Abstract
Despite documentation that the inanimate hospital environment (e.g., surfaces and medical equipment) becomes contaminated with nosocomial pathogens, the data that suggest that contaminated fomites lead to nosocomial infections do so indirectly. Pathogens for which there is more-compelling evidence of survival in environmental reservoirs include Clostridium difficile, vancomycin-resistant enterococci, and methicillin-resistant Staphylococcus aureus, and pathogens for which there is evidence of probable survival in environmental reservoirs include norovirus, influenza virus, severe acute respiratory syndrome—associated coronavirus, and Candida species. Strategies to reduce the rates of nosocomial infection with these pathogens should conform to established guidelines, with an emphasis on thorough environmental cleaning and use of Environmental Protection Agency—approved detergent-disinfectants.
The role of the inanimate hospital environment (e.g., surfaces and equipment) in the spread of nosocomial infection is controversial. Although contamination of the inanimate environment by microorganisms has long been recognized, its significance is unclear. For example, for one medical center, the decrease in environmental contamination that occurred after a move to a new hospital was not associated with any change in nosocomial infection rates [1]. Are organisms that are found in the inanimate environment “innocent bystanders,” or are they a source of patient colonization and infection?
Discrepancies between studies regarding the degree and impact of environmental contamination may reflect a complex epidemiology, differences in measurement between studies, or the variable quality of institutional cleaning, which is an important and frequently unmeasured confounder. In addition, the finding of pathogens in the hospital environment, although necessary, is not sufficient to prove a causal role in the pathogenesis of nosocomial infection. Last, observations from uncontrolled studies that outbreaks end following the implementation of improved environmental cleaning must be viewed critically, because the use of multiple infection-control measures may obscure the importance of specific infection-control activities.
The quality of the evidence that examines the contamination of the inanimate environment should be judged according to whether the following 4 factors have been measured: (1) the degree of contamination of the nosocomial environment by specific pathogens; (2) whether temporality is addressed (i.e., whether the environment is contaminated before or after patient colonization); (3) the assessment of confounders, such as hand hygiene and the quality of cleaning of fomites; and (4) whether improved cleaning, after controlling for other interventions, reduces the risk of patient infection. The best studies of cross-colonization of patients from the inanimate environment use molecular epidemiologic techniques to identify pathogens, measure the quality of environmental cleaning and hand hygiene over time, and link contaminated surfaces and cross-colonization events in geographic and temporal dimensions.
Contamination of The Hospital Environment By Nosocomial Pathogens
Viruses
Viruses can contaminate and survive in the inanimate environment (table 1). Environmental cleaning is an important part of infection-control strategies for influenza, parainfluenza, enteric viruses, hepatitis B virus, and severe acute respiratory syndrome (SARS)-associated coronavirus.
Table 1.
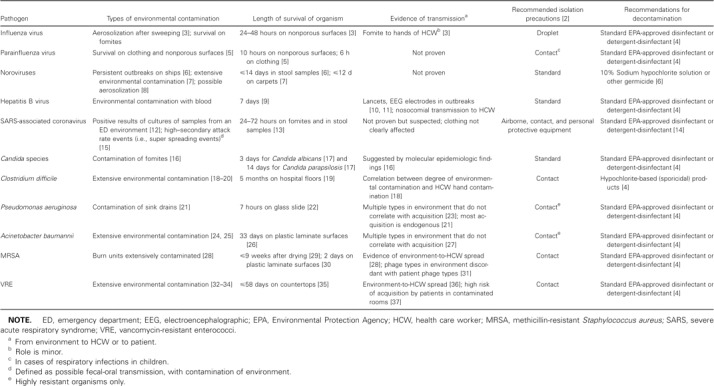
Summary of nosocomial pathogens and environmental contamination.
Influenza virus is generally spread through large respiratory droplets and, possibly, through airborne droplet nuclei. Classic studies have shown that influenza virus can contaminate the environment, persist after drying, and become re-aerosolized during floor sweeping. Influenza virus can survive for 24–48 h on nonporous surfaces, and viable virus can be spread to the skin, suggesting that environmental contamination can lead to cross-infection of patients via the hands of health care workers [3]. Similarly, parainfluenza virus is resistant to drying and can survive for 10 h on nonporous surfaces and for >6 h on clothing [5].
