In a study of 356 adults with community-acquired pneumonia, respiratory syncytial virus was a frequent pathogen (13%). Serology and real-time reverse-transcription polymerase chain reaction improved the detection of repiratory syncytial virus. High respiratory syncytial virus serum-neutralizing antibody levels protected against severe pneumonia.
Abstract
Background. Respiratory syncytial virus (RSV) has been implicated in the etiology of adult community-acquired pneumonia (CAP). We investigated RSV infection in Chilean adults with CAP using direct viral detection, real-time reverse-transcription polymerase chain reaction (rtRT-PCR), and serology (microneutralization assay).
Methods. RSV, other respiratory viruses, and bacteria were studied by conventional and molecular techniques in adults aged ≥18 years presenting with CAP to the healthcare facilities in Santiago, Chile from February 2005 through December 2007.
Results. All 356 adults with CAP enrolled had an acute blood sample collected at enrollment, and 184 had a convalescent blood sample. RSV was detected in 48 cases (13.4%). Immunofluorescence assay and viral isolation each detected only 1 infection (0.2%), whereas rtRT-PCR was positive in 32 (8.9%) cases and serology was positive in 20 (10.8%) cases. CAP clinical characteristics were similar in RSV-infected and non-RSV-infected cases. RSV-specific geometric mean serum-neutralizing antibody titer (GMST) was significantly lower at admission in the 48 RSV-infected cases compared with 308 non-RSV-infected adults (GMST in log2: RSV/A 8.1 vs 8.9, and RSV/B 9.3 vs 10.4; P < .02).
Conclusions. RSV infection is frequent in Chilean adults with CAP. Microneutralization assay was as sensitive as rtRT-PCR in detecting RSV infection and is a good adjunct assay for diagnostic research. High RSV-specific serum-neutralizing antibody levels were associated with protection against common and severe infection. The development of a vaccine could prevent RSV-related CAP in adults.
Community-acquired pneumonia (CAP) is a relevant worldwide cause of morbidity and mortality [1], and bacteria have been recognized as the main etiological agent. However, advances in diagnostic techniques have shown the potential role of viruses [2, 3]. Respiratory syncytial virus (RSV)—the main respiratory pathogen in infants [4]—has been associated with severe respiratory illness in adults [4–7], causing between 2% and 9% of adult CAP throughout the year and up to 15% during the winter months [4]. Currently, no clinical features of CAP are characteristic of viral pneumonia. Detection of RSV infection in adults is hampered by low viral shedding, and therefore the most sensitive method should be used for diagnosis [4, 8]. Polymerase chain reaction (PCR) has been shown to be more sensitive than viral isolation (VI) and immunofluorescence assay (IFA) for viral detection in adults [6, 9]; however, in some studies, PCR has not significantly increased the diagnostic serology yield [6, 7, 9, 10]. Serology for diagnosis of respiratory viruses is a sensitive technique that is primarily used in research [11]. A ≥4-fold in antibody titer in paired samples is necessary to confirm a recent infection. Although the rise in detection of virus-specific neutralizing antibodies is sensitive and provides relevant information [4], few studies of CAP have used microneutralization for RSV diagnosis [12–14]. The aim of this study was to compare standard and molecular viral diagnostic techniques with microneutralization assay for the detection of RSV infection in adults presenting with CAP in Santiago, Chile.
METHODS
Patients and Study Design
We prospectively enrolled patients ≥18 years of age presenting with CAP in 2 hospitals (Hospital Clínico Universidad de Chile and Hospital Lucio Córdova) in Santiago, Chile, between February 2005 and December 2007, covering 3 respiratory viral seasons. The study was approved by the university and institutional review boards, and all adults provided informed consent to participate in the study. CAP was defined by the presence of acute respiratory symptoms for <1 week and a chest radiograph displaying new pulmonary infiltrates. Exclusion criteria included immunodeficient conditions (ie, human immunodeficiency virus, active treatment for cancer, organ transplant, immunosuppressive therapy) and hospitalization within 30 days preceding enrollment. Clinical information at admission and hospital course were extracted from hospital charts of all patients and entered in a computer database. Patient severity was assessed during the first 48 hours after enrollment according to the pneumonia severity index described by Fine et al [15].
Sample Collection
In addition to routine laboratory tests (complete blood cell count, biochemistry panel, oxygen saturation) at enrollment, all persons had collection of urine and blood for bacterial culture, respiratory secretion for viral and bacterial diagnostics, and an acute serum sample for serology. Sputum, nasopharyngeal aspirate, or bronchoalveolar lavage fluids were obtained depending on the condition of the patient and immediately transported on ice to the laboratory. Aliquots for different diagnostic assays were prepared and stored at −80°C for later testing. Participants were contacted after 4–6 weeks for clinical follow-up and convalescent sera collection. Sera were processed immediately and stored at −20°C.
