low; (2) the efficacy of ribavirin PEP is unknown; (3) reaching the MIC or IC50 for Lassa virus is not assured with oral administration at tolerable doses; (4) adverse events are frequent and may pose a challenge to both the patient and the doctor in distinguishing them from the early symptoms of Lassa fever, which typically has an insidious onset; and (5) liberal use of ribavirin PEP might unintentionally encourage clinicians and caretakers to let down their guard regarding infection control practices, believing that infection is not necessarily a serious event, because of the availability of PEP.
We recommend ribavirin PEP for Lassa fever exclusively in the event of a definitive high-risk exposure, defined as 1 of the following: (1) penetration of skin by a contaminated sharp instrument (eg, needlestick injury), (2) contamination of mucous membranes or broken skin with blood or bodily secretions (eg, blood splashing in the eyes or mouth), (3) participation in emergency procedures (eg, resuscitation after cardiac arrest, intubation, or suctioning) without use of appropriate personal protective equipment, and (4) prolonged (ie, for hours) and continuous contact in an enclosed space without use of appropriate personal protective equipment (eg, a health care worker accompanying a patient during medical evacuation). In calculating the risk of infection, clinicians should realize that titers of Lassa virus in blood and bodily secretions correlate with disease severity [10]. The most infectious patients are those with severe clinical conditions, usually late in the course of illness. In all cases, the exposure to the index case must be during the period of acute febrile illness [48]. There is no evidence of Lassa virus transmission during the incubation period or after recovery, with the exception of sexual transmission, in which transmission rarely occurs because of delayed clearance (⩽3 months after acute infection) of Lassa virus from the gonads [79].
In the few instances in which ribavirin PEP is indicated, we propose an oral regimen of a 35-mg/kg loading dose (maximum dose, 2.5 g) followed by 15 mg/kg (maximum dose, 1 g) 3 times a day for 10 days. For a 70-kg adult, this translates to an ∼2.4-g loading dose, followed by 1 g taken 3 times a day. This regimen is derived from the standard intravenous dose, taking into account the 50% mean oral bioavailability (which theoretically requires a doubling of the intravenous dose when given orally), with modifications for tolerability and ease of administration. This represents a cumulative maintenance dose of only 54%, compared with the standard intravenous regimen, which may not achieve the target MIC for Lassa virus in all cases. However, considering that mortality in Lassa fever correlates with the viral load, prophylaxis or therapy that lowers the level of viremia could reasonably be presumed to decrease severity, even if disease still occurs [10, 62, 64]. Ribavirin is excreted primarily through the kidneys; therefore, the dose should be decreased in persons known to have significant renal insufficiecy (creatinine clearance, <50 mL/min) [78, 80, 81].
Although use of intravenous ribavirin for PEP would skirt some of the shortcomings of the oral form, including more reliably achieving the MIC for Lassa virus and avoiding gastrictoxicity, this approach comes with its own drawbacks, including more-cumbersome administration, increased hematotoxicity, and significantly increased cost (the mean wholesale price in the United States of a 10-day course of intravenous ribavirin, for which there is no generic formavailable, is >$20,000). Given these disadvantages, and again considering the low secondary attack rates, we do not recommend intravenous ribavirin PEP for Lassa fever.
Oral ribavirin should be started immediately after the highrisk exposure, but not before counseling of the patient by the physician. The drug should be taken with food. The patient should be informed that the efficacy of PEP for Lassa fever is unknown and that, although there are no major risks to its use, minor adverse effects often occur. If not already performed, the index case should be tested for Lassa fever, with cessation of ribavirin if the test results are negative.
Patients receiving oral ribavirin PEP who develop manifestations of Lassa fever should also be immediately laboratory tested for Lassa fever by the most sensitive method, usually reverse-transcriptase PCR, and treatment should be switched to the intravenous form unless the disease can be readily excluded [8, 9]. Physicians should be aware of the possibility that ribavirin PEP might prolong the incubation period for Lassa fever but might not completely prevent disease, although this has not been specifically documented for ribavirin PEP for Lassa fever or any other viral illness. The emergence of ribavirinresistant Lassa virus has not been reported.
