Abstract
This study analyzes single factors that affect the prognosis of severe acute respiratory syndrome (SARS) and establishes a prognosis model by multivariate analysis. We retrospectively analyzed the clinical features of SARS in 165 clinically confirmed severe cases. Both age and existence of other diseases before SARS were significantly correlated with prognosis (r = 0.506 and r = 0.457, respectively; P < .001). During the acute phase of SARS, lactate dehydrogenase level, degree of hypoxemia, respiratory rate, α-hydroxybutyric dehydrogenase level, creatine kinase isoenzyme—MB, platelet count, and number of involved lobes noted on chest radiographs, and so on, correlated markedly with the prognosis (r = 0.257–0.788; P < .05). The multivariate prognosis regression model was associated with degree of hypoxemia and platelet count. The model was defined by the formula Py=1 = es/(1 + es), where S is [2.490 × degree of hypoxemia]-[0.050 × number of platelets], and it had a high sensitivity (91.67%), specificity (98.33%), and accuracy (96.42%). The model could be used to effectively judge the state of illness and the prognosis.
Severe acute respiratory syndrome (SARS), which is also called “infectious atypical pneumonia” in China, is a new infectious respiratory disease with high infectivity and a state of illness that rapidly develops. It can develop into acute respiratory distress syndrome (ARDS) in some severe cases and even into multiorgan failure during the late stage in a few severe cases [1–3]. Although there have been 2 studies of the risk factors for death in cases of severe SARS [4, 5], the number of cases studied has been low (<40 cases), and few parameters have been determined. Therefore, these studies do not systematically reflect various factors that affect the prognosis in severe SARS cases. To better investigate the factors that affect the prognosis in severe SARS cases, we analyzed clinical data for 165 patients with severe SARS.
Patients Materials and Methods
General data. We collected clinical data for 165 patients with clinically confirmed severe SARS who were hospitalized for treatment in Beijing during the period of 5 March through 31 May 2003 [6]. Of the 165 patients (mean age ± SD, 37.75 ± 15.82; range, 13–93 years; ), 100 (60.6%) were male and 65 (39.4%) were female. Seven of the patients had a history of hypertension, 6 had a history of chronic liver disease (including liver cirrhosis), 3 had a history of diabetes, 3 had a history of coronary heart disease, 10 had a history of other chronic diseases, 15 had a history of ⩾2 chronic diseases, and 1 was pregnant. Sixty-seven patients (40.6%) had a history of close contact with a person with confirmed SARS. Forty patients (24.2%) had a history of close contact with a person with confirmed SARS and belonged to the group of persons with a history of infection with the SARS virus. There was definite evidence that 12 patients (7.3%) had infected other persons. Forty-six patients (27.9%) had only a history of travel to or residence in (within 2 weeks before the onset of SARS) an area where there had been recent local transmission of SARS.
Criteria for diagnosis of severe SARS. Diagnoses were made and clinical typing was conducted in accordance with the Interim Standard for Clinical Diagnosis of Infectious Atypical Pneumonia promulgated by the Office of the Chinese Ministry of Health on 4 May 2003 [6, 7]. The case definitions of SARS used were those of the World Health Organization [8] (revised on 1 May 2003; table 1).
Table 1.

Comparison between the criteria for severe acute respiratory syndrome (SARS) and severe SARS promulgated by Chinese Ministry of Health and the criteria for SARS promulgated by the World Health Organization (WHO).
Methods. The levels of alanine aminotransferase, aspartate aminotransferase, albumin, cholinesterase, total bilirubin, lactate dehydrogenase (LDH), α-hydroxybutyric dehydrogenase (HBDH), creatine kinase, creatine kinase isoenzyme—MB (CK-MB), glucose, urea, and creatinine were determined using an AU-600 automatic biochemical analyzer (Olympus). Blood gas analysis was conducted using the Omnic Blood Gas Analyzer (Roche).
Chest radiography. Chest radiography was performed once every 1–2 days during the early stage after the onset of SARS and every 3 days after achievement of a stable illness state or resolution of inflammatory lesions in lungs.