RSV Detection by VI, IFA, Real-time Reverse-Transcription PCR, and Microneutralization Assay
Respiratory secretions were inoculated onto HEp-2 cells for VI [16]. IFA and cultures were performed on all samples as described elsewhere using monoclonal antibodies (kindly provided by L. Anderson, Centers for Disease Control and Prevention, Atlanta, Georgia) and commercial conjugated antisera (Sigma) [16]. For real-time reverse-transcription (rtRT) PCR, samples were treated with the guanidinium thiocyanate-phenol-chloroform method for RNA extraction [17]. Complementary DNA (cDNA) was synthesized with 5 μL of RNA (sample) and 0.52 μM F gene primer (F844: 5′-TGTCTAACTATTTGAACA-3′) for 1 hour at 37°C, followed by 5 minutes at 95°C in a PerkinElmer gene AmpPCR System 2400. A fragment of N gene was amplified by rtPCR with 10 μM (each) N1 and N2 primers [18], 1X Master SYBR Green I (Roche), and 2 μL of cDNA. The reaction was carried out for 10 minutes at 94°C and then for 50 sequential cycles at 94°C for 10 seconds, 58°C for 5 seconds, and 72°C for 30 seconds in a LightCycler 1.5 instrument (Roche). A negative (water) and 2 positive controls (RNA from RSV working pool) were included in each assay. Heat-inactivated sera were tested by microneutralization assays for RSV/A/Tracy (A2-like virus) and RSV/B/18537 to measure RSV/A and RSV/B-specific neutralizing antibodies as described previously [12].
Detection for Other Respiratory Viruses
VI in HEp-2 and MDCK cells and IFA was used for the detection of adenovirus, influenza A and B viruses (FLU), and parainfluenza viruses types 1–3 as described previously [16, 19]. Human metapneumovirus (hMPV), coronavirus, and picornavirus (PV) were detected by rtRT-PCR by amplifying fragments of the N gene, the conserved region of the replicase 1a gene, and the 5′-noncoding conserved region, respectively, as described previously [20–22]. The 390-base pair fragment of PV was purified and sequenced using the kit ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction in the automated sequencer ABI model 377 (Applied Biosystems). Serum antibodies to hMPV subgroups A and B were detected by microneutralization and enzyme-linked immunosorbent assays [23]. Hemagglutination inhibition testing for influenza antibody was performed as described previously [24].
Seroconversion
RSV, FLU, and hMPV infection were defined by a ≥4-fold rise of antibody titer between convalescent and acute paired sera.
Detection Methods for Bacteria
Sputum samples displaying >25 leukocytes and <10 epithelial cells per 100× power field after Gram staining were processed according to standard techniques [25]. For Legionella, isolation samples were inoculated onto buffered charcoal yeast extract and GVPC media (Oxoid) and incubated for up to 10 days. Urinary antigens for Streptococcuspneumoniae and Legionella were detected using immunochromatographic tests (Binax NOW, Portland, Oregon). Chlamydia pneumoniae was detected using primers HL and HR for nested PCR amplifying a chromosomal DNA segment (PstI 474) [26]. PCR for Mycoplasma pneumoniae and Legionella was performed as described previously [27, 28]. Serum antibodies against C. pneumoniae and M. pneumoniae were detected by commercial IFA kits (SeroFIA, Sayvon, Israel and Zeus, respectively). Immunoglobulin M (IgM) ≥1:16 or a ≥4-fold rise in immunoglobulin G (IgG) titer between paired sera was regarded as an acute infection. Legionella serology was performed using a latex test kit (Legionella Oxoid).
Statistical Analysis
Analysis was performed using Z-test for categorical data and the t test and Mann-Whitney rank-sum test for continuous variables, such as geometric mean serum-neutralizing antibody titer (GMST) in log2. Logistic regression was used to determine whether RSV/A and RSV/B GMSTs were associated with the likelihood of not having an RSV infection or a hospitalization. Odds ratios (ORs) with 95% confidence intervals (CIs) were determined for RSV/A or RSV/B GMSTs. The level of significance was set at P < .05. Data were analyzed using SigmaStat software.
RESULTS
Patients
A total of 356 adults with CAP were enrolled; 330 (92.7%) were admitted to hospital, 83 (23.3%) required intensive care unit (ICU) support, 26 (7.3%) were outpatients, and 28 (7.9%) died between hospital admission and 30 days after discharge. The study population consisted of 166 (46.6%) women and 190 (53.4%) men. The mean age was 63 years (range, 18–94 years): 31.2% were 18–49 years old, 21.3% were 50–64 years old, and 47.5% were ≥65 years old. One or more predefined comorbidity was identified in 182 (53.5%) patients, cardiac disease being the most prevalent (18.2%); 78 (22.9%) had ≥2 comorbidities. One hundred twenty-nine adults (37.8%) were smokers, 72 adults (21.2%) had received antimicrobial therapy before hospital admission, and 84 (24.9%) had respiratory failure. A Fine score was determined in 341 adults with CAP: class 1 in 77 (22.6%), class 2 in 69 (20.2%), class 3 in 69 (20.2%), class 4 in 84 (24.6%), and class 5 in 42 (12.4%).