Human enteric viruses contaminate the inanimate environment and can cause institutional outbreaks [38–40]. Rotavirus is a well-known cause of outbreaks in day care centers and health care settings, extensively contaminates and survives on surfaces, and may spread after contamination of toys shared among children [38]. Norovirus has been the cause of outbreaks on cruise ships, in hospitals, and in hotels [7, 8, 39, 40]. In 2002, a total of 9 outbreaks of norovirus on cruise ships were reported [40], and outbreaks occurred on consecutive cruises, despite attempts to disinfect the ships. For 3 of 5 ships on which the outbreaks occurred, discontinuation of service and aggressive cleaning and sanitation of the ship were required to stop the outbreaks. Although no conclusive proof exists of environment-to-person transmission of norovirus, the virus has been cultured extensively from samples obtained from the inanimate environment during outbreaks [8, 39], and indirect evidence supports the idea that aerosolization of the virus can occur following emesis [8].
Individuals without immunity to hepatitis B virus (HBV) should be considered to be at risk for infection from contaminated environmental sources. Blood from infected individuals with active viral replication (i.e., hepatitis B surface antigen—positive and hepatitis B e antigen—positive individuals) may have high levels of virus, and small, visually undetectable inocula may be highly infective. HBV may survive for up to 7 days in relative humidity of 42% [9]. Outbreaks of hepatitis B that have involved fomites have been traced to contaminated electroencephalographic electrodes [10] and to lancets used in the monitoring of glucose levels [11].
SARS-associated coronavirus is believed to be spread mainly via respiratory droplets, although fecal-oral transmission and transmission via surface contamination may also occur. Current infection-control recommendations for hospitals include the use of precautions against contact, droplet, and airborne transmission [14]. The virus has been found to survive for 24–72 h on plastered walls, plastic laminate surfaces (e.g., Formica, Formica Corporation), and plastic surfaces and is viable in excreted feces and urine for at least 1–2 days at room temperature [13]. An outbreak in an apartment complex in Hong Kong may have been the result of fecal-oral transmission combined with environmental contamination [15], although the results of a modeling approach suggested an airborne mode of transmission [41]. Cleaning likely reduces surface contamination; an outbreak of cases in an emergency department in Taiwan was reported in which positive results of cultures of environmental samples obtained during the outbreak became negative after the emergency department was cleaned and the patients were isolated [12].
Fungi
Although the majority of Candida infections are likely due to endogenous sources (i.e., arising from patient colonization), molecular typing studies of yeast recovered from patients, from the hands of health care workers, and from the environment suggest that fomites may play a role in the spread of Candida albicans, Candida glabrata, and Candida parapsilosis among patients who undergo bone marrow transplantation, although the direction in which transmission occurs (i.e., from patient to environment vs. from environment to patient) has not been demonstrated conclusively [16]. Surfaces may be durably contaminated, because experimental inoculation of dry surfaces shows that C. albicans and C. parapsilosis can survive for 3 days and 14 days, respectively [17]. Epidemic spread of Candida infection has occurred in which environmental sources (e.g., a blood pressure transducer or irrigating solution) were suspected [16, 42]. Evidence of an environmental reservoir of endemic C. albicans and C. glabrata has been suggested through the use of molecular typing of Candida isolates recovered from the environment and from patients who underwent bone marrow transplantation [16]. The strain types of Candida isolates acquired by patients were identical to those found on the hospital surfaces of rooms where the patients were housed, prior to patient acquisition of infection [16].
Aspergillus and Zygomycetes species are causes of nosocomial skin infection that result from contaminated fomites. Infections have been associated with the use of arm boards or bandages by patients who have intravascular catheters, as well as with elasticized surgical bandages, hospital construction activity, and postoperative wounds [43].
Bacteria
Clostridium difficile. Spores of C. difficile are durable and are resistant to usual cleaning methods. Contamination of the inanimate environment by C. difficile has been reported to occur in areas in close proximity to infected or colonized patients. Contamination rates have been as high as 58%; commonly affected surfaces and equipment include commodes, bedpans, blood pressure cuffs, walls, floors, washbasins, and furniture [18–20]. The organism has been found in low numbers on shoes and on stethoscopes [20], and hospital floors have remained contaminated with C. difficile for up to 5 months [19]. The density of contamination is increased by the presence of colonized patients and patients with diarrhea [18, 20].
Molecular techniques provide the most concrete evidence of transmission of C. difficile from environmental surfaces to patients. The findings of a study of endemic C. difficile [18] were as follows: (1) C. difficile was present on the hands of health care workers, (2) there was a correlation between the degree of colonization of health care workers' hands and environmental contamination with C. difficile, and (3) there was differential contamination of the environment by individual strain types. Among colonized patients, a single, predominant isolate was found and was more likely to contaminate the environment than were isolates that sporadically colonized patients. This finding was reproduced in a study in which (1) despite endemicity of C. difficile, a single genotype predominated in the inanimate environment, and (2) the incidence of C. difficile infection correlated well with environmental contamination [44]. These data suggest that environmental surfaces serve as a reservoir that permits the cross-colonization of patients after they have had contact with a health care worker and that, in environments in which C. difficile is endemic, specific isolates likely predominate [18, 44].