Classification of clinical parameters. The state of illness was classified as 0 for survival or 1 for death. Hypoxemia was classified into the following 4 grades: 0, no hypoxemia; 1, partial pressure of oxygen (PO2) of ⩾70 mm Hg or sphygmous oxygen saturation of blood (SpO2) of ⩾93%; 2, PO2 of ⩾60 and <70 mm Hg or SpO2 of ⩾90% and <93%; and 3, PO2 of <60 mm Hg or SpO2 of <90%. The range of pulmonary consolidation was classified on the basis of radiograph findings as follows: 1, no consolidation or consolidation in ⩽1/4 of lung field; 2, consolidation in ⩾1/4 but <2/4 of lung field; and 3, consolidation in ⩾2/4 of lung field. The number of involved pulmonary lobes (1–5) was noted. Changes in routine urine test results were classified as follows: 0, no change; 1, RBC level of + or ++ or urinary protein level of +; and 2, RBC level of more than ++ or urinary protein level of more than + ("+" denotes mildly abnormal, “++” denotes middle-level abnormal, and “+++” denotes severely abnormal). Changes in the urea level were defined as follows: 0, normal level; 1, level of >8.2 and ⩽10 mmol/L; and 2, level of >10 mmol/L. Changes in the creatinine level were defined as follows: 0, normal level; 1, level of >106 and ⩽130 µmol/L; and 2, level of >130 µmol/L.
Statistical analysis. The SPSS software package, version 11.0 (SPSS), was used for the statistical analysis. P < .05 denotes statistical significance, and P < .01 denotes obviously important statistical significance.
Results
Analysis of General Data and Prognosis
Age and prognosis. Pearson correlative analysis showed that patient age was significantly correlated with prognosis (r = 0.506; P < .001). Only 1 patient aged <40 years (a pregnant woman aged 29 years) died. The age range for patients who survived was 13–74 years (mean ± SD, 34.3 ± 13.0 years), and it was 29–93 years (mean ± SD, 56.3 ± 17.3 years) for patients who died. There was significant difference between the 2 groups (P < .01). There was also marked difference in the mortality rate between patients aged ⩽50 years and those aged >50 years (4.08% vs. 53.3%; P < .01).
Sex and prognosis. The percentages of female patients among the patients who survived and among those who died were 40.7% and 44.4%, respectively. There was no significant difference between the 2 groups (P > .05).
Underlying conditions or risk factors before the onset of SARS and prognosis. “Underlying conditions or risk factors before the onset of SARS” refers to the history of ⩾1 disease (e.g., hypertension, chronic hepatitis [including hepatic cirrhosis], diabetes, and coronary heart disease); pregnancy is also included. There was remarkable difference in the mortality rate between patients with underlying conditions or risk factors and those without them (54.5% vs. 7.5%; P < .01).
Changes in Indices and Prognoses during the Acute Phase
Pearson's correlative analysis was used to compare prognosis during the advanced stage of SARS and the following values: peripheral WBC count, absolute neutrophil count, absolute lymphocyte count, platelet count, hemoglobin, erythrocyte sedimentation rate, routine urine test results, alanine aminotransferase, aspartate aminotransferase, total bilirubin, albumin, glucose, LDH, cholinesterase, creatine kinase, CK-MB, HBDH, urea, changes in the urea level, creatinine, changes in the creatinine level, serum iron, ratio of HBDH to LDH, prothrombin time, respiratory rate, degree of hypoxemia, degree of pulmonary consolidation, and number of involved lobes. Analysis revealed that absolute lymphocyte count, platelet count, routine urine parameters, aspartate aminotransferase level, total bilirubin level, LDH level, creatine kinase level, CK-MB level, HBDH level, urea level, changes in the urea level, changes in the creatinine level, respiratory rate, degree of hypoxemia, and number of involved lobes correlated with prognosis (table 2). As shown in table 2, platelet count and absolute lymphocyte count negatively correlated with prognosis, whereas other parameters positively correlated with prognosis. LDH level, degree of hypoxemia, and respiratory rate positively correlated with prognosis (r > 0.7; P < .01).