Overall, RSV infection was established for 48 of 356 (13.4%) adults with CAP. The characteristics of the patients are summarized in Table 1. No significant differences in demographic, clinical characteristics (Tables 1 and 2), and routine laboratory tests were observed between adults with and without RSV-related CAP. Likewise, clinical outcome was similar between both groups (Table 3), except that adults infected with RSV comprised a higher proportion of cases with disease progression on chest radiograph compared with noninfected adults (14% vs 4.8%; P = .04).
Table 1.
Characteristics of 356 Adults With Respiratory Syncytial Virus (RSV) and Non-RSV-Related Community-Acquired Pneumonia in Santiago, Chile, 2005–2007
| Characteristic | RSV(+) | RSV(−) | P Valuea |
| No. | 48 | 308 | |
| Age, years | |||
| Median | 59 | 64 | .3 |
| Range | 18–92 | 19–94 | |
| Sex | |||
| Female | 24 (50.0) | 166 (53.8) | .8 |
| Male | 24 (50.0) | 142 (46.1) | .7 |
| Current smoker | 20/45 (44.4) | 109/296 (36.8) | .4 |
| Alcohol use >80 grams/day | 8/45 (17.8) | 33/296 (11.1) | .3 |
| Comorbidity | n = 45 | n = 295 | |
| Any | 25 (55.6) | 157 (53.2) | .8 |
| Diabetes mellitus | 7 (15.6) | 47 (15.9) | .9 |
| COPD | 7 (15.6) | 52 (17.6) | .5 |
| Asthma | 1 (2.2) | 16 (5.4) | .6 |
| Cardiac failure | 10 (22.2) | 52 (17.6) | .6 |
| Liver damage | 4 (8.9) | 11 (3.7) | .2 |
| Renal disease | 0 | 12 (4.1) | .3 |
| Antibiotics before admission | 13/45 (28.9) | 59/295 (20.0) | .3 |
| Hospitalization | 44/48 (91.7) | 286/308 (92.9) | .9 |
| ICU admission | 15/48 (31.3) | 68/308 (22.1) | .2 |
| Outpatient | 4/48 (8.3) | 22/308 (7.1) | .9 |
| Cases per months | n = 48 | n = 308 | |
| January–February | 2 (4.2) | 20 (6.5) | .8 |
| March–April | 1 (2.0) | 50 (16.2) | .01 |
| May–June | 23 (47.9) | 87 (28.3) | .01 |
| July–August | 8 (16.7) | 56 (18.2) | .8 |
| September–October | 7 (14.6) | 49 (15.9) | .7 |
| November–December | 7 (14.6) | 46 (14.9) | .8 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; RSV, respiratory syncytial virus.
Z-test was applied by statistical analyses of proportions.
Table 2.
Clinical Characteristics and Severity Scores Observed in 48 Respiratory Syncytial Virus (RSV)–Infected and 308 Non-RSV-Infected Adults With Community-Acquired Pneumonia in Santiago, Chile, 2005–2007
| RSV(+) | RSV(−) | P Valuea | |
| Symptoms | |||
| Cough | 40/45 (88.9) | 256/296 (86.5) | .8 |
| Expectoration | 36/45 (80.0) | 198/296 (66.9) | .1 |
| Pleuritic chest pain | 16/45 (35.6) | 105/296 (35.5) | .8 |
| Mental confusion | 11/44 (25.0) | 66/294 (22.4) | .8 |
| Pleural effusion | 7/43 (16.3) | 55/292 (18.8) | .9 |
| Hypotension | 11/43 (25.6) | 48/295 (16.3) | .2 |
| Vital signs | |||
| Respiratory rate, No., median (range) | 38, 24.5 (18–36) | 234, 24 (15–48) | .3 |
| Pulse, No., median (range) | 43, 98 (73–142) | 290, 94 (17–180) | .1 |
| Systolic blood pressure, No., mm Hg median (range) | 36, 128 (75–213) | 241, 121 (68–198) | .7 |
| Diastolic blood pressure, No., mm Hg median (range) | 35, 72 (40–133) | 241, 69 (26–100) | .08 |
| Temperature at admission, No., °C median (range) | 44, 37 (35–40) | 292, 37.2 (35–41) | .4 |
| Chest radiograph | |||
| Interstitial patterns | 6/44 (13.6) | 38/291 (13.1) | .8 |
| Alveolar patterns | 36/44 (81.8) | 234/291 (80.4) | .6 |
| Both | 2/44 (4.6) | 19/291 (6.5) | .8 |
| Multilobar involvement (≥2 lobes) | 12/43 (27.9) | 95/286 (33.2) | .5 |
| Fine Score | n = 48 | n = 308 | |
| 1 | 10 (20.8) | 74 (24.0) | .7 |
| 2 | 13 (27.1) | 61 (19.8) | .3 |
| 3 | 10 (20.8) | 59 (19.2) | .2 |
| 4 | 7 (14.6) | 80 (26.0) | .1 |
| 5 | 8 (16.7) | 34 (11.0) | .4 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: Hg, Mercury; RSV, respiratory syncytial virus.
