Abstract
Pulmonary edema (PE) may occur with enterovirus 71 (EV71) infection. We monitored arterial pressure (AP) and heart rate (HR) in patients with EV71 infection and analyzed the variability of AP and HR. Sympathetic activity, AP, and HR increased with respiratory stress. Thereafter, parasympathetic activity increased with decreases in AP and HR. The lungs showed edema with inducible nitric oxide synthase (iNOS) expression. Destruction of the medial, ventral, and caudal medulla may lead to sympathetic overactivation, causing blood to shift to the lungs. The pathogenesis of PE may also involve iNOS and nitric oxide.
Epidemic or sporadic infection with enterovirus 71 (EV71) usually occurs during the summer months. Children infected with EV71 develop rashes on the hand, foot, and mouth. Most patients recover without complications. Some patients manifest signs such as headache, profuse sweating, lethargy, paraparesis, and weakness of the limbs. Several patients infected with EV71 have died of fulminant pulmonary edema (PE) soon after infection [1, 2]. The pathogenesis of PE is still unknown. Chang et al. [2] postulated that viral destruction of the CNS may be the cause of death. Central sympathetic activation resulting in systemic vasoconstriction and the shift of blood volume to the pulmonary region of the circulatory system, as demonstrated in earlier findings from our laboratory, was used to explain centrogenic PE [2, 3]. However, studies by Chang et al. [1, 2] did not provide pathological evidence of CNS lesions, nor did they assess autonomic activity. In the present study, we report CNS lesions in patients associated with PE due to enterovirus infection. Spectral analysis of arterial pressure (AP) and heart rate (HR) variability were employed as a measure of autonomic activities [4–7].
Expression of nitric oxide synthase (NOS) mRNA in the lungs was performed using RT-PCR. The purpose was to elucidate the possible involvement of nitric oxide in the pathogenesis of acute PE associated with enterovirus infection.
Subjects and methods. During a 2-year period, we obtained data from a total of 48 patients (29 male and 19 female subjects), aged 6–18 years, who were treated during the summer months (May–August 2001, 2002, and 2003) in hospitals in Taipei and Hualien. All subjects had developed rashes on the hand, foot, and mouth. For each of these patients, enterovirus virus infection with hand, foot, and mouth disease was suspected. Each subject underwent chest radiography and a routine physical examination at admission. Blood cell counts, blood gas levels, and biochemical factors, such as glucose level, creatinine level, urea nitrogen level, and hemoglobin level, were assessed. CSF samples were obtained and used to determine protein concentration and WBC count. Swab samples from the throat, rectum, and vesicles were obtained. Neurological and respiratory signs were observed. During episodes of acute respiratory distress syndrome (ARDS), AP and HR were continuously monitored with use of a polygraph and a tape recorder, allowing a retrospective analysis of AP and HR variability [4, 7]. Consent for autopsy of patients who died of acute PE was obtained from the patients themselves (in 4 cases) or from their relatives. Postmortem specimens obtained from the brain and lungs were examined for pathological lesions. RT-PCR was used for detection of iNOS mRNA expression in the lungs. RT-PCR was performed according to the manufacture's instructions (Alpha-Innotech). Consent for autopsy of patients who died of acute pulmonary edema was obtained from the patients themselves or from their relatives. The study was approved by the Institutional Board for Human Studies of Taipei Medical University.
Results. Isolates from swabs samples obtained from the rectum, throat, and vesicles were identified as EV71 by immunofluorescence with monoclonal antibody (Chemical International) [2]. EV71 was detected in rectal (for 4 [8%] of 48 children), throat (34 [71%] of 48), and vesicle (39 [81%] of 48) swab samples. At admission, chest radiograph findings showed clear lungs for all patients (figure 1A). Signs such as profuse sweating, insomnia, increased startle reflex, tachycardia, dyspnea, vomiting, cold skin, and elevated oral temperature developed after admission. After receipt of intensive care and respiratory therapy over a period of 8 days, the symptoms gradually subsided in 27 of the patients (15 male and 12 female patients). Twenty-one patients (14 male and 7 female patients) developed severe dyspnea, hyperglycemia, leukocytosis, and decreased blood oxygen partial pressure. AP and HR fluctuation ensued. In these 21 patients, frothy pink secretions (and even fresh blood) was expelled from the endotracheal tube. Each of these 21 patients died within 4 h after the development of ARDS. In each, the protein concentration and WBC count in CSF samples were greatly elevated. Chest radiography revealed marked lung infiltration (figure 1B).
Figure 1.