Gram-negative bacilli. Enteric gram-negative bacilli are not commonly spread to patients from the dry inanimate environment; they are generally not viable after drying, lasting 7 h or less after desiccation [22]. Infection with these organisms is thought to occur because of endogenous spread or cross-infection between patients via the hands of health care workers. However, Pseudomonas aeruginosa and Acinetobacter baumannii are strongly associated with environmental contamination.
Many studies have documented the contamination of sinks and sink drains by P. aeruginosa [21]. Whether the use of sinks leads to the nosocomial spread of P. aeruginosa is unclear. P. aeruginosa strain types that are isolated from the inanimate environment do not always match the strains that are present in incident cases [23]. In a study that examined cultures of samples of endogenous flora obtained from patients and samples obtained from the inanimate environment, results suggested that most infections with P. aeruginosa were the result of endogenous flora in patients rather than exogenous acquisition [21]. Therefore, environmental surfaces may be of variable significance in the spread of P. aeruginosa.
A. baumannii is a nonfermentative gram-negative coccobacillus that is a commensal but also causes infections (e.g., ventilator-associated pneumonia and bloodstream infections). In the past decade, A. baumannii isolates have been marked by increased resistance to antibiotics and have been the cause of recalcitrant nosocomial outbreaks. The organism has been isolated throughout the inanimate environment—on the beds of colonized patients and on nearby surfaces (e.g., on mattresses and bedside equipment), in hospital rooms (e.g., on floors, sinks, countertops, and door handles), and in room humidifiers [24, 25]. Spread of A. baumannii via droplets has been suggested by the results of air sampling with culture plates [24]. Acinetobacter species are found in soil and water and may have adapted to survive for long periods, with reports of survival of up to 3 years in hospitals [26].
Strain types of A. baumannii isolated from the inanimate environment have included strains that affect patients, as well as types that have not been found to affect patients [27]. Some studies have found no strains of A. baumannii in the inanimate environment, despite outbreaks of infection with A. baumannii among patients [45, 46], making the role of the environment in patient colonization unclear. However, the levels of hand hygiene and environmental cleaning are not commonly reported in outbreak investigations, and it is possible that the importance of environmental contamination is confounded by other interventions.
Gram-positive cocci. The major reservoirs for methicillin-resistant Staphylococcus aureus (MRSA) are colonized or infected patients and, occasionally, personnel in the hospital [47], and the major mechanism of spread is via the unwashed hands of health care workers. The role of the inanimate environment is controversial; proof of environment-to-patient transmission is not strong, the inanimate environment is variably contaminated, and the phage types of environmental isolates have not always matched the phage types isolated from colonized patients [31, 48].
The inanimate environment of burn units tends to be more heavily contaminated than that of nonburn units: MRSA contamination rates range from 1% to 18% in nonburn wards to up to 64% in burn units [28]. Hydrotherapy rooms associated with burn units have a particularly high contamination rate [47]. Rates of environmental contamination also vary on the basis of the site of infection in source patients: contamination is more common in the rooms of patients with infected urine or wounds than it is in the rooms of patients with bacteremia only [28]. Similar to other organisms (i.e., P. aeruginosa, vancomycin-resistant enterococci [VRE], and Acinetobacter species), S. aureus has been cultured from hospital mattresses during an outbreak. Moist mattress padding and leaks in mattress covers are common findings during outbreaks [49]. Other sites that have yielded MRSA include mops [50] and the gowns and gloves worn by health care workers [28]. Both MRSA and methicillin-susceptible Staphylococcus aureus have been found to be viable for as long as 9 weeks, despite drying, and have been found to survive on plastic laminate surfaces for up to 2 days under experimental conditions [29, 30].
Little evidence exists that proves that decreasing environmental contamination with MRSA leads to decreases in rates of patient infections. The most compelling are data that prove that contamination of the environment leads to contamination of health care workers' gowns and gloves, both of which could result in patient colonization [28]. Other studies have shown that cleaning the inanimate environment or isolating patients caused cessation of outbreaks of MRSA, but interpretation is limited because of the use of multiple interventions [51, 52].
The fact that VRE contaminates the inanimate environment has been well established. VRE have been found in up to 37% of samples obtained from the environment and are most often found in association with diarrhea. Environmental sites with VRE involvement have included the gowns worn by patients and health care workers, medical equipment, microsphere beds, and environmental surfaces [34]. Enterococcus species can survive for up to 58 days on experimentally inoculated countertops [35]; however, vancomycin resistance does not confer an additional advantage for survival, and routine disinfectants, heat sterilization processes, and laundry procedures all eradicate the organism [32, 53, 54].