Proportions were analyzed with the Z-test and continuous variables with the Mann-Whitney rank-sum test.
Table 3.
Clinical Outcome of 48 Respiratory Syncytial Virus (RSV)–Infected and 308 Non-RSV-Infected Adults With Community-Acquired Pneumonia in Santiago, Chile, 2005–2007
| Outcome | RSV(+) | RSV(−) | P Valuea |
| Radiological progression | 6/43 (14.0) | 14/295 (4.8) | .04 |
| Aspiration | 3/45 (6.7) | 26/296 (8.8) | .8 |
| Hepatic failure | 2/43 (4.7) | 7/295 (2.4) | .7 |
| Respiratory failure | 14/43 (32.6) | 70/295 (23.7) | .2 |
| Mechanical ventilation | 8/43 (18.6) | 37/295 (12.5) | .4 |
| Shock | 6/43 (14.0) | 24/295 (8.1) | .4 |
| Death during or 30 days after hospitalization | 3/48 (6.3) | 25/308 (8.1) | .8 |
Data are No. (%) unless otherwise indicated.
Abbreviation: RSV, respiratory syncytial virus.
Z-test was applied by statistical analyses of proportions.
Yield of RSV Infection by Diagnostic Method
IFA and cell culture identified 2 (0.2%) RSV infections, 1 by each technique. RSV was detected by rtRT-PCR in 32 (8.9%) adults, whereas serology diagnosed RSV infection in 20 of 184 (10.8%) adults with paired sera. Sensitivity of rtRT-PCR and microneutralization assays were similar (P = .5), and both were significantly better than IFA or VI (P < .001). Among the 48 adults infected with RSV, 2 (4.1%) were detected by conventional methodology (IFA and cell culture) and 32 (66.6%) by rtRT-PCR. A ≥4-fold rise in RSV-specific serum-neutralizing antibody titer occurred in 20 of 35 RSV-infected adults with paired sera: 6 by RSV/A assay, 9 by RSV/B assay, and 5 by both microneutralization assays. Paired serologic study was available in 19 adults with RSV-positive rtRT-PCR, and the results were concordant in only 4 cases (Table 4).
Table 4.
Diagnosis of Respiratory Syncytial Virus (RSV) Infection by Viral Isolation, Immunofluorescence Assay, Reverse-Transcription Polymerase Chain Reaction, and Serology in the 48 RSV-Infected Adults With Community-Acquired Pneumonia, Santiago, Chile, 2005–2007
| Method |
|||||
| Viral isolation | IFA | RT-PCR | Serology | No. | % |
| (−) | (−) | (+) | (−) | 15 | 31.2 |
| (−) | (−) | (−) | (+) | 16 | 33.3 |
| (+) | (−) | (+) | (+) | 1 | 2.1 |
| (−) | (+) | (+) | ND | 1 | 2.1 |
| (−) | (−) | (+) | (+) | 3 | 6.3 |
| (−) | (−) | (+) | ND | 12 | 25.0 |
| Total 1 | 1 | 32 | 20 | 48 | 100 |
Abbreviations: IFA, immunofluorescence assay; ND, not done; RT-PCR, reverse-transcription polymerase chain reaction.
Comparing the 26 cases detected by rtRT-PCR with the 20 patients who experienced RSV seroconversion, clinical characteristics and laboratory parameters were similar; the duration of symptoms before admission was also similar (median, 3.5 vs 4.5 days; P = 0.5), and serum samples were taken between 2–13 and 2–16 days (median, 6.0 vs 6.0 days) from the beginning of symptoms, respectively. Only the presence of comorbidity was less common in those who seroconverted (31.6% vs 69%; P = .02).
Comparing rtRT-PCR and microneutralization sensitivities, serology gave a higher yield than rtRT- PCR (P = .04) only in patients without a comorbidity (12 of 85 [14.1%] vs 9 of 158 [5.7%]).
Epidemiology of RSV-Related CAP
The majority of RSV-related CAP (30 of 42 [71.4%]) occurred from May through August (Table 1 and Figure 1). Significantly fewer non-RSV-related CAP cases (143 of 308 [46.4%]) occurred from May through August (χ2P = .004). Adults treated for CAP during the months of May through August were more likely to have an RSV-related CAP compared with other months (relative risk, 2.56 [95% CI, 1.4–4.8]).
Figure 1.