Although oral regimens similar to that recommended above have been used as treatment for Crimean-Congo hemorrhagic fever virus, with no reported side effects other than mild anemia and thrombocytosis [14, 15], frequent but generally mild adverse effects should be expected, with particular attention to anemia. Asymptomatic jaundice may develop in patients with Gilbert syndrome. Significant variability in tolerance to ribavirin may be noted, especially in patients with HIV infection, renal insufficiency, or other comorbid conditions [82]. The patient should be seen frequently after exposure and prescription of PEP, with the clinician attuned to adverse events and the potential psychological stress of Lassa virus exposure.
Relative contraindications to ribavirin PEP include severe anemia or hemoglobinopathy, pregnancy and breast-feeding, coronary artery disease, renal insufficiency, decompensated liver disease, and known hypersensitivity [39]. In these cases, the clinician should discuss the risks and benefits with the patient. Baseline hemoglobin and hematocrit levels should be measured, and therapy should be reconsidered if significant anemia is present. The complete blood count and bilirubin level should be rechecked 5–7 days after initiation of the drug, and ribavirin should be stopped or the dose should be adjusted if significant anemia is noted. The physician should be aware that the long terminal halflife (∼24 h) and the large volume of distribution of ribavirin mean that the drug may still have an effect for a time even after cessation, particularly in red blood cells, where it accumulates [72, 76–78, 83, 84].
We propose specific guidelines for the use of ribavirin PEP for Lassa fever that we hope will be useful for clinicians managing this often difficult and unfamiliar situation. These guidelines may also serve for PEP for the other arenaviruses causing hemorrhagic fever (Junin, Machupo, Guanarito, Sabiá, and Flexal viruses) as well as for Crimean-Congo hemorrhagic fever virus. Although efficacy trials involving humans are likely impossible, studies in nonhuman primates can and should be conducted to confirm the efficacy of oral ribavirin PEP for these diseases. These data could also guide decisions on whether oral ribavirin merits inclusion in the US Strategic National Stockpile. In vitro and in vivo testing should be conducted to assess the efficacy of newer antivirals against arenaviruses and other hemorrhagic fever viruses for consideration in both PEP and treatment regimens. This could include drugs that are already licensed for other indications as well as promising drugs still at the experimental stage.
Acknowledgments
This article is dedicated to our friend and colleague Dr. Aniru Conteh (1942–2004), who gave his life to the service of those with Lassa fever [85]. We thank Lucille Blumberg, Brian Gowen, Frank Maldarelli, and Nivesh Sewlall, for critical review of and commentary on the manuscript, and Andrew Bennett, Nell Bond, and Catherine Pruszynski, for assistance with its preparation.
Financial support. Tulane University.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.McCormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155:437–444. doi: 10.1093/infdis/155.3.437. [DOI] [PubMed] [Google Scholar]
- 2.Monath TP, Newhouse VF, Kemp GE, Setzer HW, Cacciapuoti A. Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone. Science. 1974;185:263–265. doi: 10.1126/science.185.4147.263. [DOI] [PubMed] [Google Scholar]