The degree of environmental contamination with VRE correlates with the number of body site that have been colonized with VRE [55]. Environmental sites closest to the patient(e.g., bed rails, bedside tables, and pullover sheets) have the greatest likelihood of being contaminated with VRE [56]. The quantity of VRE in the environment is less than that on the skin of patients (e.g., the inguinal area will have a much higher colony count than will the nearby environment) [56]. Transmission from surfaces to patients might occur: contact with contaminated surfaces alone is almost as likely to lead to contamination of the hands of health care workers as is contact with a colonized patient [36]. Other data supporting environment-to-patient transmission demonstrate that noncolonized patients who were admitted to contaminated rooms had highly increased odds of acquisition of VRE [37].
In monoclonal outbreaks of VRE, the strain isolated from patients during the outbreak (hereafter known as the “outbreak strain”) contaminates environmental surfaces, which suggests that the environment may be a common source of VRE [33]. For example, outbreaks have been associated with thermometers carrying VRE strains that were clonally identical to outbreak strains [33]. However, studies to clarify the role of the environment in outbreaks need to be performed.
The behavior of VRE in environments in which VRE is endemic shows a more complex epidemiology. The diversity of clones of VRE emerges through importation by colonized patients or through genetic changes—in other words, through mutation or genetic transfer of resistance elements to vancomycin-susceptible organisms [57]. When multiple strains occur in a hospital, strain types that are isolated from patient rooms either may be the same as the strains isolated from colonized patients housed within the rooms [58, 59] or may differ substantially [37, 60]. It has been suggested that the behavior of VRE is similar to that of C. difficile, in that, despite endemicity, clustering of strains isolated from patients and from the environment occurs [37, 61]. Environmental contamination with VRE followed by patient acquisition of an indentical strain type has also been reported [55]. More data are needed to clarify the behavior of VRE, but it is likely that such factors as degree of cleaning, compliance with gown use and hand hygiene, and presence of common sources of VRE interact in the spread of the organism.
Intervention Strategies
Two major categories for the intensity of cleaning exist: sterilization and disinfection. Sterilization destroys all microbial life on an object or surface and occurs through the use of heat, pressure, or chemical methods. Disinfection eliminates most microbes, excluding bacterial spores, and typically involves the use of chemical agents. The degree of destruction of organisms depends on their sensitivity to chemical disinfection. High-level disinfection involves the elimination of all but large quantities of spores, intermediate-level disinfection leads to destruction of all life except spores, and low-level disinfection will not reliably kill mycobacteria or spores. “Cleaning” is the process of removal of foreign material from a surface or object and may involve both mechanical processes and the use of detergents with water. Cleaning, alone, can reduce the organism load on a surface and, if used in conjunction with disinfection, may lead to significant reductions in organism load in shorter spans of time [62]. Three types of available solutions can be used during cleaning: detergents, which remove organic material and suspend grease or oil; disinfectants, which rapidly kill or inactivate infectious particles; and detergent-disinfectants, which achieve both aims. Conclusive data do not exist to prove that the routine disinfection of hospital surfaces is preferable to the use of detergent alone [63], and, therefore, routine use of detergent-disinfectants is based largely on consensus and logistic considerations [4].
In 2003, the Healthcare Infection Control Practice Advisory Committee of the Centers for Disease Control and Prevention (CDC/HICPAC; Atlanta, GA) issued updated guidelines for environmental infection control in health care facilities [4]. As a part of these recommendations, strategies for the cleaning of patient care areas were enumerated. The objective of cleaning efforts should be to keep surfaces visibly clean, to disinfect high-contact surfaces more frequently than non—high-contact surfaces, and to clean up spills promptly. For patient care areas, it is suggested that environmental services workers select Environmental Protection Agency (EPA)-registered detergent-disinfectants to clean inanimate environmental surfaces. This is a controversial recommendation [63], but the CDC/HICPAC guidelines note that this recommendation accommodates situations in which uncertainty exists regarding the nature of the contaminants on inanimate environmental surfaces (e.g., blood or body fluid contamination vs. routinely accumulated dust or dirt) or regarding the presence of multidrug-resistant organisms on such surfaces [4]. No specific recommendations were given regarding the frequency of cleaning, only that it should occur on a regular basis. In hospitals, patient rooms should be cleaned on a daily basis and undergo “terminal cleaning” after patient discharge from the hospital. During terminal cleaning, noncritical surfaces in the inanimate environment may be thoroughly cleaned using a disinfectant, typically a quaternary ammonium compound or phenolics (the latter is not advised for use in nurseries or infant care areas). Terminal cleaning may be more efficacious for degerming the environment because of its greater thoroughness.