Table 2.
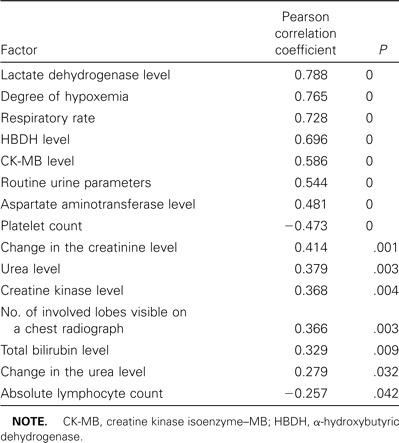
Relationship between factors observed during the advanced stage of severe acute respiratory syndrome (SARS) and the prognosis for SARS.
We also calculated LDH levels, respiratory rates, HBDH levels, CK-MB levels, aspartate aminotransferase levels, platelet count, urea levels, creatine kinase levels, total bilirubin levels, and absolute lymphocyte count and compared them those for patients who survived with those for patients who died. The results are shown in table 3, which shows that LDH level, respiratory rate, HBDH level, CK-MB level, and aspartate aminotransferase level were significantly higher in the group of patients who died than in the group of those who survived (P < .001). The platelet count was significantly lower among patients who died than among those who survived (P < .001).
Table 3.
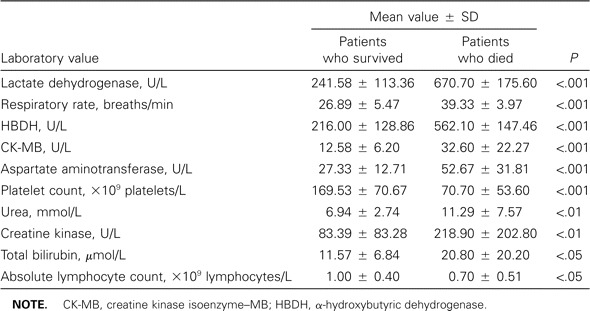
Laboratory values for peripheral blood during the acute phase of severe acute respiratory syndrome (SARS) in 165 patients with severe SARS.
Multivariate Analysis of Factors Affecting Prognosis
On the basis of analysis of general data and single-factor analysis during the acute phase, we selected factors that closely correlated with prognosis and illness state (age, underlying conditions or risk factors before the onset of SARS, LDH level, degree of hypoxemia, respiratory rate, HBDH level, CK-MB level, aspartate aminotransferase level, platelet count, and number of involved lobes) during the acute phase for multivariate analysis. Logistic gradual regression analysis was conducted to establish 3 prognosis regression models that included 5 variables for prognosis.
It was very interesting to find that the platelet count was selected twice in the 3 prognosis regression models. The first model included 2 variables (degree of hypoxemia and platelet count), as did the second model (platelet count and LDH level). The third model included 3 variables (age, underlying conditions or risk factors before the onset of SARS, and degree of hypoxemia). We decided to use the first model because the correlation of the second model with prognosis was not as high as that for the first model, the accuracy of prediction was lower for the third model (only 84.1%) than for the other 2 models, and platelet count and degree of hypoxemia were both selected twice in the 3 models (table 4). After performing logistic gradual regression analysis, we established the following multivariate regression model: Py=1 = es/(1 + es), where S is [2.490 × degree of hypoxemia]-[0.050 × platelet count]). If S is >0, then the value for Py=1 is >0.5, which means that the patient has died. If S is <0, then the value for Py=1 is <0.5, which means that the patient has survived.
Table 4.

Findings of multivariate analysis of factors affecting prognosis for 165 patients with severe acute respiratory syndrome (SARS).
Sensitivity, Specificity, and Accuracy of the Model for SARS Prognosis
Using this model, we conducted prospective analysis for 60 randomly selected patients in the surviving group and all 24 patients who died. Of the 60 patients who survived, 59 received a prognosis of survival and 1 received a prognosis of death. Of the 24 patients who died, 2 received a prognosis of survival of 2 and 22 received a prognosis of death. The model had a high rate of conformity (sensitivity, 91.67%; specificity, 98.33%; positive predictive value, 95.65%; negative predictive value, 96.72%; accuracy, 96.42%).