- 3.Enria D, Mills JN, Flick R, et al. Arenavirus Infections. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases: principles, pathogens, and practice. 2nd ed. Vol. 1. Philadelphia: PA: Elsevier; 2006. pp. 734–755. [Google Scholar]
- 4.Fisher-Hoch SP, Tomori O, Nasidi A, et al. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. BMJ. 1995;311:857–859. doi: 10.1136/bmj.311.7009.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Update on Lassa fever in West Africa. Wkly Epidemiol Rec. 2005;80:86–88. [PubMed] [Google Scholar]
- 6.Carey DE, Kemp GE, White HA. Lassa fever: epidemiological aspects of the 1970 epidemic, Jos, Nigeria. Trans R Soc Trop Med Hyg. 1972;66:402–408. doi: 10.1016/0035-9203(72)90271-4. [DOI] [PubMed] [Google Scholar]
- 7.Monath TP, Mertens PE, Patton R, et al. A hospital epidemic of Lassa fever in Zorzor, Liberia, March-April 1972. Am J Trop Med Hyg. 1973;22:773–779. doi: 10.4269/ajtmh.1973.22.773. [DOI] [PubMed] [Google Scholar]
- 8.Bausch DG, Rollin PE, Demby AH, et al. Diagnosis and clinical virology of Lassa fever as evaluated by enzyme-linked immunosorbent assay, indirect fluorescent-antibody test, and virus isolation. J Clin Microbiol. 2000;38:2670–2677. doi: 10.1128/jcm.38.7.2670-2677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drosten C, Kummerer BM, Schmitz H, Gunther S. Molecular diagnostics of viral hemorrhagic fevers. Antiviral Res. 2003;57:61–87. doi: 10.1016/s0166-3542(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 10.McCormick JB, King IJ, Webb PA, et al. Lassa fever: effective therapy with ribavirin. N Engl J Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 11.Barry M, Russi M, Armstrong L, et al. Brief report: treatment of a laboratory-acquired Sabia virus infection. N Engl J Med. 1995;333:294–296. doi: 10.1056/NEJM199508033330505. [DOI] [PubMed] [Google Scholar]
- 12.Kilgore PE, Ksiazek TG, Rollin PE, et al. Treatment of Bolivian hemorrhagic fever with intravenous ribavirin. Clin Infect Dis. 1997;24:718–722. doi: 10.1093/clind/24.4.718. [DOI] [PubMed] [Google Scholar]
- 13.Huggins JW, Hsiang CM, Cosgriff TM, et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis. 1991;164:1119–1127. doi: 10.1093/infdis/164.6.1119. [DOI] [PubMed] [Google Scholar]
- 14.Fisher-Hoch SP, Khan JA, Rehman S, Mirza S, Khurshid M, McCormick JB. Crimean Congo-haemorrhagic fever treated with oral ribavirin. Lancet. 1995;346:472–475. doi: 10.1016/s0140-6736(95)91323-8. [DOI] [PubMed] [Google Scholar]
- 15.Mardani M, Jahromi MK, Naieni KH, Zeinali M. The efficacy of oral ribavirin in the treatment of Crimean-Congo hemorrhagic fever in Iran. Clin Infect Dis. 2003;36:1613–1618. doi: 10.1086/375058. [DOI] [PubMed] [Google Scholar]
- 16.Picardi A, Gentilucci UV, Zardi EM, D'Avola D, Amoroso A, Afeltra A. The role of ribavirin in the combination therapy of hepatitis C virus infection. Curr Pharm Des. 2004;10:2081–2092. doi: 10.2174/1381612043384330. [DOI] [PubMed] [Google Scholar]
- 17.Ventre K, Randolph A. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev. 2004;((4)):CD000181. doi: 10.1002/14651858.CD000181.pub2. [DOI] [PubMed] [Google Scholar]
- 18.McJunkin JE, Khan R, delos Reyes EC, et al. Treatment of severe La Crosse encephalitis with intravenous ribavirin following diagnosis by brain biopsy. Pediatrics. 1997;99:261–267. doi: 10.1542/peds.99.2.261. [DOI] [PubMed] [Google Scholar]
- 19.Mustafa MM, Weitman SD, Winick NJ, Bellini WJ, Timmons CF, Siegel JD. Subacute measles encephalitis in the young immunocompromised host: report of two cases diagnosed by polymerase chain reaction and treated with ribavirin and review of the literature. Clin Infect Dis. 1993;16:654–660. doi: 10.1093/clind/16.5.654. [DOI] [PubMed] [Google Scholar]