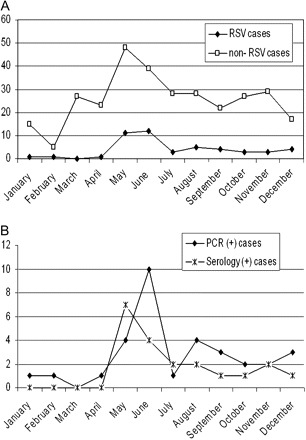
A, Respiratory syncytial virus (RSV)–related and non-RSV-related adult community-acquired pneumonia (CAP) by month categories, Santiago, Chile, 2005–2007. B, RSV-related CAP by type of diagnostic method by month categories, Santiago, Chile, 2005–2007. PCR indicates polymerase chain reaction.
RSV-Specific Serum-Neutralizing Antibody Titers
All 356 adults with CAP had neutralizing antibodies to RSV/A and RSV/B. The acute serum samples were obtained on the day of admission in 35.3% of cases and during the first week after illness onset in 66% (range, 1–22 days; median, 6.67 days). The GMSTs to RSV/A and RSV/B during the acute illness phase were significantly lower in adults with RSV infection compared with non-RSV-infected adults (8.1 ± 2.2 vs 9.3 ± 1.6 and 8.9 ± 1.9 vs 10.4 ± 1.9 for RSV/A and RSV/B, respectively; P ≤ .028). These significant differences also remained when the analysis was limited to cases with paired sera (Table 5).
Table 5.
Geometric Mean Serum Antibody Titers for Respiratory Syncytial Virus (RSV) Subgroups A and B in Adults With RSV-Related and Non-RSV-Related Community-Acquired Pneumonia in Santiago, Chile, 2005–2007
| RSV/A |
RSV/A |
RSV/B |
RSV/B |
||||||
| 1st Sample |
2nd Sample |
1st Sample |
2nd Sample |
||||||
| Group | n | GMST | n | GMST | n | GMST | n | GMST | |
| All patients | 355 | 8.8 ± 1.9a | 184 | 8.9 ± 1.9a | 355 | 10.3 ± 1.9a | 184 | 10.5 ± 1.8a | |
| RSV patients | 48 | 8.1 ± 2.2b | 35 | 9.2 ± 2.4 | 48 | 9.3 ± 1.6b | 35 | 10.7 ± 1.9 | |
| Non-RSV patients | 308 | 8.9 ± 1.9b | 149 | 8.8 ± 1.8 | 308 | 10.4 ± 1.9b | 149 | 10.5 ± 1.8 | |
| Patients with RT-PCR(+) | Total | 32 | 8.1 ± 2.1 | 19 | 9.0 ± 2.4 | 32 | 9.5 ± 1.7 | 19 | 10.3 ± 1.9 |
| Without seroconversion | 15 | 7.7 ± 2.0 | 15 | 8.2 ± 1.7c,d | 15 | 9.5 ± 1.8 | 15 | 9.8 ± 1.6e | |
| With seroconversion | 4 | 10.0 ± 1.2 | 4 | 12.3 ± 2.0d | 4 | 10.7 ± 1.1 | 4 | 12.3 ± 1.7e | |
| Patients with seroconversion | Total | 20 | 8.4 ± 2.3 | 20 | 10.1 ± 2.6c | 20 | 9.4 ± 1.4 | 20 | 11.3 ± 1.8 |
| RT-PCR(−) | 16 | 8.0 ± 2.3 | 16 | 9.5 ± 2.5 | 16 | 9.0 ± 1.3 | 16 | 11.2 ± 1.8 | |
Geometric mean serum antibody titers (GMSTs) are in log2 and standard deviation. RSV/A: neutralizing antibody titer to RSV/A; RSV/B: neutralizing antibody titer to RSV/B. Seroconversion: Respiratory syncytial virus (RSV) infection based on serology was defined by a ≥4-fold rise of antibody titer in the convalescent serum sample compared with the acute serum sample. Differences between groups were determined by Mann-Whitney rank-sum and t tests. P ≤ .05 was considered significant.
Abbreviation: RT-PCR, reverse-transcription -polymerase chain reaction.
GMST to RSV/A in acute and convalescent serum of all studied patients was significantly lower than GMST to RSV/B (P < .00).
RSV-infected patients had significantly lower GMST to RSV/A and RSV/B in acute serum compared with non-RSV-infected patients (P < .02).
GMST to RSV/A in convalescent serum of patients with seroconversion was significantly higher than GMST to RSV/A in patients without seroconversion (P = .02, t test).
GMST to RSV/A in convalescent serum of patients with RT-PCR(+) and seroconversion was significantly higher than GMST to RSV/A in patients with RT-PCR(+) without seroconversion (P = .00, t test).
GMST to RSV/B in convalescent serum of patients with RT-PCR(+) and seroconversion was significantly higher than GMST to RSV/B in patients with RT-PCR(+) without seroconversion (P = .00, t test).