Discussion
SARS is a new infectious disease with high infectivity and fatality rates. Now the WHO has confirmed that the pathogen responsible is a new type of coronavirus [1–3]. The illness state is complicated, treatment is difficult, and the prognosis is bad for patients with severe SARS cases. Through careful analysis, we found that the outcome of SARS is related to several risk factors.
Univariate Analysis
Age. With increasing age, the compensatory functions of the heart, lungs, kidneys, and other organs of patients with SARS gradually decline. Meanwhile, their tolerance of hypoxia also gradually decreases. For a given degree of hypoxia, the compensatory functions of the heart, lungs, and kidneys in patients aged ⩾50 years are worse than those of patients aged <50 years. Therefore, it is easy for multiorgan failure to occur in these patients and to increase the risk factors for death. In addition, the percentage of persons with chronic diseases (e.g., hypertension, coronary heart disease, and diabetes) increases with increasing age; this undoubtedly increases the risk of death for these patients. According to our analysis of the clinical data, the mortality rate among patients aged ⩾50 years is 13.06 times that for patients aged <50 years (P < .01); this is consistent with the results reported by Lee et al. [1], Liu Xiaoqing et al. [4], and He Weiqun et al. [5].
Underlying conditions or risk factors before the onset of SARS. The presence of underlying conditions or risk factors before the onset of SARS in patients with severe SARS will increase the risk of death, which is in consistent with the results reported by Hoheisel et al. [9], Liu Xiaoqing et al. [4], and He Weiqun et al. [5]. Because high doses of glucocorticoid hormones are necessary for the treatment of severe SARS, if a patient has the underlying condition of diabetes, his or her illness state will be aggravated, because glucocorticoid hormones can increase the levels of hepatic and muscular glycogen and the blood sugar level. If a patient has the underlying condition of hepatitis or hepatic cirrhosis, his or her illness state will also be aggravated, because glucocorticoid hormones can inhibit the immune system, resulting in active replication of hepatitis viruses or even hepatic necrosis. In patients with hypertension, the illness state can be aggravated, because glucocorticoid hormones can increase blood pressure by causing storage of sodium and discharge of potassium.
Decrease in the platelet count. Although Peiris et al. [10] did not find thrombocytopenia in 50 patients with SARS, Hoheisel et al. [9] reported that thrombocytopenia was the most prominent laboratory finding, reflecting a more severe clinical course. Lee et al. [1] reported that 62 (44.8%) of 138 patients with SARS had thrombocytopenia. However, in a multivariate analysis, the independent predictors of an adverse outcome did not include thrombocytopenia. Liu Xiaoqing et al. [4] and He Weiqun et al. [5] reported that thrombocytopenia was found to be closely related to prognosis in multivariate analysis. The clinical data we collected for this study also indicated that the platelet count had decreased in >90% of the patients who died; in these patients, the platelet count progressively decreased and reached a level of <10 × 109 platelets/L in the late stage of disease. For example, the platelet count at hospital admission was 90 × 109 platelets/L in 1 patient who died. However, the platelet count progressively decreased with the aggravation of illness of state (from 90, to 89, to 75, to 20, to 17, to 15, and then to 6 × 109 platelets/L) in the 23 days after the onset of SARS.
It was very interesting to find that the platelet count was selected twice in the 3 prognosis regression models by multivariate analysis. This implies that a decrease in the platelet count—especially a gradual decrease—is a marker of aggravation of illness state and suggests that disseminated intravascular coagulation occurs in the patients, and that appropriate emergency measures should be taken immediately to prevent such patients from dying. The major reason is that the pathological basis of severe SARS is similar to that of severe ARDS: damage to the pulmonary capillary membrane caused by pulmonary local inflammatory reaction, which is mediated by various inflammatory cells (e.g., macrophages, neutrophils, and lymphocytes) and loses control of inflammatory reaction [11–13]. The damage to the pulmonary capillary membrane usually causes platelet aggregation and microthrombus formation in pulmonary micrangia, resulting in a decrease in the platelet count and in platelet consumption [14].