- 20.Uyeki TM. Influenza diagnosis and treatment in children: a review of studies on clinically useful tests and antiviral treatment for influenza. Pediatr Infect Dis J. 2003;22:164–177. doi: 10.1097/01.inf.0000050458.35010.b6. [DOI] [PubMed] [Google Scholar]
- 21.Gavin PJ, Katz BZ. Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children. Pediatrics. 2002;110:e9. doi: 10.1542/peds.110.1.e9. [DOI] [PubMed] [Google Scholar]
- 22.Snell NJ. Ribavirin—current status of a broad spectrum antiviral agent. Expert Opin Pharmacother. 2001;2:1317–1324. doi: 10.1517/14656566.2.8.1317. [DOI] [PubMed] [Google Scholar]
- 23.Crotty S, Cameron CE, Andino R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad SciUS A. 2001;98:6895–6900. doi: 10.1073/pnas.111085598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotty S, Maag D, Arnold JJ, et al. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 25.Cameron CE, Castro C. The mechanism of action of ribavirin: lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr Opin Infect Dis. 2001;14:757–764. doi: 10.1097/00001432-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Tam RC, Lau JY, Hong Z. Mechanisms of action of ribavirin in antiviral therapies. Antivir Chem Chemother. 2001;12:261–272. doi: 10.1177/095632020101200501. [DOI] [PubMed] [Google Scholar]
- 27.Patterson JL, Fernandez-Larsson R. Molecular mechanisms of action of ribavirin. Rev Infect Dis. 1990;12:1139–1146. doi: 10.1093/clinids/12.6.1139. [DOI] [PubMed] [Google Scholar]
- 28.Crowcroft NS, Meltzer M, Evans M, et al. The public health response to a case of Lassa fever in London in 2000. J Infect. 2004;48:221–228. doi: 10.1016/j.jinf.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Holmes GP, McCormick JB, Trock SC, et al. Lassa fever in the United States: iInvestigation of a case and new guidelines for management. N Engl J Med. 1990;323:1120–1123. doi: 10.1056/NEJM199010183231607. [DOI] [PubMed] [Google Scholar]
- 30.Fisher-Hoch SP, Gborie S, Parker L, Huggins J. Unexpected adverse reactions during a clinical trial in rural West Africa. Antiviral Res. 1992;19:139–147. doi: 10.1016/0166-3542(92)90073-e. [DOI] [PubMed] [Google Scholar]
- 31.Haas WH, Breuer T, Pfaff G, et al. Imported Lassa fever in Germany: surveillance and management of contact persons. Clin Infect Dis. 2003;36:1254–1258. doi: 10.1086/374853. [DOI] [PubMed] [Google Scholar]
- 32.Chapman LE, Mertz GJ, Peters CJ, et al. Ribavirin Study Group Intravenous ribavirin for hantavirus pulmonary syndrome: safety and tolerance during 1 year of open-label experience. Antivir Ther. 1999;4:211–219. doi: 10.1177/135965359900400404. [DOI] [PubMed] [Google Scholar]
- 33.Hillyard IW. The preclinical toxicology and safety of ribavirin. In: Smith W, Kirkpatrick W, editors. Ribavirin: a broad spectrum antiviral agent. New York: Academic Press; 1980. pp. 59–71. [Google Scholar]
- 34.Japour AJ, Lertora JJ, Meehan PM, et al. AIDS Clinical Trials Group 231 Protocol Team A phase-I study of the safety, pharmacokinetics, and antiviral activity of combination didanosine and ribavirin in patients with HIV-1 disease. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:235–246. doi: 10.1097/00042560-199611010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Bodenheimer HC, Jr, Lindsay KL, Davis GL, Lewis JH, Thung SN, Seeff LB. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial. Hepatology. 1997;26:473–477. doi: 10.1002/hep.510260231. [DOI] [PubMed] [Google Scholar]