Logistic regression analysis detected a protective role of RSV-specific serum-neutralizing antibodies against RSV infection (RSV/A: OR, 0.81 [95% CI, .696–.955]; P = .011 and RSV/B: OR, 0.72 [95% CI, .613–.860]; P < .001), and against ICU admission (RSV/A: OR, 0.85 [95% CI, .756–.977]; P = .02 and RSV/B: OR, 0.84 [95% CI, .738–.965]; P = .01). Previously reported minimal protective threshold titers for neutralizing antibodies to RSV A and B (≥6.0 log2 and ≥8.0 log2, respectively) against RSV-related hospitalization [13] were used to evaluate the risk for RSV-related CAP. A significantly higher proportion of adults with RSV-related CAP had RSV/A-specific GMST <6.0 log2 compared with those with non-RSV-related CAP (17% vs 2%; P = .02). Likewise, a significantly higher proportion of adults with RSV-related CAP had RSV/B GMST <8 log2 compared with non-RSV-related CAP (18% vs 6%; P = .03).
Coinfection
Respiratory secretions and urine were obtained from all 356 patients for detection of other viruses and bacteria. Blood culture was performed in 241 (67%) cases, and picornavirus and coronaviruses were studied in a subset (268 cases) from August 2005 to December 2007. Cases with RSV-related CAP frequently had another agent identified (30 of 48 [62.5%]): respiratory viruses in 12 (25%), bacteria in 8 (16.7%), and both viruses and bacteria in 10 cases (20.8%). Interestingly, in 4 patients, 3 different agents were identified, and in 1 adult with RSV-related CAP, 4 viruses were identified in addition to S. pneumoniae (Table 6).
Table 6.
Agents Detected in Addition to Respiratory Syncytial Virus in 31 Adults With Community-Acquired Pneumonia in Santiago, Chile, 2005–2007
| Other Viruses | No. | Bacteria | No. | Other Viruses + Bacteria | No. |
| Picornavirus | 4 | Streptococcus pneumoniae | 2 | S. pneumoniae | 1 |
| Influenza | 4 | Legionella pneumophila | 2 | +Flu | 1 |
| hMPV | 2 | Mycoplasma pneumoniae | 2 | + HCoV | 2 |
| + hMPV | 1 | ||||
| Adenovirus | 1 | Moraxella catarrhalis | 1 | + hMPV + Flu + PV | 2 |
| + Chlamydia pneumoniae + hMPV | 1 | ||||
| +C. pneumoniae + PV | 1 | ||||
| hMPV + Flu | 1 | S. pneumoniae + BGN | 1 | M. pneumoniae + hMPV | 1 |
| Total | 12 | Total | 8 | C. pneumoniae + Staphy + HCoV | 1 |
Abbreviations: BGN, Gram-negative bacillus; Flu, influenza virus; HCoV, human coronavirus; hMPV, human metapneumovirus; PV, picornavirus; Staphy, Staphylococcus.
DISCUSSION
This prospective study of CAP in adults used a comprehensive diagnostic approach in identifying the etiologic agents in Santiago, Chile. A relevant proportion of adults with CAP were hospitalized and some died, indicating that our findings are biased toward the spectrum of severe disease.
RSV was identified in 48 of 356 patients (13.4%). Because RSV detection in this population has varied from 0% to 14% [4, 5], depending mainly on the diagnostic method used, the high frequency found can be explained by application of a sensitive molecular diagnostic method and RSV-specific serology. Here, rtRT-PCR showed significantly better diagnostic performance than did IFA and viral culture methods. It is the preferred diagnostic test for identifying RSV in adults with CAP who are expected to shed low amounts of virus [4]. However, rtRT-PCR did not identify a significant proportion of RSV-infected adults (16 of 20) who were diagnosed by serology. In agreement with Falsey et al [11], these findings highlight the need to combine rtRT-PCR with serology to optimize the diagnosis of RSV infection in adults.
Age and comorbidities appear to influence the diagnostic sensitivity of rtRT-PCR and serology. It is well known that age may influence virus-specific antibody response [4, 29]. Serology gave a higher yield than did rtRT-PCR in the detection of RSV in healthy adults with CAP (14.1% vs 5.7%; P = .04); this group was significantly younger than adults with comorbidities (median age, 48 vs 72 years; P < .001). Also, the 20 patients with seroconversion had a trend toward being younger compared with 15 nonseroresponders (45% vs 26.7% <40 years; P = .4). In addition, the presence of comorbidity showed a similar tendency, being less frequent among the seroresponders (31% vs 66%; P = .09). It has been documented that underlying disease represents a significant risk factor for severe RSV illness [14]. This inability to develop or to sustain an adequate RSV-specific neutralizing antibody response could also contribute to the pathogenesis of the disease.