Changes in cleaning products or cleaning practices are generally not required to eliminate specific pathogens. Areas with high rates of C. difficile infection may warrant the use of hypochlorite-based products because of the more reliable sporicidal activity of these agents. Most commercial disinfectants used for environmental cleaning have activity against viruses; enveloped viruses are more susceptible to detergents than are nonenveloped viruses [9]. Most viruses, including SARS-associated coronavirus, may be eliminated through the use of EPA-approved disinfectants or detergent-disinfectants that are prepared according to the manufacturers' instructions [14]. Decontamination performed after outbreaks of norovirus should involve the use of a germicidal product, such as 10% sodium hypochlorite solution (i.e., household bleach), and closure of an affected institution or facility may be necessary [6].
Effective cleaning of the hospital environment would seem, intuitively, to be an important factor in the control of resistant organisms. One study evaluated a more intense method of environmental cleaning that allowed inanimate environmental surfaces to have prolonged exposure to the cleaning agent and that eliminated environmental VRE [64]. Enhancements in cleaning adherence have also affected environmental hygiene. In a study of cleaning behaviors, constructive feedback given to housekeeping staff led to improved environmental cleaning and a 3-fold reduction in environmental VRE contamination. This change occurred through the use of conventional cleaning methods and materials only [65]. Whether these improvements translate into diminished rates of nosocomial infection is unclear.
Discussion
Although much about the spread of nosocomial infection remains unknown, several facts have been established by existing data: (1) inanimate environmental surfaces can become durably contaminated after exposure to colonized patients; (2) although an organism may be endemic within an institution, specific isolates may predominate in the inanimate environment (as shown for C. difficile and VRE); and (3) contaminated rooms may be a risk factor for the acquisition of nosocomial pathogens by unaffected patients. The use of molecular epidemiology has helped to enhance understanding of the role of the environment in nosocomial infection by confirming that isolates in the environment either are the same as isolates recovered from patients (as shown for C. difficile, Candida species, or VRE) or differ (as shown in the case of Acinetobacter species). It is difficult, given the existing data, to draw conclusions from many existing outbreak reports or studies of the inanimate environment, because the levels of hand hygiene or environmental cleaning are rarely measured and may represent important confounders of the environment-transmission association. Studies conclusively demonstrating an improvement in nosocomial infection rates following improved cleaning need to be performed. Future studies to elaborate on the role of the inanimate environment must include measures of when, where, and how: in other words, when the environment was contaminated and patients acquired organisms; where the the patients were located during acquisition, with respect to contaminated rooms; and how well hand hygiene and environmental cleaning were practiced.
The importance of understanding the role of the inanimate environment derives from continued problems in compliance with infection control measures and hand hygiene. The advent of alcohol gels may lead to increased hand hygiene compliance and may diminish the effect of contact with colonized walls, bed rails, or medical equipment. However, it may be that an additional cost-effective infection-control measure in hospitals will be better, more thorough, and more frequent environmental cleaning that reduces the risk of cross-colonization.
Acknowledgment
Conflict of interest. B.H.: No conflict.
References
- 1.Maki DG, Alvarado CJ, Hassemr CA, Zilz MA. Relation of the inanimate hospital environment to endemic nosocomial infection. N Engl J Med. 1982;307:1562–6. doi: 10.1056/NEJM198212163072507. [DOI] [PubMed] [Google Scholar]
- 2.Garner JS. Guideline for isolation precautions in hospitals. Infect Control Hosp Epidemiol. 1996;17:53–80. doi: 10.1086/647190. [DOI] [PubMed] [Google Scholar]