- 36.Dusheiko G, Main J, Thomas H, et al. Ribavirin treatment for patients with chronic hepatitis C: results of a placebo-controlled study. J Hepatol. 1996;25:591–598. doi: 10.1016/s0168-8278(96)80225-x. [DOI] [PubMed] [Google Scholar]
- 37.Tod M, Farcy-Afif M, Stocco J, et al. Pharmacokinetic/pharmacodynamic and time-to-event models of ribavirin-induced anaemia in chronic hepatitis C. Clin Pharmacokinet. 2005;44:417–428. doi: 10.2165/00003088-200544040-00006. [DOI] [PubMed] [Google Scholar]
- 38.McKee KT, Jr, Huggins JW, Trahan CJ, Mahlandt BG. Ribavirin prophylaxis and therapy for experimental Argentine hemorrhagic fever. Antimicrob Agents Chemother. 1988;32:1304–1309. doi: 10.1128/aac.32.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russmann S, Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr Med Chem. 2006;13:3351–3357. doi: 10.2174/092986706778773059. [DOI] [PubMed] [Google Scholar]
- 40.Canonico PG. Efficacy, toxicology and clinical applications of ribavirin against virulent RNA viral infections. Antiviral Res. 1985;(Suppl 1):75–81. doi: 10.1016/s0166-3542(85)80011-5. [DOI] [PubMed] [Google Scholar]
- 41.Price ME, Fisher-Hoch SP, Craven RB, McCormick JB. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. BMJ. 1988;297:584–587. doi: 10.1136/bmj.297.6648.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frame JD. Clinical features of Lassa fever in Liberia. Rev Infect Dis. 1989;11((Suppl 4)):S783–S789. doi: 10.1093/clinids/11.supplement_4.s783. [DOI] [PubMed] [Google Scholar]
- 43.Huggins JW. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev Infect Dis. 1989;11((Suppl 4)):S750–S761. doi: 10.1093/clinids/11.supplement_4.s750. [DOI] [PubMed] [Google Scholar]
- 44.Huggins JW, Jahrling PB, Kende M, Canonico PG. Efficacy of ribavirin against virulent RNA virus infections. In: Smith RA, Knight V, Smith JAD, editors. Clinical applications of ribavirin. New York: Academic Press; 1984. pp. 49–63. [Google Scholar]
- 45.Bossi P, Tegnell A, Baka A, et al. Bichat guidelines for the clinical management of haemorrhagic fever viruses and bioterrorism-related haemorrhagic fever viruses. Euro Surveill. 2004;9:E11–E12. [PubMed] [Google Scholar]
- 46.Johnson K. Lassa fever: life saving therapy with ribavirin. In: Stapleton T, editor. Studies with broad spectrum antiviral agent. Vol. 108. London: Royal Society of Medicine Services; 1986. pp. 25–36. [Google Scholar]
- 47.Crowcroft NS. Management of Lassa fever in European countries. Euro Surveill. 2002;7:50–52. doi: 10.2807/esm.07.03.00337-en. [DOI] [PubMed] [Google Scholar]
- 48.Johnson KM, Monath TP. Imported Lassa fever—reexamining the algorithms. N Engl J Med. 1990;323:1139–1141. doi: 10.1056/NEJM199010183231611. [DOI] [PubMed] [Google Scholar]
- 49.Cummins D. Lassa fever. Br J Hosp Med. 1990;43:186–188. 190, 192. [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention Management of patients with suspected viral hemorrhagic fever. MMWR Morb Mortal Wkly Rep. 1988;37((Suppl 3)):1–16. [Google Scholar]
- 51.Fisher-Hoch SP, Price ME, Craven RB, et al. Safe intensive-care management of a severe case of Lassa fever with simple barrier nursing techniques. Lancet. 1985;2:1227–1229. doi: 10.1016/s0140-6736(85)90752-4. [DOI] [PubMed] [Google Scholar]
- 52.Helmick CG, Webb PA, Scribner CL, Krebs JW, McCormick JB. No evidence for increased risk of Lassa fever infection in hospital staff. Lancet. 1986;2:1202–1205. doi: 10.1016/s0140-6736(86)92206-3. [DOI] [PubMed] [Google Scholar]
- 53.Frame JD, Baldwin JM, Jr, Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg. 1970;19:670–676. doi: 10.4269/ajtmh.1970.19.670. [DOI] [PubMed] [Google Scholar]