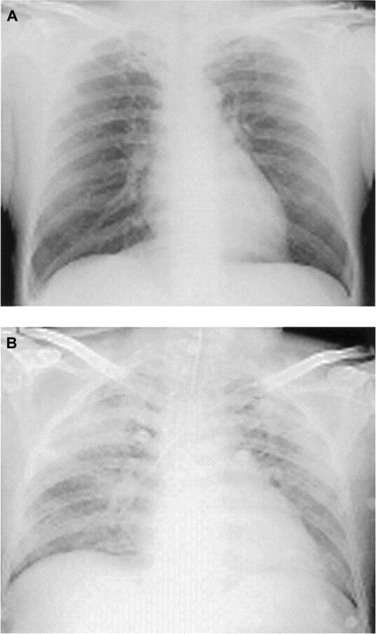
Chest radiographs showing a clear lung at admission (A) and severe pulmonary infiltration after the onset of acute respiratory distress syndrome (B).
Autopsy was performed with prior consent for 4 of the 21 patients who died of acute PE. Figure 2 shows an immunohistochemical stain of brain lesions using an antienterovirus monoclonal antibody [8, 9]. The brain lesions were characterized by the formation of nodules in axonal and dendritic processes (figure 2A). Distribution of the viral lesions was predominantly in the medial and caudal medulla (figure 2B). A horizontal section (figure 2C) revealed the destruction of the ventral medulla. These regions are considered to be the central depressor areas [10]. Accordingly, the sympathetic drive was increased following the destruction of the depressor areas. This contention was confirmed by spectral analysis of the AP and HR variabilities that occurred during the episode of ARDS. Figures 3 and 4 show changes in AP and HR in a representative patient at the onset of illness (A), 1 h after onset (B), and 30 min before death (C).
Figure 2.

Brain lesions in patients with enterovirus infection. An iimmunohistochemical stain (A) shows the formation of nodules in neuronal cell bodies and dendrites. The horizontal section (B) indicates that viral destruction is predominantly located in the medial and caudal areas of the medulla oblongata. The cross-section (C) illustrates the lesions in the ventral medulla. A few lesions are observed in the cortex, pons, cerebellum, and other areas.
Figure 3.
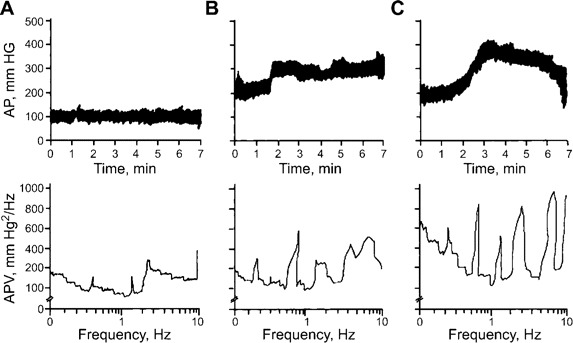
Arterial pressure (AP) and AP variability in a patient with acute respiratory distress at the onset of respiratory distress (A), 1 h after the onset (B), and 30 min before death (C). Note the increase and fluctuation in AP during the course of the episode, with an increase in low frequency power spectrum 30 min before death.
Figure 4.
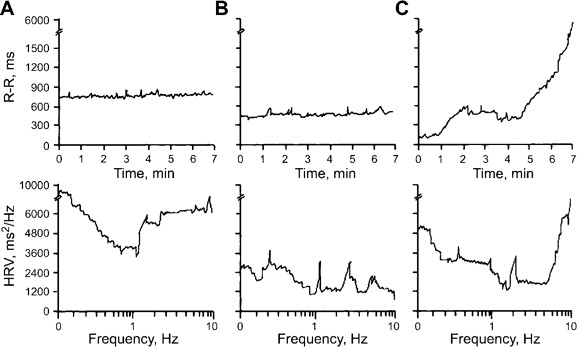
Heart rate (HR; the reverse of the R-R interval) and HR variability (HRV) in a patient with acute respiratory distress syndrome at the onset of dyspnea (A), 1 h after onset (B), and 30 min before death (C). Note the dominant high frequency power spectrum at the onset of dyspnea and the decrease in HR before death, with a further increase in the high frequency domain.