- 3.Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in health care settings. Clin Infect Dis. 2003;37:1094–101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- 4.Sehulster L, Chinn RY, Centers for Disease Control and Prevention, HICPAC Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52(RR-10):1–42. [PubMed] [Google Scholar]
- 5.Brady MT, Evans J, Cuartas J. Survival and disinfection of parainfluenza viruses on environmental surfaces. Am J Infect Control. 1990;18:18–23. doi: 10.1016/0196-6553(90)90206-8. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Norwalk like viruses: public health consequences and management. MMWR Recomm Rep. 2001;50(RR-09):1–18. [PubMed] [Google Scholar]
- 7.Cheesbrough JS, Green J, Gallimore CI, et al. Widespread environmental contamination with Norwalk-like viruses detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol Infect. 2000;125:93–8. doi: 10.1017/s095026889900432x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks PJ, Vipond IB, Carlisle D, et al. Evidence for airborne transmission of Norwalk-like virus in a hotel restaurant. Epidemiol Infect. 2000;124:481–7. doi: 10.1017/s0950268899003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaidi M, Wenzel RP. Disinfection, sterilization, and control of hospital waste. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. pp. 3000–2. [Google Scholar]
- 10.Hepatitis B Outbreak Investigation Team An outbreak of hepatitis B associated with reusable subdermal electroencephalogram electrodes. CMAJ. 2000;162:1127–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Polish LB, Shapiro CN, Bauer F, et al. Nosocomial transmission of hepatitis B virus associated with the use of a spring-loaded finger-stick device. N Engl J Med. 1992;326:721–5. doi: 10.1056/NEJM199203123261101. [DOI] [PubMed] [Google Scholar]
- 12.Chen YC, Huang LM, Chan CC, et al. Emerg Infect Dis [serial online] May. 2004. SARS in hospital emergency room. Available at: http://www.cdc.gov/ncidod/EID/vol10no5/03-0579.htm. Accessed 29 February 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network. Available at: http://www.who.int/csr/sars/survival_2003_05_04/en/index.html. Accessed 29 February 2004. [Google Scholar]
- 14.Centers for Disease Control and Prevention . Public health guidance for community-level preparedness and response to severe acute respiratory syndrome (SARS) Version 2. Available at: http://www.cdc.gov/ncidod/sars/guidance/I/pdf/healthcare.pdf. Accessed 20 September 2004. [Google Scholar]
- 15.Sampathkumar P, Temesgen Z, Smith TF, et al. SARS: epidemiology, clinical presentation, management, and infection control measures. Mayo Clin Proc. 2003;78:882–90. doi: 10.4065/78.7.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vazquez JA, Dembry LM, Sanchez V, et al. Nosocomial Candida glabrata colonization: an epidemiologic study. J Clin Microbiol. 1998;36:421–6. doi: 10.1128/jcm.36.2.421-426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traore O, Springthorpe VS, Sattar SA. A quantitative study of the survival of two species of Candida on porous and non-porous environmental surfaces and hands. J Appl Microbiol. 2002;92:549–55. doi: 10.1046/j.1365-2672.2002.01560.x. [DOI] [PubMed] [Google Scholar]
- 18.Samore MH, Venkataraman L, DeGirolami PC, et al. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med. 1996;100:32–40. doi: 10.1016/s0002-9343(96)90008-x. [DOI] [PubMed] [Google Scholar]
- 19.Kim KH, Fekety R, Batts DH, et al. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J Infect Dis. 1981;143:42–50. doi: 10.1093/infdis/143.1.42. [DOI] [PubMed] [Google Scholar]
- 20.Fekety R, Kim KH, Brown D, et al. Epidemiology of antibiotic-associated colitis: isolation of Clostridium difficile from the hospital environment. Am J Med. 1981;70:906–8. doi: 10.1016/0002-9343(81)90553-2. [DOI] [PubMed] [Google Scholar]
- 21.Olson B, Weinstein RA, Nathan C, et al. Epidemiology of endemic Pseudomonas aeruginosa: why infection control efforts have failed. J Infect Dis. 1984;150:808–16. doi: 10.1093/infdis/150.6.808. [DOI] [PubMed] [Google Scholar]
- 22.Hirai Y. Survival of bacteria under dry conditions: from a viewpoint of nosocomial infection. J Hosp Infect. 1991;19:191–200. doi: 10.1016/0195-6701(91)90223-u. [DOI] [PubMed] [Google Scholar]
- 23.Orsi GB, Mansi A, Tomao P, et al. Lack of association between clinical and environmental isolates of Pseudomonas aeruginosa in hospital wards. J Hosp Infect. 1994;27:49–60. doi: 10.1016/0195-6701(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 24.Simor AE, Lee M, Vearncombe M, et al. An outbreak due to multiresistant A. bauman nii in a burn unit: risk factors for acquisition and management. Infect Control Hosp Epidemiol. 2002;23:261–7. doi: 10.1086/502046. [DOI] [PubMed] [Google Scholar]