Pulmonary changes and degree of hypoxemia. According to studies published previously [9–13], massive formation of transparent membrane in the alveolar cavity is found in dead patients who had SARS. This results in a decrease in pulmonary compliance, an increase in intrapulmonary shunting, and an imbalance in ventilation and blood flow. The clinical manifestations included massive consolidation visible on chest radiographs, breathing difficulty, respiratory distress, and obstinate hypoxemia. The larger that the range of consolidation range in lungs and the number of involved lobes were, the higher the respiratory rate was. Furthermore, the prognosis worsened with an increase in the degree of severity of hypoxemia. We classified hypoxemia into 4 grades (see Patients, Materials, and Methods). Grade 2 hypoxemia conforms with the standard for mild SARS, grade 3 conforms with the standard for severe SARS, and grade 4 (severe hypoxemia) conforms with the standard for type I respiratory failure. Using this classification system, the degree of hypoxemia can be easily judged in clinical practice.
Changes in serum enzyme levels. Studies by Zhao et al. [11, 13] and Ding [12] reported that SARS coronavirus particles were present in some myocardial cells and renal tubular epithelial cells, and vacuolation was seen in few myocardial cells. The researchers also found that the liver and spleen were also involved. Damage to the lungs, heart, liver, kidneys, or spleen can result in an increase in the levels of LDH, aspartate aminotransferase, and creatine kinase. The more serious is the pulmonary consolidation or damage of organs (other than the lungs), the higher are the levels of LDH, aspartate aminotransferase, and creatine kinase. Our study showed that LDH level, aspartate aminotransferase level, and creatine kinase level during the advanced stage of SARS were significantly increased in patients who died, compared with those who survived (P < .01). It is well known that CK-MB is a specific enzyme that reflects myocardial condition. In this study, it was found that the CK-MB level during the advanced stage of disease was significantly elevated in patients who died, compared with those who survived (P < .001). This suggests that myocardial damage was more serious in patients who died than in those who did not. It also indicates that increased CK-MB levels in patients with SARS indicates myocardial damage and a bad prognosis.
Renal function changes. Studies by Zhao et al. [11, 13] and Ding [12] found that particles of SARS coronavirus were present in renal tubular epithelial cells. In addition, there was obvious swelling of the renal tubular epithelial cells, occasional renal tubular necrosis, focal hemorrhage, and infiltration of a few lymphocytes into interstitial tissue, which indicate pathological changes associated with renal damage. Because hypoxia is more serious in severe cases of SARS, the percentage of patients with abnormal urea levels is significantly higher among those with severe SARS than among those with mild SARS (P < .05). Moreover, the level of internally originated toxic substances is high in patients with severe cases. These high levels aggravate renal dysfunction, which might result in renal functional failure, which affects recovery and prognosis of the patients. In this study, we found that changes in both creatinine and urea levels during the advanced stage significantly correlated with prognosis (P < .01 and <.05, respectively). The urea level in patients who died was markedly elevated, compared with the level in survivors (P < .01).
Multivariate Analysis
It was found that age, underlying conditions or risk factors before the onset of SARS, absolute lymphocyte count, platelet count, routine urine test results, aspartate aminotransferase level, total bilirubin level, LDH level, creatine kinase level, CK-MB level, HBDH level, urea level, changes in the urea level, changes in the creatinine level, respiratory rate, degree of hypoxemia, and number of involved lobes during the acute phase, as selected by univariate analysis, correlated with the prognosis for SARS. However, logistic gradual regression analysis showed that the 2 variables of degree of hypoxemia and platelet count were of regressive to prognosis for SARS. This suggests that parameters selected by univariate analysis can only reflect certain aspects of SARS pathogenesis and prognosis and that establishment of a multivariate regression biomathematical model is necessary for all-around and more accurate judgment of the prognosis for patients with SARS.