Delay in the evaluation of CAP may hinder the diagnosis of a viral pathogen because the virus may no longer be present and because of the difficulty in demonstrating a seroconversion between an acute and convalescent blood sample. Falsey et al [11] have suggested that young, healthy individuals may rapidly clear RSV infection, reducing the viral shedding period. Thus, a delay in the collection of a respiratory sample could explain a lower yield by rtRT–PCR than by serology in RSV-infected adults without preexisting conditions. Nevertheless, in our study, the delay in the sample collection was similar for adults with or without comorbidity (median, 5 days; P = .3); young people tended to consult earlier than adults >50 years of age (median, 4 and 5 days, respectively; P = .05); and the sensitivity of rtRT-PCR was similar between age groups. On the other hand, 40.7% (11 of 27) of cases with positive rtRT-PCR and 27.8% of seroresponders (P = .4) had ≥7 days of symptoms at the time of sample collection. This emphasizes the need of getting specimens for specific diagnosis despite late consultation.
In the 20 cases in which serology identified RSV infection, the explanation could be that lower antibody titers in acute phase sera could facilitate detecting a seroconversion; however, the mean antibody titer was similar (P = .07) among 20 cases with seroconversion and 164 nonseroresponders. In addition, the neutralizing antibody titers were comparable in patients with RSV-positive rtRT-PCR compared with seroresponders (Table 5).
The fact that approximately 10%–30% of adults with documented respiratory viral infection do not have a seroconversion could partially explain the absence of serologic response in patients with positive rtRT-PCR [8]. Also, having in place the appropriate rtRT-PCR procedures and quality controls to reduce contamination reduces the likelihood of false-positive rtRT-PCR results.
Low serum-neutralizing antibody titers against RSV have been associated with severe RSV disease in children and adults [13, 14]. In our study, serum-neutralizing antibody titers to RSV/A and RSV/B were significantly lower in RSV-infected adults compared with those not infected (RSV/A: 8.1 log2 vs 8.9 log2; RSV/B: 9.3 log2 vs 10.4 log2, respectively; P < .02). Likewise, subjects with titers below the minimal protective threshold titer against RSV hospitalization reported by Piedra et al [13] (<6.0 log2 and <8.0 log2 for RSV/A and B, respectively) were detected more frequently in adults with RSV-related CAP than in those with non-RSV CAP (RSV/A: 17% vs 2%, P = .02; RSV/B: 18% vs 6%, P = .03). Furthermore, logistic regression analysis showed a protective role of higher levels of RSV/A- and RSV/B-specific serum-neutralizing antibody against RSV-related CAP and admission to ICU. Thus, a RSV vaccine for increasing neutralizing antibodies titers should reduce hospitalization and death associated with RSV-related CAP in adults.
The epidemiology of RSV-related CAP in adults in Santiago, Chile, mirrors the months when the RSV outbreak occurs in Chile [16], mainly from May through August. Although the majority (62.5%) of RSV-related CAP in adults occurred during the RSV season, many cases were also detected outside this period, stressing the importance of considering RSV etiology even when the outbreak has ended.
In conclusion, RSV infection is frequent in adults with CAP in Santiago, Chile. Sensitive complementary methodologies for detecting RSV should be included in the laboratory tests for identifying the etiologic agents of pneumonia in adults. Molecular diagnostics such as rtRT-PCR is one such test that should be implemented in clinical viral laboratories to improve the detection of respiratory viral pathogens. In addition, serologic assays, especially the microneutralization test, complement molecular diagnostics. Newer, faster, and easier serologic methods, such as flow cytometry–based assay to detect RSV-specific neutralizing antibody [30], could facilitate the use of serology as a reliable diagnostic method. A safe and effective RSV vaccine would also be beneficial for the adult population.
Notes
Acknowledgments.
We thank Dr María Luisa Garmendia for excellent statistical advice and Cristian Moreno for helpful technical assistance.
Financial support.