Before the onset of ARDS, the patient's AP and HR were within normal limits. Later, the AP and HR (the reverse of the R-R interval) increased and fluctuated. Before death, there was a dramatic decrease in AP and HR. The patient's blood glucose level (± SE) was 7.6 ± 0.4 mmol/L at admission. Hyperglycemia (blood glucose level ± SE, 26.7 ± 2.5 mmol/L) developed. At admission, the patient's WBC count ± SE was 15.6 ± 3.2 × 109 cells/L. An elevated WBC count (WBC count ± SE, 48.6 ± 9.4 × 109 cells/L) was noted at the onset of ARDS. In samples of the CSF, WBC count and protein concentration were 15.3 ± 1.9 × 107 cells/L and 45 ± 6 mg/dL, respectively. During the episode of ARDS, WBC count and protein concentration in CSF samples increased to 105.3 ± 8.7 × 107 cells/L and 210 ± 8 mg/dL, respectively.
Frequency domain analysis of the AP and HR variabilities revealed increases in very low frequency and low frequency at the onset of ARDS. The very low frequency and low frequency continuously increased during the episode of ARDS (figures 2 and 3). Pathological examinations of the lungs revealed severe hemorrhagic edema (not shown). The iNOS expression in the lungs was greatly increased (figure 5).
Figure 5.
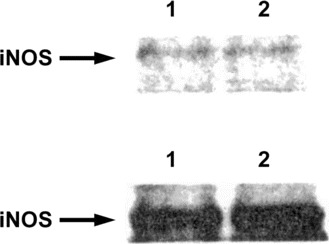
Inducible nitric oxide synthase (iNOS) mRNA expression in the lung of a patient with acute respiratory distress syndrome (lower panel) and in the lung of a patient who died of another disease (upper panel). Biopsy specimens from 2 sites within each lung (labelled 1 and 2) were examined. Note the elevated iNOS expression in the lung of the patient with acute respiratory distress syndrome (lower panel), compared with that in the lung of the patient who died of another disease (upper panel).
Discussion. Our laboratory has performed a series of studies on the mechanisms of acute PE induced by cerebral compression or increased intracranial pressure [3, 11–13]. The PE associated with ARDS is usually fatal. In brief, central sympathetic overactivation produces systemic hypertension and vasoconstriction. These changes cause a shift of the blood to the pulmonary circulatory system [3]. We extended the study from animals to human subjects. Acute PE can be induced in rare cases of Japanese B encephalitis, fat embolism, breast cancer with lymphangitis, and rupture of intracranial aneurysm [14]. The lesions in the CNS following EV71 infection that were observed in the current study matched those that we have previously observed in patients who died of Japanese B encephalitis [14]. In a recent review article [13], we described and discussed early and recent studies of ARDS in animals and humans and recommended further studies on the mechanism, prevention, and treatment of ARDS in the clinical practice of intensive care. The present study also supports the contention that hyperglycemia, leukocytosis, and elevated protein levels in CSF are risk factors for the development of ARDS due to enterovirus infection [1, 2]. The relationship between hyperglycemia and ARDS remains an interesting topic for future study.
Our research demonstrated enhanced iNOS mRNA expression in the hemorrhagic lung. The increase in iNOS expression does not definitely indicate that nitric oxide formation is detrimental to the lung. Our previous studies [15, 16] found that exogenous and endogenous nitric oxide were toxic to the lung in cases of sepsis and of ischemia and reperfusion. Therefore, nitric oxide production may be responsible for the pathogenesis of ARDS due to EV71 infection, and selective iNOS blockers may be therapeutic agents for ARDS induced by EV71 or by other viral infections.
In the present report, we did not measure the pulmonary AP, which was demonstrated to be high during the development of PE [3, 11, 14, 16]. It is well known that nitric oxide exerts vasodilatory effects in various vascular beds. The production of nitric oxide through iNOS could reduce pulmonary hypertension. On the other hand, it may increase the oxygen free radicals and recruit more capillaries to increase pulmonary microvascular permeability. In this connection, we found that endogenous and exogenous nitric oxide reduced pulmonary hypertension but increased the capillary filtration coefficient and the extent of injury in an isolated rat's lung that was subjected to ischemia and reperfusion [16].
Viral lesions in the brain were found in the cortex, pons, medulla, cerebellum, and spinal cord. The brain stem lesions were predominantly in the ventral, medial, and caudal medulla. These areas have been considered to the central depressor or sympathetic inhibitory mechanisms in the vasomotor center [10]. Destruction of these areas causes an increase in the sympathetic drive. In a recent report from our laboratory [14], we also found that viral lesions caused by Japanese B encephalitis were similar to those caused by EV71 infection. However, whether the same brain stem lesions are caused by other neurotropic viruses (such as coronavirus, rabies, and others) remains to be investigated.