- 54.McCormick JB, King IJ, Webb PA, et al. A case-control study of the clinical diagnosis and course of Lassa fever. J Infect Dis. 1987;155:445–455. doi: 10.1093/infdis/155.3.445. [DOI] [PubMed] [Google Scholar]
- 55.Bausch DG, Demby AH, Coulibaly M, et al. Lassa fever in Guinea. I. Epidemiology of human disease and clinical observations. Vector Borne Zoonotic Dis. 2001;1:269–281. doi: 10.1089/15303660160025903. [DOI] [PubMed] [Google Scholar]
- 56.Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol. 2003;170:2797–2801. doi: 10.4049/jimmunol.170.6.2797. [DOI] [PubMed] [Google Scholar]
- 57.Baize S, Kaplon J, Faure C, Pannetier D, Georges-Courbot MC, Deubel V. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J Immunol. 2004;172:2861–2869. doi: 10.4049/jimmunol.172.5.2861. [DOI] [PubMed] [Google Scholar]
- 58.Bowen MD, Rollin PE, Ksiazek TG, et al. Genetic diversity among Lassa virus strains. J Virol. 2000;74:6992–7004. doi: 10.1128/jvi.74.15.6992-7004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knobloch J, McCormick JB, Webb PA, Dietrich M, Schumacher HH, Dennis E. Clinical observations in 42 patients with Lassa fever. Tropenmed Parasitol. 1980;31:389–398. [PubMed] [Google Scholar]
- 60.Monson MH, Frame JD, Jahrling PB, Alexander K. Endemic Lassa fever in Liberia. I. Clinical and epidemiological aspects at Curran Lutheran Hospital, Zorzor, Liberia. Trans R Soc Trop Med Hyg. 1984;78:549–553. doi: 10.1016/0035-9203(84)90082-8. [DOI] [PubMed] [Google Scholar]
- 61.Jahrling PB, Frame JD, Smith SB, Monson MH. Endemic Lassa fever in Liberia. III. Characterization of Lassa virus isolates. Trans R Soc Trop Med Hyg. 1985;79:374–379. doi: 10.1016/0035-9203(85)90386-4. [DOI] [PubMed] [Google Scholar]
- 62.Jahrling PB, Hesse RA, Eddy GA, Johnson KM, Callis RT, Stephen EL. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J Infect Dis. 1980;141:580–589. doi: 10.1093/infdis/141.5.580. [DOI] [PubMed] [Google Scholar]
- 63.Jahrling PB, Peters CJ, Stephen EL. Enhanced treatment of Lassa fever by immune plasma combined with ribavirin in cynomolgus monkeys. J Infect Dis. 1984;149:420–427. doi: 10.1093/infdis/149.3.420. [DOI] [PubMed] [Google Scholar]
- 64.Stephen EL, Jahrling PB. Experimental Lassa fever virus infection successfully treated with ribavirin. Lancet. 1979;1:268–269. doi: 10.1016/s0140-6736(79)90790-6. [DOI] [PubMed] [Google Scholar]
- 65.Weissenbacher MC, Calello MA, Merani MS, McCormick JB, Rodriguez M. Therapeutic effect of the antiviral agent ribavirin in Junin virus infection of primates. J Med Virol. 1986;20:261–267. doi: 10.1002/jmv.1890200308. [DOI] [PubMed] [Google Scholar]
- 66.Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;2:1216–1217. doi: 10.1016/s0140-6736(79)92335-3. [DOI] [PubMed] [Google Scholar]
- 67.Gunther S, Asper M, Roser C, et al. Application of real-time PCR for testing antiviral compounds against Lassa virus, SARS coronavirus and Ebola virus in vitro. Antiviral Res. 2004;63:209–215. doi: 10.1016/j.antiviral.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Connor J, Hintz M, van Dyke R. Ribavirin pharmacokinetics in children and adults during therapeutic trials. In: Smith R, Knight V, Smith J, editors. Clinical applications of ribavirin. Orlando: Academic Press; 1984. pp. 107–123. [Google Scholar]