This work was supported by the Fondo Nacional de Ciencia y Tecnología (grant number 1050734) and Fondo Nacional de Investigación en Salud (grant number SA04 I 2084).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Vila-Corcoles A, Ochoa-Gondar O, Rodríguez-Blanco T, Raga-Luria X, Gómez-Bertomeu F EPIVAC Study Group. Epidemiology of community-acquired pneumonia in older adults: a population-based study. Respir Med. 2009;103:309–16. doi: 10.1016/j.rmed.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone J, Majumdar S, Fox J, Marrie T. Viral infection in adults hospitalized with community- acquired pneumonia. Chest. 2008;134:1141–8. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcos MA, Esperatti M, Torres A. Viral pneumonia. Curr Op Infect Dis. 2009;22:143–7. doi: 10.1097/QCO.0b013e328328cf65. [DOI] [PubMed] [Google Scholar]
- 4.Falsey A. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med. 2007;28:171–81. doi: 10.1055/s-2007-976489. [DOI] [PubMed] [Google Scholar]
- 5.Falsey A, Hennessey P, Formica M, Cox C, Walsh E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 6.Jennings L, Anderson T, Beynon K, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42–8. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 7.Johannson N, Kalin M, Tiveljung-Lindell A, Giske C, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–9. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrickson K, Breese C. Diagnostic assays for respiratory syncytial virus disease. Pediatr Infect Dis J. 2007;26:S36–40. doi: 10.1097/INF.0b013e318157da6f. [DOI] [PubMed] [Google Scholar]
- 9.Templeton K, Scheltinga S, van den Eeden W, Graffelman W, van den Broek P, Claas E. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–51. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcos MA, Camps M, Pumarola T, et al. The role of viruses in the aetiology of community-acquired pneumonia in adults. Antivir Ther. 2006;11:351–9. [PubMed] [Google Scholar]
- 11.Falsey A, Formica M, Walsh E. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral cultures and serology in adults with respiratory illness. J Clin Microbiol. 2002;40:817–20. doi: 10.1128/JCM.40.3.817-820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piedra P, Cron S, Jewell A, et al. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine. 2003;21:2448–60. doi: 10.1016/s0264-410x(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 13.Piedra P, Jewell A, Cron S, Atmar R, Glezen P. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine. 2003;21:3479–82. doi: 10.1016/s0264-410x(03)00355-4. [DOI] [PubMed] [Google Scholar]
- 14.Walsh E, Peterson D, Falsey A. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189:233–8. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- 15.Fine M, Auble T, Yaely D, et al. A prediction rule to identify low risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 16.Avendaño LF, Palomino MA, Larrañaga C. Surveillance for respiratory syncytial virus in infants hospitalized for acute lower respiratory infection in Chile (1989 to 2000) J Clin Microbiol. 2003;41:4879–82. doi: 10.1128/JCM.41.10.4879-4882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Cane P, Pringle C. Molecular epidemiology of respiratory syncytial virus: rapid identification of subgroup A lineages. J Virol Methods. 1992;40:297–306. doi: 10.1016/0166-0934(92)90088-u. [DOI] [PubMed] [Google Scholar]
- 19.Larrañaga C, Kajon A, Villagra E, Avendaño LF. Adenovirus surveillance on children hospitalized for acute lower respiratory infections in Chile (1988–1996) J Med Virol. 2000;60:342–6. [PubMed] [Google Scholar]
- 20.Escobar C, Luchsinger V, de Oliveira DB, Durigon E, Chnaiderman J, Avendaño LF. Genetic variability of human metapneumovirus isolated from Chilean children, 2003–2004. J Med Virol. 2009;81:340–4. doi: 10.1002/jmv.21399. [DOI] [PubMed] [Google Scholar]
- 21.Esper F, Weibel C, Ferguson D, Landry ML, Kahn J. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. 2005;191:492–8. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitkäranta A, Arruda E, Maimberg H, Hayden FG. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791–3. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García D, Hiatt P, Jewell A, et al. Human metapneumovirus and respiratory syncytial virus infections in older children with cystic fibrosis. Pediatr Pulmonol. 2007;42:66–74. doi: 10.1002/ppul.20546. [DOI] [PubMed] [Google Scholar]
- 24.Harmon M, Kendal A. Influenza viruses. In: Schmidt N, Emmons R, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. 6th ed. Washington, DC: APHA; 1989. pp. 631–68. [Google Scholar]
- 25.Bartlett JG, Breiman RF, Mandell LA, File TM. Community acquired pneumonia in adults: guidelines for management. Clin Infect Dis. 1998;26:811–38. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 26.Maass M, Bartels C, Kruger S, et al. Endovascular presence of Chlamydia pneumoniae DNA is a generalised phenomenon in atherosclerotic vascular disease. Atherosclerosis. 1998;140:S25–30. doi: 10.1016/s0021-9150(98)00117-8. [DOI] [PubMed] [Google Scholar]
- 27.Tjhie JH, Van Kuppeveld FJ, Roosendaal R, et al. Direct PCR enables detection of Mycoplasma pneumoniae in patients with respiratory tract infections. J Clin Microbiol. 1994;32:11–6. doi: 10.1128/jcm.32.1.11-16.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonas D, Rosenbaum A, Weyrich S, Bhakdi S. Enzyme-linked immunoassay for detection of PCR-amplified DNA of Legionellae in bronchoalveolar fluid. J Clin Microbiol. 1995;33:1247–52. doi: 10.1128/jcm.33.5.1247-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terrosi C, Di Genova G, Martorelli B, Valentini M, Cusi M. Humoral immunity to respiratory syncytial virus in young and elderly adults. Epidemiol Infec. 2009;137:1684–6. doi: 10.1017/S0950268809002593. [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Chang J, Nason M, et al. A flow cytometry-based assay to assess RSV-specific neutralizing antibody is reproducible, efficient and accurate. J Immunol Methods. 2010;362:180–4. doi: 10.1016/j.jim.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