- 25.Das I, Lambert P, Hill D, et al. Carbapenem-resistant Acinetobacter and role of curtains in an outbreak in intensive care units. J Hosp Infect. 2002;50:110–4. doi: 10.1053/jhin.2001.1127. [DOI] [PubMed] [Google Scholar]
- 26.Jawad A, Seifert H, Snelling AM, et al. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J Clin Microbiol. 1998;36:1938–41. doi: 10.1128/jcm.36.7.1938-1941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster CA, Crowe M, Humphreys H, et al. Surveillance of an adult intensive care unit for long-term persistence of a multi-resistance strain of A. baumann ii. Eur J Clin Microbiol Infect Dis. 1998;17:171–6. doi: 10.1007/BF01691113. [DOI] [PubMed] [Google Scholar]
- 28.Boyce JM, Potter-Bynoe G, Chenevert C, et al. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997;18:622–7. [PubMed] [Google Scholar]
- 29.Beard-Pegler MA, Stubbs E, Vickery AM. Observations on the resistance to drying of staphylococcal strains. J Med Microbiol. 1988;26:251–5. doi: 10.1099/00222615-26-4-251. [DOI] [PubMed] [Google Scholar]
- 30.Duckworth GJ, Jordens JZ. Adherence and survival properties of an epidemic methicillin-resistant strain of Staphylococcus aureus compared with those of methicillin-sensitive strains. J Med Microbiol. 1990;32:195–200. doi: 10.1099/00222615-32-3-195. [DOI] [PubMed] [Google Scholar]
- 31.Bradley SF, Terpenning MS, Ramsey MA, et al. Methicillin-resistant Staphylococcus aureus : colonization and infection in a long-term care facility. Ann Intern Med. 1991;115:417–22. doi: 10.7326/0003-4819-115-6-417. [DOI] [PubMed] [Google Scholar]
- 32.Weber DJ, Rutala WA. Role of environmental contamination in the transmission of vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 1997;18:306–9. doi: 10.1086/647616. [DOI] [PubMed] [Google Scholar]
- 33.Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol. 1997;18:771–3. doi: 10.1086/647535. [DOI] [PubMed] [Google Scholar]
- 34.Gould FK, Freeman R. Nosocomial infection with microsphere beds. Lancet. 1993;342:241–2. doi: 10.1016/0140-6736(93)92333-o. [DOI] [PubMed] [Google Scholar]
- 35.Bonilla HF, Zervos MJ, Kauffman CA. Long-term survival of vancomycin-resistant Enterococcus faecium on a contaminated surface. Infect Control Hosp Epidemiol. 1996;17:770–2. doi: 10.1086/647230. [DOI] [PubMed] [Google Scholar]
- 36.Duckro AN, Blom DW, Lyle EA, et al. Program and abstracts of the 13th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America (Arlington, Virginia) Mt. Royal, NJ: Society for Healthcare Epidemiology of America; 2003. Frequency of environmental sites and patient skin as sources of VRE transmission; p. 64. [Google Scholar]
- 37.Martinez JA, Ruthazer R, Hansjosten K, et al. Role of environmental contamination as a risk factor for acquisition of vancomycin-resistant enterococci in patients treated in a medical intensive care unit. Arch Intern Med. 2003;163:1905–12. doi: 10.1001/archinte.163.16.1905. [DOI] [PubMed] [Google Scholar]
- 38.Rogers M, Weinstock DM, Eagan J, et al. Rotavirus outbreak on a pediatric oncology floor: possible association with toys. Am J Infect Control. 2000;28:378–80. doi: 10.1067/mic.2000.109908. [DOI] [PubMed] [Google Scholar]
- 39.Green J, Wright PA, Gallimore CI, et al. The role of environmental contamination with small round structured viruses in a hospital outbreak investigated by reverse-transcriptase polymerase chain reaction assay. J Hosp Infect. 1998;39:39–45. doi: 10.1016/s0195-6701(98)90241-9. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention Outbreaks of gastroenteritis associated with noroviruses on cruise ships—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1112–5. [PubMed] [Google Scholar]
- 41.Yu ITS, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. New Engl J Med. 2004;350:1731–9. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez JA, Sanchez V, Dmuchowski C, et al. Nosocomial acquisition of Candida albicans: an epidemiologic study. J Infect Dis. 1993;168:195–201. doi: 10.1093/infdis/168.1.195. [DOI] [PubMed] [Google Scholar]
- 43.Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fawley WN, Wilcox MH. Molecular epidemiology of endemic Clostridium difficile infection. Epidemiol Infect. 2001;126:343–50. doi: 10.1017/s095026880100557x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Agata EM, Thayer V, Schaffner W. An outbreak of Acinetobacter baumannii: the importance of cross-transmission. Infect Control Hosp Epidemiol. 2000;21:588–91. doi: 10.1086/501808. [DOI] [PubMed] [Google Scholar]