The model we established has the following advantages: (1) It is a simple calculation to make, because only 2 variables are needed for judgment of survival or death by calculation of the value S < 0 or S > 0. (2) The data are easy to obtain. The platelet count can be easily determined in every hospital. It is also easy to determine the degree of hypoxemia by the simple determination of the SpO2 value, with no necessity for an invasive examination. (3) The model has a high conformity rate: its sensitivity is >90%, and its specificity and accuracy are >95%.
However, this model for SARS prognosis has its limitations. The model does not work for patients with SARS who had such underlying conditions as severe chronic pulmonary diseases (grade 3 or higher severity), hypoxemia, or primary or secondary thrombocytopenia before SARS onset. This multivariate regression, biomathematical model provides a more objective basis for judgment of the prognosis for patients with SARS. Combination of univariate analysis with multivariate analysis is helpful for determination of both the prognosis of SARS and the efficacy of various treatments.
In short, this model for SARS prognosis may be a new, simple, and cost-effective index. It has high sensitivity, specificity, and accuracy, and it was established on the basis of SARS pathophysiology and biomathematics. It could be used to effectively judge the state of illness and the prognosis for patients with SARS, and it may have important clinical significance for guiding treatment and determining the efficacy of therapy in clinical practice.
Acknowledgments
We would like to express our heartfelt gratitude to all the front-line medical workers at our hospital and some of the health care workers at other hospitals who gathered these precious data at the risk of their lives. We also wish to pay lofty tribute to the medical personnel who worked at the anti-SARS front line and to their family members.
Footnotes
Financial support: The Foundation for Key Science and Technology Research Projects of “The Tenth Five-Year Plan” 863 Hi-Tech Researches Program of China (grants 2003AA208106).
References
- 1.Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–94. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 2.Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Poutanen SM, Low DE, Henry B, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 4.Liu XQ, Chen SP, He GQ, et al. Management of critical severe acute respiratory syndrome and risk factors for deat. Zhonghua Jie He He Hu Xi Za Zhi. 2003;26:329–33. [PubMed] [Google Scholar]
- 5.He WQ, Chen SP, Liu XQ, et al. Death risk factors of severe acute respiratory syndrome with acute respiratory distress syndrome. Chinese Critical Care Medicine. 2003;15:326–7. [PubMed] [Google Scholar]
- 6.Office of Chinese Ministry of Health. Interim standard for clinical diagnosis of infectious atypical pneumonia: advancements in research of SAR. China Medical Tribune. 2003;2:44. [Google Scholar]
- 7.Office of Chinese Ministry of Health Standard for clinical diagnosis of severe infectious atypical pneumonia: advancements in research of SARS, China Medical Tribune. 2003;2:44. [Google Scholar]
- 8.World Health Organization. Severe acute respiratory syndrome. Wkly Epidemiol Rec. 2003;78:97–120. [Google Scholar]
- 9.Hoheisel G, Wu A, Lee N, Chan CH, et al. Severe acute respiratory syndrome (SARS) Pneumologie. 2003;57:315–21. doi: 10.1055/s-2003-40049. [DOI] [PubMed] [Google Scholar]
- 10.Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–25. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao JM, Zhou GD, Sun YL, et al. Studies of pathological changes of extra-lung organs in a case of severe acute respiratory syndrome. Med J Chin PLA. 2003;28:480–1. [Google Scholar]
- 12.Ding YQ. Preliminary discussion of etiology and pathogenesis of severe acute respiratory syndrome. Med J Chin PLA. 2003;28:475–6. [Google Scholar]
- 13.Zhao JM, Zhou GD, Sun YL, et al. Pathological and etiological findings in a case of severe acute respiratory syndrome of China. Med J Chin PLA. 2003;28:379–82. [Google Scholar]
- 14.Zhu YY. Role of prostaglandins in rabbits after acute pulmonary damage. Zhonghua Yi Xue Za Zhi. 1987;67:456–9. [PubMed] [Google Scholar]