- 69.Andrei G, De Clercq E. Inhibitory effect of selected antiviral compounds on arenavirus replication in vitro. Antiviral Res. 1990;14:287–299. doi: 10.1016/0166-3542(90)90009-v. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez M, McCormick JB, Weissenbacher MC. Antiviral effect of ribavirin on Junin virus replication in vitro. Rev Argent Microbiol. 1986;18:69–74. [PubMed] [Google Scholar]
- 71.Huggins JW, Robins RK, Canonico PG. Synergistic antiviral effects of ribavirin and the C-nucleoside analogs tiazofurin and selenazofurin against togaviruses, bunyaviruses, and arenaviruses. Antimicrob Agents Chemother. 1984;26:476–480. doi: 10.1128/aac.26.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandez H, Banks G, Smith R. Ribavirin: a clinical overview. Eur J Epidemiol. 1986;2:1–14. doi: 10.1007/BF00152711. [DOI] [PubMed] [Google Scholar]
- 73.Austin RK, Trefts PE, Hintz M, Connor JD, Kagnoff MF. Sensitive radioimmunoassay for the broad-spectrum antiviral agent ribavirin. Antimicrob Agents Chemother. 1983;24:696–701. doi: 10.1128/aac.24.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lertora JJ, Rege AB, Lacour JT, et al. Pharmacokinetics and long-term tolerance to ribavirin in asymptomatic patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 1991;50:442–449. doi: 10.1038/clpt.1991.162. [DOI] [PubMed] [Google Scholar]
- 75.Glue P. The clinical pharmacology of ribavirin. Semin Liver Dis. 1999;19((Suppl 1)):17–24. [PubMed] [Google Scholar]
- 76.Catlin DH, Smith R, Samuels AI. 14C-ribavirin: distribution and pharmacokinetic studies in rats, baboon and man. In: Smith RA, Kirkpatrick W, editors. Ribavirin: a broad spectrum antiviral agent. New York: Academic Press; 1980. pp. 83–98. [Google Scholar]
- 77.Preston SL, Drusano GL, Glue P, Nash J, Gupta SK, McNamara P. Pharmacokinetics and absolute bioavailability of ribavirin in healthy volunteers as determined by stable-isotope methodology. Antimicrob Agents Chemother. 1999;43:2451–2456. doi: 10.1128/aac.43.10.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paroni R, Del Puppo M, Borghi C, Sirtori CR, Galli Kienle M. Pharmacokinetics of ribavirin and urinary excretion of the major metabolite 1,2,4-triazole-3-carboxamide in normal volunteers. Int J Clin Pharmacol Ther Toxicol. 1989;27:302–307. [PubMed] [Google Scholar]
- 79.McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- 80.Maeda Y, Kiribayashi Y, Moriya T, et al. Dosage adjustment of ribavirin based on renal function in Japanese patients with chronic hepatitis C. Ther Drug Monit. 2004;26:9–15. doi: 10.1097/00007691-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 81.Bruchfeld A, Lindahl K, Schvarcz R, Stahle L. Dosage of ribavirin in patients with hepatitis C should be based on renal function: a population pharmacokinetic analysis. Ther Drug Monit. 2002;24:701–708. doi: 10.1097/00007691-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Roberts RB, Laskin OL, Laurence J, et al. Ribavirin pharmacodynamics in high-risk patients for acquired immunodeficiency syndrome. Clin Pharmacol Ther. 1987;42:365–373. doi: 10.1038/clpt.1987.165. [DOI] [PubMed] [Google Scholar]
- 83.Sidwell RW, Robins RK, Hillyard IW. Ribavirin: an antiviral agent. Pharmacol Ther. 1979;6:123–146. doi: 10.1016/0163-7258(79)90058-5. [DOI] [PubMed] [Google Scholar]
- 84.Canonico PG, Kende M, Luscri BJ, Huggins JW. In-vivo activity of antivirals against exotic RNA viral infections. J Antimicrob Chemother. 1984;14((Suppl A)):27–41. doi: 10.1093/jac/14.suppl_a.27. [DOI] [PubMed] [Google Scholar]
- 85.Bausch DG, Sesay SS, Oshin B. On the front lines of Lassa fever: Aniru Conteh (1942–2004) Emerg Infect Dis. 2004;10:1889–1890. doi: 10.3201/eid1010.IM1010. [DOI] [PMC free article] [PubMed] [Google Scholar]