- 46.Mah MW, Memish ZA, Cunningham G, et al. Outbreak of Acinetobacter baumannii in an intensive care unit associated with tracheostomy. Am J Infect Control. 2001;29:284–8. doi: 10.1067/mic.2001.114232. [DOI] [PubMed] [Google Scholar]
- 47.Boyce JM. Methicillin-resistant Staphylococcus aureus in hospitals and long-term care facilities: microbiology, epidemiology, and preventive measures. Infect Control Hosp Epidemiol. 1992;13:725–37. doi: 10.1086/648346. [DOI] [PubMed] [Google Scholar]
- 48.Cookson B, Peters B, Webster M, et al. Staff carriage of epidemic methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1989;27:1471–6. doi: 10.1128/jcm.27.7.1471-1476.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ndawula EM, Brown L. Mattresses as reservoirs of epidemic methicillin-resistant Staphylococcus aureus. Lancet. 1991;337:488. doi: 10.1016/0140-6736(91)93420-e. [DOI] [PubMed] [Google Scholar]
- 50.Oie S, Kamiya A. Survival of methicillin-resistant Staphylococcus aureus (MRSA) on naturally contaminated dry mops. J Hosp Infect. 1996;34:145–9. doi: 10.1016/s0195-6701(96)90140-1. [DOI] [PubMed] [Google Scholar]
- 51.Fitzpatrick F, Murphy OM, Brady A, et al. A purpose built MRSA cohort unit. J Hosp Infect. 2000;46:271–9. doi: 10.1053/jhin.2000.0838. [DOI] [PubMed] [Google Scholar]
- 52.Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2001;49:109–16. doi: 10.1053/jhin.2001.1013. [DOI] [PubMed] [Google Scholar]
- 53.Bradley CR, Fraise AP. Heat and chemical resistance of enterococci. J Hosp Infect. 1996;34:191–6. doi: 10.1016/s0195-6701(96)90065-1. [DOI] [PubMed] [Google Scholar]
- 54.Orr KE, Holliday MG, Jones AL, et al. Survival of enterococci during hospital laundry processing. J Hosp Infect. 2002;50:133–9. doi: 10.1053/jhin.2001.1137. [DOI] [PubMed] [Google Scholar]
- 55.Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet. 1996;348:1615–9. doi: 10.1016/S0140-6736(96)02331-8. [DOI] [PubMed] [Google Scholar]
- 56.Blom DW, Lyle EA, Weinstein RA, et al. Program and abstracts of the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) (Toronto, Canada) Washington, DC: American Society for Microbiology Press; 2000. The relationship between environmental contamination with vancomycin-resistant Enterococcus and patient colonization in a medical intensive care unit; p. 432. [Google Scholar]
- 57.Kim WJ, Weinstein RA, Hayden MK. The changing molecular epidemiology and establishment of endemicity of vancomycin resistance in enterococci at one hospital over a 6-year period. J Infect Dis. 1999;179:173–1. doi: 10.1086/314564. [DOI] [PubMed] [Google Scholar]
- 58.Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med. 1996;125:448–56. doi: 10.7326/0003-4819-125-6-199609150-00004. [DOI] [PubMed] [Google Scholar]
- 59.Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med. 1999;131:269–72. doi: 10.7326/0003-4819-131-4-199908170-00006. [DOI] [PubMed] [Google Scholar]
- 60.Shay DK, Maloney SA, Montecalvo M, et al. Epidemiology and mortality risk of vancomycin-resistant enterococcal bloodstream infections. J Infect Dis. 1995;172:993–1000. doi: 10.1093/infdis/172.4.993. [DOI] [PubMed] [Google Scholar]
- 61.Edmond MB, Ober JF, Weinbaum DL, et al. Vancomycin-resistant Enterococcus faecium bacteremia: risk factors for infection. Clin Infect Dis. 1995;20:1126–33. doi: 10.1093/clinids/20.5.1126. [DOI] [PubMed] [Google Scholar]
- 62.Rutala WA. APIC guideline for selection and use of disinfectants. 1994, 1995, and 1996 APIC Guidelines Committee. Association for Professionals in Infection Control and Epidemiology, Inc. Am J Infect Control. 1996;24:313–42. doi: 10.1016/s0196-6553(96)90066-8. [DOI] [PubMed] [Google Scholar]
- 63.Allerberger F, Ayliffe G, Bassetti M, et al. Routine surface disinfection in health care facilities: should we do it? Am J Infect Control. 2002;30:318–9. doi: 10.1067/mic.2002.127205. [DOI] [PubMed] [Google Scholar]
- 64.Byers KE, Durbin LJ, Simonton BM, et al. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol. 1998;19:261–4. doi: 10.1086/647806. [DOI] [PubMed] [Google Scholar]
- 65.Hota B, Blom DW, Weinstein RA, et al. Program and abstracts of the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) (Chicago) Washington DC: American Society for Microbiology Press; 2003. The effect of observation of environmental workers on thoroughness and outcome of environmental cleaning; p. 369. [Google Scholar]