In conclusion, the present study suggests that death due to EV71 infection likely results from brain stem lesions. The production of nitric oxide through iNOS may also be involved in the pathological changes in the lung.
Acknowledgments
We are grateful to Dr. C. T. Hu (Skanhex Technology) for the spectral analysis of AP and HR variabilities and to Ms. C. C. Chang for the preparation of this manuscript.
Financial support. National Science Council (grants NSC 90-2320-B-320-04 and NSC 91-2320-B-320-008), Shin-Kong Wu-Ho Su Memorial Foundation, and Outstanding Scholarship Development Foundation (1998–2003).
References
- 1.Chang LY, Huang YC, Lin TY. Fulminant neurogenic pulmonary edema with hand, foot and mouth disease. Lancet. 1998;352:367–8. doi: 10.1016/S0140-6736(98)24031-1. [DOI] [PubMed] [Google Scholar]
- 2.Chang LY, Lin TY, Hsu KH, et al. Clinical feature and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet. 1999;354:1682–6. doi: 10.1016/S0140-6736(99)04434-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen HI, Liao JF, Kuo L, Ho ST. Centrogenic pulmonary edema induced by cerebral compression in rats. Circ Res. 1980;47:366–73. doi: 10.1161/01.res.47.3.366. [DOI] [PubMed] [Google Scholar]
- 4.Yang CCH, Chao TC, Kuo TJB, et al. Preeclamptic pregnancy is associated with increased sympathetic and decreased parasympathetic control of HR. Am J Physiol. 2000;278:1269–73. doi: 10.1152/ajpheart.2000.278.4.H1269. [DOI] [PubMed] [Google Scholar]
- 5.Yien HW, Hseu SS, Lee LC. Spectral analysis of systemic arterial pressure and heart rate signals as a prognostic tool for the prediction of patient outcome in intensive care unit. Crit Care Med. 1997;25:258–66. doi: 10.1097/00003246-199702000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Baillard C, Vivien B, Mansier P. Brain death assessment using instant spectral analysis of heart rate variability. Crit Care Med. 2002;30:306–10. doi: 10.1097/00003246-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kuo TBJ, Yien HW, Hseu SS, et al. Diminished vasomotor component of systemic arterial pressure signals and baroreflex in brain death. Am J Physiol. 1997;273:1291–8. doi: 10.1152/ajpheart.1997.273.3.H1291. [DOI] [PubMed] [Google Scholar]
- 8.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase technique: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:557–80. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RT, Burke DS, Elweel M. Japanese encephalitis: immunocytochemical studies of viral antigen and inflammatory cells in fatal case. Ann Neurol. 1985;18:567–73. doi: 10.1002/ana.410180510. [DOI] [PubMed] [Google Scholar]
- 10.Chai CY, Wang SC. Localization of central cardiovascular control mechanism in lower brain stem of the cat. Am J Physiol. 1962;202:25–30. doi: 10.1152/ajplegacy.1962.202.1.25. [DOI] [PubMed] [Google Scholar]
- 11.Chen HI, Sun SC, Chai CY. Pulmonary edema and hemorrhage resulting from cerebral compression. Am J Physiol. 1973;224:223–9. doi: 10.1152/ajplegacy.1973.224.2.223. [DOI] [PubMed] [Google Scholar]
- 12.Chen HI, Lin JD, Liao JF. Participation of regional sympathetic outflows in the centrogenic pulmonary pathology. Am J Physiol. 1981;240:109–15. doi: 10.1152/ajpheart.1981.240.1.H109. [DOI] [PubMed] [Google Scholar]
- 13.Chen HI, Kao SJ, Wang D, et al. Acute respiratory distress syndrome. J Biomed Sci. 2003;10:588–92. doi: 10.1007/BF02256308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu YH, Kao SJ, Lee RP, Chen HI. Acute pulmonary oedema: rare causes and possible mechanisms. Clin Sci. 2003;104:259–64. doi: 10.1042/CS20020166. [DOI] [PubMed] [Google Scholar]
- 15.Lee LP, Wang D, Kao SJ, Chen HI. The lung is the major site that produces acute pulmonary oedema in endotoxin shock. Clin Exp Pharmacol Physiol. 2001;28:315–20. doi: 10.1046/j.1440-1681.2001.03446.x. [DOI] [PubMed] [Google Scholar]
- 16.Kao SJ, Peng TC, Lee RP, et al. Nitric oxide mediates lung injury induced by ischemia-reperfusion in rats. J Biomed Sci. 2003;10:58–64. doi: 10.1007/BF02255998. [DOI] [PubMed] [Google Scholar]


